Abstract
Background: Human immunodeficiency virus (HIV) is a virus that affects the immune system, the body’s natural defence system. It is a virus spreading through certain body fluids that attacks the body’s immune system, specifically the Cluster of Differentiation 4 (CD4) T-cells. Anemia is a common manifestation of pediatric HIV infection and is a significant negative predictor of survival. Moreover, undernutrition is the underlying cause of death among 35% of children aged under 5 years, and it has been negatively implicated with antiretroviral therapy (ART) outcomes, particularly in developing countries. The aim of this study was to determine the magnitude of anemia and undernutrition among HIV-infected children within the first year of ART initiation at University of Gondar comprehensive specialized hospital ART clinic. Methods: Records of 200 children aged <15 years old who were on ART at the University of Gondar comprehensive specialized hospital from 2005 to 2017 were retrospectively reviewed in 2017. Baseline characteristics and one-year flow-up data after ART initiation were extracted from the medical records. Anemic status was determined based on the hemoglobin (Hb) level in accordance with World Health Organization (WHO) guideline. The nutritional status was calculated based on anthropometric measurements. Generalized Estimating Equation (GEE) was fitted to identify factors associated with anemia and undernutrition. Odds ratio with the corresponding 95% Confidence interval (CI) was reported. Results: Of the total children, 75 (37.5%) (95% CI: 30.73-44.27%) were anemic at the baseline of ART initiation. The magnitude of anemia has shown a persistent decline from the baseline to 12th months of ART initiation. At ART initiation, the magnitude of undernutrition was high, 64% (95% CI: 57.3-70.7%). Similarly, the magnitude of undernutrition showed decrement during a one year ART initiation period. Stunting was the most common type of undernutrition at baseline (49.5%), 6 months (44%), 9 months (41%), and 12 months (39%) of ART initiation. Baseline CD4 count, Baseline WHO clinical stage and age at enrollment to the care were significantly associated with anemia within the first year of ART initiation. Conclusion: Despite a decline in the first year of ART initiation, anemia and undernutrition were public health problems in HIV-infected children. Hence, for HIV-infected children taking HAART, emphasis should be given to manage anemia and undernutrition within the first year of ART initiation.
Keywords: Anemia, ART, children, HAART, HIV, undernutrition
Introduction
HIV is one of the world’s most serious health and development challenges. There were an estimated 37.9 million (32.7-44 million) people living with HIV/AIDS at the end of 2018 globally; of which, 1.7 million (1.3-2.2. million) were children (<15 years old). In 2018, around 1.7 million (1.4-2.3 million) individuals were newly infected with HIV globally. This includes 160,000 (110,000-260,000) children (<15 years) [1]. Most of these children were living in sub-Saharan Africa and acquired the infection from their HIV-positive mother during pregnancy, childbirth (labour and delivery) and breastfeeding [2].
In the WHO African Region, there are an estimated 25.7 million people living with HIV. This region also accounts for almost two-thirds of the global total of new HIV infections. In 2018, about 1.1 million people were newly infected with HIV. Ten countries including Ethiopia were accounting for 80% of all people living with HIV in the region [3]. Based on the United Nation on HIV/AIDS estimation, about 700,000 (1.2%) of people were living with HIV/AIDS in Ethiopia by the end of 2018 [4]. In the latest WHO estimate, there were 62,000 (42,000-84,000) children aged 0-14 year living with HIV in 2016; of which 21,700 (35%) of them were receiving ART [5].
Anemia is a common manifestation of pediatric HIV infection and is a significant negative predictor of survival. It occurs 50%-90% of children living in both resource-limited and resource-rich settings [6]. It is the commonest hematological disorder affecting people living with HIV. Studies have shown that the prevalence of anemia in HIV/AIDS disease could exceed 80% depending on the region and threshold used to define anemia. HIV-related anemia may be as a result of opportunistic infections, side effect of chemotherapeutic treatment, changes in cytokine expression with a resultant decrease in blood cell production, and a consequence of micronutrient deficiencies [7].
Undernutrition is the underlying cause of death among 35% of children aged <5 years and could lead to irreversible damages such as cognitive impairment, chronic diseases and growth failure [8]. It is a major public health problem for children and especially for HIV-infected children since it creates a vicious circle with HIV infection. Indeed, on the one hand, undernutrition worsens HIV/AIDS disease as it has similar effects on the immune system as HIV infection does. For example, among undernourished people, lymphoid tissues are damaged, and CD4 T-cell concentration is decreased [9]. Wasting and weight loss are common features of HIV infection, especially in resource-limited settings [10]. Evidence revealed that undernutrition is common in HIV-infected patients and is a challenge to achieve the desired outcome of ART, particularly in Africa [8,11]. A study done in Central and West African countries revealed that approximately 42% (95 CI: 40%-44%) of HIV-infected children were undernourished [8]. Undernutrition has been strongly associated with mortality in children infected with HIV after initiation of ART [12] and is an indicator of impaired response to ART [13].
Even though there are cross-sectional level studies on the magnitude of anemia and undernutrition among HIV-infected children in Ethiopia, there is a limited data about the changes within the first year of ART initiation, particularly in Northwest part of Ethiopia. Therefore, this study aimed at determining the magnitude and change of anemia and undernutrition in HIV-infected children within a one-year of highly active antiretroviral therapy (HAART) initiation at the University of Gondar comprehensive specialized hospital pediatric ART clinic.
Materials and methods
Study area, and design
An institutional-based retrospective follow-up study was conducted at the University of Gondar comprehensive specialized hospital pediatrics ART clinic from March to May 2017. The medical records of HIV-infected children who registered for ART from 2005 to 2017 were reviewed. The hospital is located in Gondar town, Amhara regional state, Northwest Ethiopia, which is 750 km from Addis Ababa, the capital city of Ethiopia; and 180 km from Bihar Dar, the capital city of the region. The ART service of the hospital was initiated in 2005, and it has 7 outpatient rooms, one voluntary testing and counselling room, one pharmacy, and one laboratory. The ART service has three adult ART clinics, one pediatric, one voluntary counselling and testing and 2 adherence counselling clinics. Based on the information obtained from the hospital, since the hospital started ART service, 7581 adults and 738 pediatric HIV-infected patients had been enrolled for ART service until the end of 2016.
Source and study population
All HIV-infected children who took HAART at the University of Gondar comprehensive specialized hospital pediatric ART clinic were source population. The study population included all HIV-infected children who took HAART for at least one year at the University of Gondar comprehensive specialized hospital Pediatric ART clinic.
Sample size, and sampling technique
Since the study was retrospective, the sample size calculation was not applicable. A total of 540 HIV-infected children who took or were taking HAART were registered. From those children, 200 of them who took HAART for at least one year and had a complete information for key variables were included in this study.
Inclusion and exclusion criteria
All data from the HIV-infected children who took HAART for one year at the University of Gondar comprehensive specialized hospital pediatric ART clinic were included; whereas those HIV-infected children who took HAART for at least one year with incomplete data on Hb, weight, height and age were excluded from the study.
Data collection
All available information on patient records were checked. Data extraction format was prepared based on the Ethiopian Federal Ministry of Health National Guidelines for Comprehensive HIV Prevention, Care and Treatment, 2014 [14]. Besides, the extraction checklist was further customized based on the children ART monitoring record book. The tool was used to extract data like socio-demographic status, Hb value, weight, height, CD4 cell count, type of ART regimens used, WHO clinical stages, tuberculosis (TB) screening results, and other characteristics at baseline (before initiation of ART), and at 6 months, 9 months and one year after ART initiation.
Definition of outcomes
The anemia among children was defined based on the altitude adjusted Hb values as follows: Hb<11 g/dl for children <5 years old, Hb<11.5 g/dl for children 5-11.9 years old and <12 g/dl for children 12-14.9 years old. Similarly, the severity of anemia was categorized based on the recommendation of WHO guideline taking age into consideration [15]. According to the WHO child growth standards, undernutrition in children was defined as anthropometric indices less than -2 standard deviations. Children were categorized as underweight (Z-score of weight-for-age (WAZ) or body mass index (BMI)-for-age <-2), stunted (Z score of height-for-age (HAZ) <-2), and wasted (Z score of weight-for-height (WHZ) <-2) [16].
Data analysis, and interpretation
Data were cleared, entered into Epi info version 3.5.3 and then transferred to Statistical Package SPSS version 20 software for analysis. Descriptive analyses were conducted to describe the characteristics of study participants, and the results were presented in tables. Undernutrition and anemia were described in relation to demographic and clinical characteristics of the study participants at baseline, 6 months, 9 months and 12 months of ART initiation. The proportion with its corresponding 95% CI was estimated for undernutrition and anemia. The change in the magnitude of undernutrition and anemia during the first one year of ART initiation period was presented in a line graph. Bivariable correlation analysis was done to see the correlation of Hb and nutritional indices with time within one year of ART initiation. As anemia and undernutrition were binary outcome variables with repeated measurement over one year of ART initiation, we fitted a GEE to identify factors associated them. All variables with p-value <0.25 in the bivariable GEE model were fitted into multivariable GEE model. In bivariable GEE, both crude odds ratio (COR) and unadjusted beta coefficient (B) with the corresponding 95% CI were reported. Similarly, in multivariable GEE model, both adjusted odds ratio (AOR) and adjusted B and the corresponding 95% CIs were reported to see the strength of association. A p-value of <0.05 in multivariable analysis was considered statistically significant.
Ethics approval
The ethical approval was obtained from the Ethics and Research Review committee of the School of Biomedical and Laboratory Science, College of Medicine and Health Sciences, the University of Gondar (Ref. No: SBLS/659/09; Issue date: 13 March, 2017). Letter of permission was obtained from the clinical director office of the University of Gondar comprehensive specialized hospital. The ART clinics and Medical record office had been communicated before data collection. Study participants’ name and identification number were not extracted to ensure confidentiality.
Results
Characteristics of study population
Among 200 children included, 117 (58.5%) were females; 118 (59%) were below the age of five years at enrollment; and 181 (90.5%) were from urban area. The majority, 164 (67%), of the HIV-infected children had a biological relationship with their caregivers (Table 1). Out of the total children, 129 (64.5%) and 18 (9%) of them were at WHO clinical stage I and II, and had a history of tuberculosis treatment, respectively. Concerning the functional status before the initiation of HAART, 89 (44.5%), 105 (52.5%) and 6 (3%) were working/appropriate, ambulatory/delayed and bedridden/regression, respectively (Table 1).
Table 1.
Socio-demographic and Clinical characteristics of HIV-infected children who were on HAART and their caregivers at university of Gondar comprehensive specialized hospital ART clinics
| Characteristics | Frequency (%) | |
|---|---|---|
| Age at enrollment (year) | <5 | 118 (59) |
| ≥5 | 82 (41) | |
| Sex | Male | 83 (41.5) |
| Female | 117 (58.5) | |
| Residence | Urban | 181 (90.5) |
| Rural | 19 (9.5) | |
| Caregiver’s religion | Orthodox | 152 (76) |
| Muslim | 48 (24) | |
| Caregiver’s marital status | Single | 18 (9) |
| Married | 150 (75) | |
| Divorced/widowed | 32 (16) | |
| Caregiver’s educational status | Unable to read and write | 59 (29.5) |
| Primary school | 141 (70.5) | |
| Caregiver’s occupation | Unemployed and daily laborer | 48 (24) |
| Government employed | 13 (6.5) | |
| Farmer | 7 (3.5) | |
| Merchant | 54 (27) | |
| House wife | 78 (39) | |
| Caregiver’s relation with child | Biological | 134 (67) |
| Relatives | 66 (33) | |
| WHO clinical stage | Stage I and II | 129 (64.5) |
| Stage III and IV | 71 (35.5) | |
| Functional status of child | Working/appropriate | 89 (44.5) |
| Ambulatory/delayed | 105 (52.5) | |
| Bedridden/regression | 6 (3) | |
| Tb treatment history | Yes | 18 (9) |
| No | 182 (91) | |
Magnitude of anemia
Of the total children, 75 (37.5%) (95% CI: 30.73-44.27%) were anemic at the baseline of ART initiation. Likewise, 33 (40.2%) and 42 (35.6%) of HIV-infected children aged greater than or equal to five years, and aged less than five years old were anemic at the baseline, respectively. The magnitude of anemia in male (31 (37.3%)) and female (44 (37.6%)) was nearly the similar at baseline. The magnitude of anemia at 6, 9 and 12 months after ART initiation was 44 (22%) (95% CI: 16.21-27.79%), 41 (20.5%) (95% CI: 14.86-26.14%) and 36 (18%) (95% CI: 12.63-23.37%), respectively (Table 2). This indicates that the magnitude of anemia had shown decrement after ART initiation (Figure 1).
Table 2.
Anemia prevalence according to Socio-demographic characteristics and WHO clinical stage of children who took HAART at least 12 months at University of Gondar comprehensive specialized hospital ART clinic
| Characteristics | Anemic status during ART follow-up | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| At baseline of ART initiation | At 6 months of ART Initiation | At 9 months of ART initiation | At 12 months of ART | ||||||
|
|
|
|
|
||||||
| Anemic (n (%)) | Non-anemic (n (%)) | Anemic (n (%)) | Non-anemic (n (%)) | Anemic (n (%)) | Non-anemic (n (%)) | Anemic (n (%)) | Non-anemic (n (%)) | ||
| Age at enrolment (year) | <5 years | 42 (35.6) | 76 (64.6) | 25 (21.2) | 93 (78.8) | 22 (18.6) | 96 (81.4) | 21 (17.8) | 97 (82.2) |
| ≥5 years | 33 (40.2) | 49 (59.8) | 19 (23.2) | 63 (76.8) | 19 (23.2) | 63 (76.8) | 15 (18.3) | 67 (81.7) | |
| Sex | Male | 31 (37.3) | 52 (62.7) | 21 (25.3) | 62 (74.7) | 23 (27.7) | 60 (72.3) | 20 (24.1) | 63 (75.9) |
| Female | 44 (37.6) | 73 (62.4) | 23 (19.7) | 94 (80.3) | 18 (15.4) | 99 (84.6) | 16 (13.7) | 101 (86.3) | |
| Residence | Urban | 67 (37) | 114 (63) | 38 (21) | 143 (79) | 38 (21) | 143 (79) | 32 (17.7) | 149 (82.3) |
| Rural | 8 (42.1) | 11 (57.9) | 6 (31.6) | 13 (68.4) | 3 (15.8) | 16 (84.2) | 4 (21.1) | 15 (78.9) | |
| Religion of caregiver | Orthodox | 54 (35.5) | 98 (64.5) | 33 (21.7) | 119 (78.3) | 4 (22.4) | 118 (77.6) | 32 (21.1) | 120 (78.9) |
| Muslim | 21 (43.8) | 27 (56.2) | 11 (22.9) | 37 (77.1) | 7 (14.6) | 41 (85.4) | 4 (8.3) | 44 (91.7) | |
| Occupation of caregiver | Non-employee | 16 (12.8) | 8 (10.7) | 20 (12.8) | 4 (9.1) | 19 (11.9) | 5 (12.2) | 20 (12.2) | 4 (11.1) |
| Gov’t employee | 7 (5.6) | 6 (8) | 9 (5.8) | 4 (9.1) | 10 (6.3) | 3 (7.3) | 11 (6.7) | 2 (5.6) | |
| Farmer | 5 (4) | 2 (2.7) | 5 (3.2) | 2 (4.8) | 5 (3.1) | 2 (4.9) | 5 (3) | 2 (5.6) | |
| Merchant | 30 (24) | 24 (32) | 45 (28.8) | 9 (20.5) | 45 (29.3) | 9 (22) | 48 (29.3) | 6 (16.7) | |
| Daily laborer | 13 (13.6) | 7 (9.3) | 19 (12.2) | 5 (11.4) | 20 (12.6) | 4 (9.8) | 21 (12.8) | 3 (8.3) | |
| Housewife | 50 (40) | 28 (37.3) | 58 (37.2) | 20 (45.5) | 60 (37.7) | 18 (43.9) | 59 (36) | 19 (52.8) | |
| WHO clinical stage | I | 16 (23.2) | 53 (76.8) | 13 (15.1) | 73 (84.9) | 19 (16.5) | 96 (83.5) | 18 (14.1) | 110 (85.9) |
| II | 29 (48.3) | 31 (51.7) | 15 (28.8) | 37 (71.2) | 13 (30.2) | 30 (69.8) | 14 (38.9) | 22 (61.1) | |
| III | 21 (37.5) | 35 (62.5) | 11 (23.9) | 35 (76.1) | 6 (16.7) | 30 (83.3) | 2 (16.5) | 29 (93.5) | |
| IV | 9 (60) | 6 (40) | 5 (31.2) | 11 (68.8) | 3 (50) | 3 (50) | 2 (40) | 3 (60) | |
Figure 1.
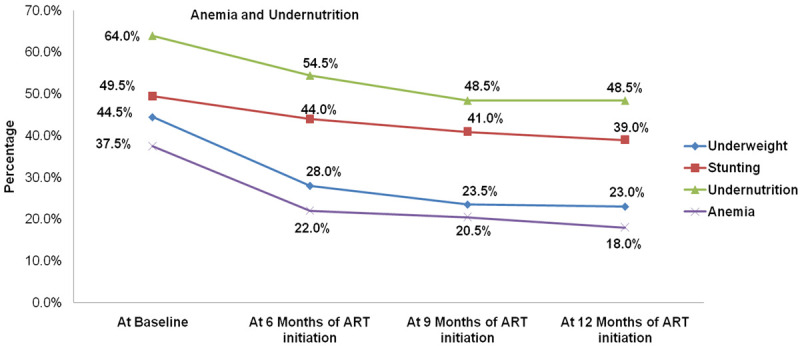
Magnitude of anemia and undernutrition of study participants at baseline, 6 months, 9 months and 12 months of ART initiation. The picture depicts the overtime change of anemia and undernutrition within one year of ART initiation among children who had been taking ART medication at University of Gondar compressive specialized hospital ART clinic.
In this study, anemia was classified into mild, moderate and severe based on the altitude adjusted Hb values [12]. Of the total anemic children, 41 (54.7%) and 34 (45.3%) of them had mild and moderate anemia at the baseline of ART initiation, respectively. At the 6 months of ART initiation, 18 (40.9%) of them were mildly anemic whereas 26 (59.1%) of them were moderately anemic. Moreover, at 9 and 12 months of ART initiation, 41 (75.6%) and 21 (60.0%) were mildly anemic; and 10 (24.4%) and 14 (40.0%) were moderately anemic, respectively (Table 3).
Table 3.
Severity of anemia with respect to ART follow-up period at the University of Gondar comprehensive specialized hospital pediatrics ART clinic
| Anemic status | Anemia severity | |
|---|---|---|
|
| ||
| Mild anemia, n (%) | Moderate anemia, n (%) | |
| At baseline of initiation | 41 (54.7) | 34 (45.3) |
| At 6 months of ART initiation | 18 (40.9) | 26 (59.1) |
| At 9 months of ART initiation | 31 (75.6) | 10 (24.4) |
| At 12 months of ART initiation | 21 (60.0) | 14 (40.0) |
At the initiation of ART, the magnitude of anemia with WHO clinical stage I, II, III and IV was 23.2%, 48.3%, 37.5% and 60%, respectively. With respect to the WHO clinical stage, anemia was more common in advanced and severe stages than stage I and II. Furthermore, as depicted in Table 2, the magnitude of anemia had shown a decrement after 6 months, 9 months and 12 months of ART initiation with respect to the WHO clinical stage (Table 2). In addition to this, Hb concentration had shown a significant positive correlation with time of ART initiation within one year of treatment (Spearman’s rank-order correlation (rho) = 0.192, P<0.01).
Magnitude of undernurition
The magnitude of underweight, stunting and undernutrition had shown decrement during the first year of ART initiation. At the baseline, the magnitude of underweight, stunting and undernutrition was 44.5% (95% CI: 37.6-51.5%), 49.5% (95% CI: 42.5-56.5%) and 64.0% (95% CI: 57.3-70.7%), respectively. Figure 1 showed that the magnitudes of underweight, stunting and undernutrition have declined at 6 and 9 months of ART initiation compared to the baseline. However, there was no such large difference between 9 and 12 months of ART initiation (Figure 1). Besides, the bivariable correlation analyses had showed an overall weak negative correlations between time and nutritional indices over one year of ART initiation.
Factors associated with anemia and undernutrition
Compared to children at WHO clinical stage I at baseline of ART initiation, those who were at stage II-IV had higher risk of anemic within one year of ART initiation (AOR = 1.73; 95% CI: 1.02-3.0; P = 0.048). However, a unit month increased in age of children at enrollment to the care (AOR = 0.97; 95% CI: 0.95-0.99; P = 0.026) and baseline CD4 count (AOR = 0.95; 95% CI: 0.93-0.98; P<0.01) had reduced the risk of anemia. Regarding undernutrition, there was not statistically significant factor in multivariable GEE model (Table 4).
Table 4.
Factors associated the magnitude of anemia and undernutrition in HIV-infected children within one year of HAART initiation using GEE model
| Factors associated with magnitude of anemia from baseline to 12 months of ART initiation | |||||||
|
| |||||||
| Variables | Crude model | Adjusted model | |||||
|
|
|
||||||
| B (95% CI) | COR (95% CI) | P-value | Adjusted B (95% CI) | AOR (95% CI) | p-value | ||
|
| |||||||
| Sex | Male | 0 | 1 | 0 | 1 | ||
| Female | -0.38 (-0.88, 0.12) | 0.68 (0.42, 1.13) | 0.141 | -0.44 (-0.95, 0.08) | 0.65 (0.39, 1.08) | 0.095 | |
| Child residence | Urban | 0 | 1 | ||||
| Rural | -0.18 (-0.613, 0.974) | 1.20 (0.54, 2.65) | 0.656 | - | - | - | |
| Baseline WHO clinical stage | Stage I | 0 | 1. | 0 | 1 | ||
| Stage II, III and IV | 0.59 (0.04, 1.14) | 1.81 (1.04, 3.13) | 0.035 | 0.55 (0.02, 1.10) | 1.73 (1.02, 3.0) | 0.048 | |
| Baseline Functional status | Appropriate/working | 0 | 1. | - | - | ||
| Ambulatory or regression | -0.07 (-0.58, 0.43) | 0.93 (0.56, 1,54) | 0.775 | - | - | - | |
| Age at enrolment (month) | Continuous as a covariate | -0.04 (-0.02, 0.005) | 0.96 (0.98, 1.005) | 0.241 | -0.03 (-0.05, -0.001) | 0.97 (0.95, 0.99) | 0.026 |
| Baseline CD4 count | Continuous as a covariate | -0.001 (-0.002, 0.001) | 1.0 (0.998, 1.001) | 0.239 | -0.05 (-0.07, -0.02) | 0.95 (0.93, 0.98) | <0.01 |
|
| |||||||
| Factors associated with magnitude of undernutrition from baseline to 12 months of ART initiation | |||||||
|
| |||||||
| Sex | Male | 0 | 1 | ||||
| Female | 0.045 (-0.359, 0.449) | 1.05 (0.7, 1.57) | 0.827 | - | - | - | |
| Child residence | Urban | 0 | 1 | ||||
| Rural | -0.07 (-0.685, 0.545) | 0.932 (0.50, 1.72) | 0.823 | - | - | - | |
| Baseline WHO clinical stage | Stage I | 0 | 1 | ||||
| Stage II, III and IV | 0.399 (-0.022, 0.819) | 1.49 (0.98, 2.27) | 0.063 | 0.003 (-0.001, 0.006) | 1.003 (0.99, 1.01) | 0.693 | |
| Baseline Functional status | Appropriate/working | 0 | 1 | ||||
| Ambulatory or regression | 0.083 (-0.317, 0.483) | 1.09 (0.73, 1.62) | 0.685 | - | - | - | |
| Age at enrolment (month) | Continuous as a covariate | -0.004 (-0.011, 0.003) | 0.996 (0.989, 1.003) | 0.222 | .004 (-0.012, 0.003) | 0.99 (0.98, 1.003) | 0.221 |
| Baseline CD4 count | Continuous as a covariate | -0.003 (-0.004, 0.001) | 0.997 (0.995, 1.001) | 0.229 | 0.002 (-0.004, 0.001) | 1.002 (0.99, 1.01) | 0.693 |
AOR: Adjusted odds ratio; B: Beta Coefficient; COR: Crude odds Ration; Italicized P-value in adjusted model shows a significant at P<0.05.
Discussion
The presence of anemia in HIV-infected patients has serious implications, which vary from functional and quality-of-life decrement to disease progression and decreased survival. An obvious cause of anemia in patients with HIV infection is blood loss. Blood loss may be associated with such conditions as neoplastic disease or gastrointestinal lesions that accompany opportunistic infections. Other than blood loss, the pathophysiology of HIV-associated anemia may involve 3 basic mechanisms: decreased red blood cell (RBC) production, increased destruction of matured RBC and erythroid precursor cells, and ineffective RBC production [17]. One of the etiological agents of anemia in HIV-infected children is the suppression of erythropoiesis by cytokines which is produced by HIV-infected lymphocyte and macrophage [18].
In this study, the overall magnitude of anemia was 37.5%, 22%, 20.5% and 18% at the baseline, 6 months, 9 months, and 12 months of ART initiation, respectively. The magnitude gradually decreased within one year of ART initiation. Similar to our study, studies showed a decrement of anemia prevalence after ART initiation. A study done by Mocroft et al indicated that 65.5% of patients were anemic before HAART initiation, 53% were anemic after 6 months of HAART and 46% were anemic after 12 months of HAART [19]. Similarly, a study done at Yekatit 12 Hospital, Addis Ababa, Ethiopia had reported a declined magnitude of anemia during the course of 12 months of ART initiation (18.9% at the baseline, 12.3% at 6 months and 10.4% at 12 months ART initiation) [20]. The reason for a decline in the magnitude of anemia during the course of HAART is that ART medication reduces viral replication, increases the CD4 lymphocyte count, improves CD4 lymphocyte function, and reduces the incidence of opportunistic infections. This ultimately reduces mortality and HIV-related complication including anemia [21].
In this study, the magnitude of anemia at the baseline of ART initiation was 37.5%. It is comparable with study reported from Kenya in which 35.9% of the study population had anemia [22]. However, it was higher compared to the study done at Yekatit 12 Hospital, Addis Ababa, Ethiopia (18.9%) [20], Enugu, Nigeria (3%) [23] and Jimma, Ethiopia (21.9%) [24]. High prevalence of anemia in this study may be due to differences in socio-economic factors across the study settings and study design. The low prevalence of anemia in the other study such as Yekatit 12 Hospital Ethiopia (18.9%) [20] may be due to the difference in the study design (cross-sectional study in Yekatit 12 hospital but retrospective follow-up in our case), age difference of study subjects (study subject up to 18 years included in Yekatit 12 hospital, but age less than 15 years included in our study) and sample size variation (106 study subject in Yekatit 12 hospital but 200 study participants in our case). The reason why our finding was higher than a study done in Jimma, Ethiopia (21.9%) might be due to differences in study design (cross-sectional in Jimma but retrospective follow-up in our case), sample size variation (64 study subject in Jimma but 200 in our study) and socio-economic status of the study participants.
On the other hand, the magnitude of anemia is lower than the research done in India (66%) [25], Ile-Ife, Nigeria (70%) [26], Cape Town, South Africa (73%) [27], Lagos, Nigeria (77.9) [28], Harar, Ethiopia (54.4%) [6], Jimma, Ethiopia (53.1%) [24]. The differences in the magnitude of anemia might be attributed to the sample size variation, the variability of nutritional factors, variation in burden of opportunistic infections, variability in age limit of included children and the time difference where the studies were conducted.
In this study, at 6 months of ART medication, the magnitude of anemia was decreased from 37.5% at baseline to 22%. This decrement is similar to other similar studies report in Kenya (from 35.9 to 16.6%) [22] and in Addis Ababa, Ethiopia (from 18.9 to 12.35%) [20]. Moreover, at 12 months of ART treatment, anemia was also declined from 37.5% at baseline to 18%. This improvement of Hb value of the study participants was similar to other study done in Harar, Ethiopia (from 54.4 to 39.2%) [6] and Addis Ababa, Ethiopia (from 18.9 to 10.4%) [20]. The decrement of anemia during the course of ART medication might be related to the positive effect of HAART, the reduction in viral load, increase in CD4 count, and childcare service and nutritional supplementation provided for children during follow-up. These may reduce the destruction of maturing hematopoietic cells of multiple lineages and improve the erythropoietin response of hematopoietic organs [19].
The current study showed that a unit increase in baseline CD4 count reduced the risk of anemia by 2-7% (AOR = 0.95; 95% CI: 0.93-0.98). The possible reason for reduced risk of anemia might be due to good immunological recovery during the course of ART medication in children with high baseline CD4 count. For HIV-infected patients who had a higher baseline CD4 cell count, it might be easier to reach to the normal CD4 level within a short period of time after initiation of treatment. Studies also reported that high baseline CD4 cell count was positively associated with a better immunological response in HIV-infected patients who are taking HAART [29,30]. This ultimately reduces the viral load and HIV/AIDS-related complications including anemia.
This study showed that children who were at WHO clinical stage II-IV at baseline of ART initiation were more likely to be anemic. The possible justification might be related to poor immunological recovery in children with advanced and severe immunosuppression at baseline of ART initiation. Evidence also revealed that HIV-infected patients with low CD4 count at baseline are less like to achieve good immunological response [31]. In this case, the possibility of achieving the desired ART medication outcomes such as reducing viral load, improving quality of life, reducing HIV/AIDS-related complications etc could not be met.
In the current study, age at enrollment to the HIV care service was one of the factors significantly associated with anemia. A unit increase in month of child age during enrollment to HIV care service reduced the risk of anemia by 3% (95% CI: 1-5%) (AOR = 0.97; 95% CI: 0.95-0.99). Previous study also affirmed that younger age was a risk factor of anemia in HIV-infected children [32]. The reason might be related to the developmental and behavioral changes of children. As the child age advances, the growth requirement becomes optimal and the adherence to ART medication becomes good compared to those whose age is younger. This could possibly reduce the risk HIV/AIDS-related complications including anemia.
In our study, the magnitude of anemia was high among under 5 years of age compared to HIV-infected children who were in the age of 5-14.9 years old. This was similar to other studies [25,33]. This may be due to the fact that children aged less 5 years old do have high nutritional demand for their rapid growth and RBC production; and it could be accompanied with a high frequency of gastrointestinal infections which leading to anemia of blood and nutritional deficiencies [6,25].
In HIV-infected children, undernutrition is a major public health problem which limits the desirable success of HAART, particularly in developing nations like Ethiopia. Undernutrition together with HIV infection negatively affects the immunity, leading to severe immune-dysfunction and susceptibility to infectious diseases [34]. In our study, the magnitude of undernutrition was high at baseline of ART initiation. At baseline, the magnitude of underweight and stunting were found to be 44.5% (95% CI: 37.7-51.5%), and 49.5% (95% CI: 42.5-56.5%), respectively. These figures were comparable with a study done in Harar, Ethiopia [6] revealing that the prevalence of underweight and stunting were 51.6% and 49.1%, respectively [6]. A high magnitude of undernutrition at the baseline of ART might be due to a high viral replication that might cause an immune-dysfunction leading to susceptibility to infectious diseases and malabsorption of micronutrients.
In this study, the magnitude of undernutrition had declined during a one year course of HAART medication. This is comparable with other studies conducted in Harar, Eastern Ethiopian [6] and Dar es Salaam, Tanzania [35]. The possible reason might be due to the reduction of viral replication, and the immune recovery after ART-initiation. This, in turn, decreases the risk of HIV-associated comorbidities, which improves micronutrient absorption [36]. Moreover, the integration of nutritional supplementation and health education during ART follow-up may improve the nutritional status of children [37].
Conclusion
In general, there was a high prevalence of anemia and undernutrition in HIV-infected children who took HAART at the University of Gondar comprehensive specialized hospital ART clinic. Baseline WHO clinical stage, Baseline CD4 count and age at enrollment to the care were associated with anemia. Despite anemia and undernutrition declined after initiation of ART which highlights the importance of the ART medication in reducing HIV/AIDS-related complications, the magnitude remained high and were the public health problems. Therefore, emphasis should be given to manage anemia and undernutrition. Besides, continuous assessment of hematological and nutritional parameters before and after initiation of ART, giving adherence counselling to strengthen the uptake of ART regime, providing health education about personal and environmental hygiene for the caregivers, and providing nutritional supplementation for the HIV-infected children are advisable to reduce the burden of anemia and undernutrition.
Acknowledgements
We would like to thank the University of Gondar for logistic support, and staff members of the University of Gondar comprehensive specialized hospital ART clinic for their cooperation during data collection.
Disclosure of conflict of interest
None.
Abbreviations
- AIDS
Acquired Immune Deficiency Syndrome
- AOR
Adjusted Odds Ratio
- ART
Anti-retroviral Therapy
- B
Beta coefficient
- BMI
Body Mass Index
- CD
Cluster Differentiation
- CI
Confidence Interval
- COR
Crude Odds Ratio
- GEE
Generalized Estimating Equations
- HAART
Highly Active Antiretroviral Therapy
- HAZ
Z score of height-for-age
- Hb
Hemoglobin
- HIV
Human Immunodeficiency Virus
- RBC
Red blood cell
- TB
Tuberculosis
- WAZ
Z-score of weight-for-age
- WHO
World Health Organization
- WHZ
Z-score of weight-for-height
References
- 1.UNAIDS. Global HIV and AIDS statistics - 2019 [Google Scholar]
- 2.AVERT. Information on HIV: prevention of mother-to-child transmission (PMTCT) of HIV, December 2018 [Google Scholar]
- 3.WHO. HIV in the WHO African Region. 2018 [Google Scholar]
- 4.UNAIDS. Ethiopian - UNAIDS. 2019 [Google Scholar]
- 5.WHO. Ethiopia HIV Profile - World Health Organization. 2016 [Google Scholar]
- 6.Teklemariam Z, Mitiku H, Mesfin F. Prevalence of anemia and nutritional status among HIV-positive children receiving antiretroviral therapy in Harar, eastern Ethiopa. HIV/AIDS (Auckl) 2015;7:191–196. doi: 10.2147/HIV.S78845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahumareze RE, Rankin J, David A, Wapmuk A, Disu E, Balogun Y, Adetunji A, MacArthur E, Amoo O. Prevalence of anaemia and the relationship between haemoglobin concentration and CD4 count in HIV positive children on highly active antiretroviral therapy (HAART) in Lagos, Nigeria. Curr Pediatr Res. 2016;20:29–36. [Google Scholar]
- 8.Jesson J, Masson D, Adonon A, Tran C, Habarugira C, Zio R, Nicimpaye L, Desmonde S, Serurakuba G, Kwayep R, Sare E, Konate T, Nimaga A, Saina P, Kpade A, Bassuka A, Gougouyor G, Leroy V Growing Up Working Group. Prevalence of malnutrition among HIV-infected children in central and West-African HIV-care programmes supported by the growing up programme in 2011: a cross-sectional study. BMC Infect Dis. 2015;15:216. doi: 10.1186/s12879-015-0952-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunningham-Rundles S, McNeeley DF, Moon A. Mechanisms of nutrient modulation of the immune response. J Allergy Clin Immunol. 2005;115:1119–1128. doi: 10.1016/j.jaci.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 10.Arpadi SM, Cuff PA, Kotler DP, Wang J, Bamji M, Lange M, Pierson RN, Matthews DE. Growth velocity, fat-free mass and energy intake are inversely related to viral load in HIV-infected children. J Nutr. 2000;130:2498–2502. doi: 10.1093/jn/130.10.2498. [DOI] [PubMed] [Google Scholar]
- 11.Jesson J, Leroy V. Challenges of malnutrition care among HIV-infected children on antiretroviral treatment in Africa. Med Mal Infect. 2015;45:149–156. doi: 10.1016/j.medmal.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Callens SF, Shabani N, Lusiama J, Lelo P, Kitetele F, Colebunders R, Gizlice Z, Edmonds A, Van Rie A, Behets F SARA team. Mortality and associated factors after initiation of pediatric antiretroviral treatment in the Democratic Republic of the Congo. Pediatr Infect Dis. 2009;28:35–40. doi: 10.1097/INF.0b013e318184eeb9. [DOI] [PubMed] [Google Scholar]
- 13.Muenchhoff M, Healy M, Singh R, Roider J, Groll A, Kindra C, Sibaya T, Moonsamy A, McGregor C, Phan MQ, Palma A, Kloverpris H, Leslie A, Bobat R, LaRussa P, Ndung’u T, Goulder P, Sobieszczyk ME, Archary M. Malnutrition in HIV-infected children is an indicator of severe disease with an impaired response to antiretroviral therapy. AIDS Res Hum Retroviruses. 2018;34:46–55. doi: 10.1089/aid.2016.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.FDRE/MoH. National guidelines for comprehensive HIV prevention, care and treatment. Federal Democratic Republic of Ethiopia: 2014. [Google Scholar]
- 15.WHO. Hemoglobin concentrations for the diagnosis of anemia and assessment of severity. Vitamin and mineral nutrition information system. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 16.WHO, UNICEF. WHO child growth standards and the identification of severe acute malnutrition in infants and children: joint statement by the World Health Organization and the United Nations Children’s Fund. WHO, UNICEF; 2009. [PubMed] [Google Scholar]
- 17.Volberding PA, Levine AM, Dieterich D, Mildvan D, Mitsuyasu R, Saag M Anemia in HIV Working Group. AiHW. Anemia in HIV infection: clinical impact and evidence-based management strategies. Clin Infect Dis. 2004;38:1454–1463. doi: 10.1086/383031. [DOI] [PubMed] [Google Scholar]
- 18.Gökengin D, Doroudi F, Tohme J, Collins B, Madani N. HIV/AIDS: trends in the Middle East and North Africa region. Int J Infect Dis. 2016;44:66–73. doi: 10.1016/j.ijid.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Mocroft A, Kirk O, Barton SE, Dietrich M, Proenca R, Colebunders R, Pradier C, Ledergerber B, Lundgren JD. Anaemia is an independent predictive marker for clinical prognosis in HIV-infected patients from across Europe. EuroSIDA study group. AIDS. 1999;13:943–950. doi: 10.1097/00002030-199905280-00010. [DOI] [PubMed] [Google Scholar]
- 20.Debasu M, Menon MK, Belayneh Y, Abebe W, Jerene D, Seifu D. Anti-retroviral treatment related haematological disorders among HIV-infected children attending HIV clinic at yekatit 12 hospital, Addis Ababa, Ethiopia. Int Blood Res Rev. 2015;4:1–18. [Google Scholar]
- 21.Candiani TM, Pinto J, Araujo Cardoso CA, Carvalho IR, Dias AC, Carneiro M, Goulart EA. Impact of HAART on the incidence of opportunistic infectios, hospitalization and mortality among children and dolescents living with HIV/AIDS in Belo Horizonte, Minas Gerais State, Brazil. Can Saude Publica. 2007;23:S414–423. doi: 10.1590/s0102-311x2007001500009. [DOI] [PubMed] [Google Scholar]
- 22.Kibaru EG, Nduati R, Wamalwa D, Kariuki N. Baseline hematological indices among HIV-1 infected children at Kenyatta National Hospital. Int J Novel Res Healthcare Nursing. 2014;1:21–26. doi: 10.1186/s12981-015-0069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ezeonwu BU, Ikefuna AN, Oguonu T, Okafor HU. Prevalence of hematological abnormalities and malnutrition in HIV-infected under five children in Enugu. Niger J Clin Pract. 2014;17:303–308. doi: 10.4103/1119-3077.130230. [DOI] [PubMed] [Google Scholar]
- 24.Abebe M, Alemseged F. Hematologic abormalities among children on HAART, in Jimma university specialized hospital, southwestern ethiopia. Ethiop J Health Sc. 2009;19:83–89. [Google Scholar]
- 25.Shet A, Mehta S, Rajagopalan N, Dinakar C, Ramesh E, Samuel NM, Indumathi CK, Fawzi WW, Kurpad AV. Anemia and growth failure among HIV-infected children in India: a retrospective analysis. BMC pediatr. 2009;9:37. doi: 10.1186/1471-2431-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anyabolu HC, Adejuyigbe EA, Adeodu OO. Undernutrition and anaemia among HAART-naïve HIV infected children in Ile-Ife, Nigeria: a case-controlled, hospital based study. Pan Afri Med Journ. 2014;18:77. doi: 10.11604/pamj.2014.18.77.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eley BS, Sive AA, Shuttleworth M, Hussey GD. A prospective, cross-sectional study of anaemia and peripheral iron status in antiretroviral naive, HIV-1 infected children in Cape Town, South Africa. BMC Infect Dis. 2002;2:3. doi: 10.1186/1471-2334-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adetifa IM, Temiye EO, Akinsulie AO, Ezeaka VC, Iroha EO. Haematological abnormalities associated with paediatric HIV/AIDS in Lagos. Ann Trop Paediatr. 2006;26:21–25. doi: 10.1179/146532806X107467. [DOI] [PubMed] [Google Scholar]
- 29.Moore DM, Harris R, Lima V, Hogg B, May M, Yip B, Justice A, Mocroft A, Reiss P, Lampe F, Chene G, Costagliola D, Elzi L, Mugavero MJ, Monforte AD, Sabin C, Podzamczer D, Fatkenheuer G, Staszewski S, Gill J, Sterne JA. Effect of baseline CD4 cell counts on the clinical significance of short-term immunologic response to antiretroviral therapy in individuals with virologic suppression. J Acquir Immune Defic Syndr. 2009;52:357–363. doi: 10.1097/QAI.0b013e3181b62933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asfaw A, Ali D, Eticha T, Alemayehu A, Alemayehu M, Kindeya F. CD4 cell count trends after commencement of antiretroviral therapy among HIV-infected patients in Tigray, Northern Ethiopia: a retrospective cross-sectional study. PLoS One. 2015;10:e0122583. doi: 10.1371/journal.pone.0122583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mussini C, Cossarizza A, Sabin C, Babiker A, De Luca A, Bucher HC, Fisher M, Rezza G, Porter K, Dorrucci M. Decline of CD4(+) T-cell count before start of therapy and immunological response to treatment in antiretroviral-naive individuals. AIDS. 2011;25:1041–1049. doi: 10.1097/QAD.0b013e3283463ec5. [DOI] [PubMed] [Google Scholar]
- 32.Ruhinda EN, Bajunirwe F, Kiwanuka J. Anaemia in HIV-infected children: severity, types and effect on response to HAART. BMC Pediatr. 2012;12:170. doi: 10.1186/1471-2431-12-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsegay YG, Tadele A, Addis Z, Alemu A, Melku M. Magnitude of cytopenias among HIV-infected children in Bahir Dar, northwest Ethiopia: a comparison of HAART-naïve and HAART-experienced children. HIV AIDS (Auckl) 2017;9:31–42. doi: 10.2147/HIV.S125958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taye B, Shiferaw S, Enquselassie F. The impact of malnutrition in survival of HIV infected children after initiation of antiretroviral treatment (ART) Ethiop Med J. 2010;48:1–10. [PubMed] [Google Scholar]
- 35.Sunguya BF, Poudel KC, Otsuka K, Yasuoka J, Mlunde LB, Urassa DP, Mkopi NP, Jimba M. Undernutrition among HIV-positive children in Dar es Salaam, Tanzania: antiretroviral therapy alone is not enough. BMC Public Health. 2011;11:869. doi: 10.1186/1471-2458-11-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ubesie AC, Ibe BC, Emodi IJ, Iloh KK. Serum selenium status of HIV-infected children on care and treatment in Enugu, Nigeria. S Afr J Child Health. 2017;11:21–25. [Google Scholar]
- 37.Scarcella P, Buonomo E, Zimba I, Doro Altan AM, Germano P, Palombi L, Marazzi MC. The impact of integrating food supplementation, nutritional education and HAART (Highly Active Antiretroviral Therapy) on the nutritional status of patients living with HIV/AIDS in Mozambique: results from the DREAM Programme. Ig Sanita Pubbl. 2011;67:41–52. [PubMed] [Google Scholar]


