ABSTRACT
Background
Human milk (HM) lipid content is highly variable, and infants consume different volumes of milk. This makes precise sampling and calculation of the infant lipid intake problematic.
Objectives
In order to describe inaccuracies of estimates of lipid content introduced by various sampling protocols, we compared the true infant lipid intake with estimated intakes using different milk sampling protocols.
Methods
Monthly milk samples (n = 1026) from months 1 to 6 of lactation were collected from 20 healthy, exclusively breastfeeding women. Infant lipid intake was measured by 24-hour test-weighing at month 3. Total lipid content was measured by creamatocrit. Concentrations and infant lipid intakes were calculated using 11 sampling protocols, using either the true milk intake or an average of 800 mL/d. These estimates were compared with the true infant lipid intake using repeated-measures ANOVA and linear mixed modeling with multiple comparisons.
Results
The mean maternal age was 32.0 years (SD ± 3.10), and infants were born term (40.1 ± 1.1 weeks) with a mean birth weight of 3.87 kg (SD ± 0.39). The mean true infant lipid intake was 28.6 g/d (SD ± 9.8). The mean estimated lipid intake using 1 morning pre-feed sample underestimated intake by >8.0 g/d. Estimates of infant lipid intake using other sampling protocols and an assumed intake volume of 800 mL/d also resulted in a wide range of differences (0.8–18.1 g/d) from the true intake. Use of 6 daily pre- and post-feed milk samples had a mean difference of only 0.1 g/d (95% CI, −2.9 to 2.7) from the true intake.
Conclusions
A sampling protocol with 6 pre- and post-feed samples provides the most accurate estimate of lipid intake if it is not possible to perform 24-hour test weights. The potential inaccuracies of sampling protocols should be taken into consideration in the interpretation and translation of infant lipid intake results.
Keywords: lactation, breastfeeding, fat, lipids, dose, breast milk
Introduction
Human milk (HM) provides human infants with the best start at life, delivering a multitude of nutritive and nonnutritive factors for optimal health and growth (1). An important yet complex factor is the HM lipid content. HM lipids are highly variable, differing between women, increasing through a feed, and displaying differences throughout the day (2). The reason for this variability has not been comprehensively investigated, but is likely due to the adsorption of milk fat globules to alveoli or ductal walls, with globule displacement as the breast empties (3). Because of this variation, sampling should be carefully considered for research involving the HM lipidome for both total lipids and individual lipid classes, such as triglycerides and phospholipids.
HM studies have used a wide variety of sampling protocols: some that account for lipid variability, but many that do not consider the changes that occur. Sample collection methods reported include pre-feed, pre-feed and post-feed, mid-feed, or full expressions. Samples may be collected at 1 time point, several time points, or at every feed over a 24-hour period (2, 4–8). In addition to the sampling protocol, the volume of HM delivered to the infant is also an important consideration for HM studies, as thriving infants may receive as little as 478 mL/d or as much as 1356 mL/d throughout lactation (9, 10). Due to the variability of both lipid concentrations and intake volumes, daily infant intake is a more appropriate way to investigate HM lipids and their impact on infant growth and development. The milk volume ingested by the infant can be measured by 24-hour test weighing of the infant pre- and post-feed. Combining the feed volumes with the lipid content of all pre- and post-feed samples across a 24-hour period is currently the most representative way to measure infant lipid intake, but this approach is more labor intensive and requires more sensitive scales to complete than other protocols (9). For this reason, studies often use 800 mL/d as a mean intake volume or focus solely on lipid concentration. Inadequate sampling protocols may result in large discrepancies from the true infant lipid intake and, therefore, the infant energy intake, so it is important to determine sampling protocols that are appropriate for estimating the true infant lipid intake.
Therefore, the objective of this study was to estimate infant lipid intake using 11 different sampling protocols, and compare these estimates with the true infant lipid intake. Dose estimates using each sampling protocol were carried out assuming an 800 mL/d intake volume. The sampling protocols were selected to be representative of existing protocols reported in the literature (8). Using this methodology, we describe the considerable discrepancies that arise from inadequate sampling, and suggest the most accurate protocol for estimations of infant lipid intake.
Methods
Women who intended to exclusively breastfeed were recruited in pregnancy for a longitudinal study. Participants were excluded from the study if their infant was born preterm, if the infant was fed any supplementary foods before 6 months of age, or if there were any infant health or growth concerns. There were 20 mother-infant dyads who participated in this study. Infant weight, maternal BMI, and maternal age at birth were recorded, and HM samples were collected monthly from 1 to 6 months. At months 1, 2, 4, 5, and 6, participants collected left and right pre-feed samples (2 mL) by manual expression into sterile vials at 3 time points: morning (06:00–09:00), afternoon (13:00–16:00), and evening (19:00–22:00). At 3 months, participants weighed their infant before and after every feed for a 24-hour period, using electronic baby weight scales (accuracy ± 2 g; Medela Inc.), in order to measure the infant's intake. Scales were calibrated regularly. In conjunction with test-weighing at 3 months, pre-feed and post-feed samples (2 mL) were collected at every feed, by manual expression, into sterile vials (9). All samples were stored immediately at −20°C for less than 48 hours, prior to storage at −80°C. Details recorded for each sample were collection breast (left or right), collection time, whether collection was pre- or post-feed, and whether the infant was fed from that breast. Each sample was subjected to 1 freeze-thaw cycle. Informed written consent was obtained from all study participants. All research was carried out in accordance with relevant guidelines and regulations. This study was approved by The University of Western Australia Human Ethics Research Office (RA/4/20/4023).
Total lipid content was measured using the creamatocrit method, with samples analyzed in triplicate with relative standard deviation (RSD) <3%. The mean creamatocrit (percentage) from 3 samples was converted to concentration using the following equation (11):
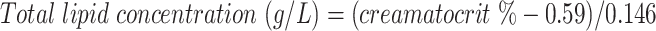 |
(1) |
The true infant lipid intake was calculated at 3 months by multiplying the mean lipid concentration of a feed (mean of pre- and post-feed samples) by the volume of the feed, for all feeds in the 24-hour period. Estimated infant intake was calculated using 11 different sampling protocols, designed to be representative of sampling protocols used in previous HM lipid studies (2, 4–8). The first feed of the day was defined as the first infant feed after 06:00 h. The first breast was defined as the breast (left or right) that the infant fed from first during the feed. All protocols sampled the first breast, unless otherwise stated. Sampling protocols containing post-feed samples (2/prepost/second and 6/prepost/daily) were only investigated at 3 months. Sampling protocols 3/pre/daily/L, 3/pre/daily/R, and 6/pre/daily/LR were not calculated from the 3-month data, as it was not a true comparison. Estimations of infant lipid intake were made using these protocols and assuming an average 800 mL/d intake, as many studies have previously used an average value if the volume was not measured (10). Estimations were also carried out using each sampling protocol and the infant true volume intake that was measured at 3 months. Sampling protocols are outlined in Table 1.
TABLE 1.
Description of sampling protocols
| Protocol | Description |
|---|---|
| True | Mean of pre- and post-feed samples (multiplied by feed volume) for every feed in 24 hours |
| 1/pre/am | 1 pre-feed sample for the first feed of the day (06:00–09:00) from the first breast |
| 1/pre/am/L | 1 pre-feed sample before the first feed of the day (06:00–09:00) from the left breast |
| 1/pre/am/R | 1 pre-feed sample before the first feed of the day (06:00–09:00) from the right breast |
| 2/pre/am/LR | Mean of 2 pre-feed samples before the first feed of the day (06:00–09:00), 1 from the left breast and 1 from the right breast |
| 3/pre/daily | Mean of 3 pre-feed samples before morning (06:00–09:00), afternoon (13:00–16:00), and evening (19:00–22:00) feeds, each from the first breast |
| 3/pre/daily/L1 | Mean of 3 pre-feed samples before morning (06:00–09:00), afternoon (13:00–16:00), and evening (19:00–22:00) feeds, from the left breast |
| 3/pre/daily/R1 | Mean of 3 pre-feed samples before morning (06:00–09:00), afternoon (13:00–16:00), and evening (19:00–22:00) feeds, from the right breast |
| 6/pre/daily/LR1 | Mean of 6 pre-feed samples before morning (06:00–09:00), afternoon (13:00–16:00), and evening (19:00–22:00) feeds, from both the left and right breasts |
| 1/pre/drained | 1 pre-feed sample from the most drained breast (highest lipid concentration) at any time through the day |
| 2/prepost/second2 | Mean of 2 samples, pre-feed and post-feed, from the second feed of the day |
| 6/prepost/daily2 | Mean of 6 samples, pre-feed and post-feed, from morning (06:00–09:00), afternoon (13:00–16:00), and evening (19:00–22:00) feeds, from the first breast |
Not conducted at 3-month lactation.
Conducted only at 3-month lactation.
The statistical analysis was carried out using IBM SPSS Statistics for Windows, version 25.0 (IBM Corp.). The concentrations of pre-feed and post-feed samples were compared using all samples (n = 438) at 3 months, with repeated-measures ANOVA. The concentration of left vs. right breast samples was compared using 6 monthly pre-feed samples (n = 720) from each mother, with repeated-measures ANOVA. The concentration of samples each month was compared using all samples collected (n = 1026), including post-feed samples, as well as using 3 monthly pre-feed samples (n = 360). The concentration of morning vs. afternoon vs. evening was made using 1 pre-feed sample (n = 720) for each time point each month. Concentrations were compared using linear mixed models and pairwise comparisons, with mother as a random effect and breast, month, or time of day as a fixed effect. The concentration and intake for each sampling protocol was compared against the true intake at 3 months, as the infant lipid intake does not change through lactation. Linear mixed models and pairwise comparisons were used to compare the average concentration or intake resulting from each sampling protocol, with protocol included as a fixed effect and the mother as a random effect. Standard graphical methods were used to check the model assumptions. Results are presented as means ± SDs (ranges) and with 95% CIs for comparisons. In all cases, P values < 0.05 were considered significant, and Bonferroni adjustments were carried out to account for multiple comparisons.
Results
Complete lactation sample sets were provided by 20 healthy, exclusively breastfeeding women with term infants for months 1 to 6, including 24-hour infant test-weighing with pre- and post-feed samples at 3 months (Table 2).
TABLE 2.
Cohort characteristics
| Variable | Value |
|---|---|
| Maternal age at delivery, y | 32 ± 3 (26–37) |
| Infant birth age, weeks | 40 ± 1 (38–42) |
| Maternal BMI at delivery | 28.3 ± 6.0 (21.8–43.6) |
| Infant weight at birth, kg | 3.87 ± 0.39 (3.30–4.66) |
| Number of breastfeeds, n | 11 ± 3 (6–16) |
| Breastfeed volume, mL | 71 ± 27 (30–158) |
| Infant milk intake, mL/24 h | 728 ± 163 (419–946) |
Data are for 20 exclusively breastfeeding women and their infants. Values are mean ± SD (range), n = 20.
All participant mothers were normal and healthy, with steady weight loss during lactation. Mothers had parity of 1 (n = 5), 2 (n = 10), and 3 (n = 5). All infants were born healthy and term (>37 weeks; Table 2), and were growing appropriately on their growth trajectories. There were 10 female and 10 male infants.
Total lipid concentration
Based on the intensive 24-hour sampling at 3 months, the variation of lipid concentrations through the day was 3.5 to 106 g/L. The total lipid concentration was significantly higher in post-feed samples than in pre-feed samples (P < 0.001; Supplemental Table 1).
There were no significant differences between months for the lipid concentrations of all collected samples (n = 1026; Table 3; Supplemental Table 2). However, when the concentrations of only 3 daily pre-feed samples were compared (Table 3), concentrations were significantly different between 3 months and all other months (P < 0.05), with the exception of month 4 (P = 0.10; Supplemental Table 3).
TABLE 3.
Human milk lipid concentration
| Month | All samples, g/L | 3 daily pre-feed samples, g/L |
|---|---|---|
| 1 | 35.8 ± 11.0 (22.0–62.5) | 37.2 ± 19.3 (4.9–88.8) |
| 2 | 32.0 ± 10.0 (19.4–50.8) | 31.6 ± 16.0 (4.2–70.3) |
| 3 | 38.5 ± 9.2 (21.6–53.5) | 22.1 ± 11.7 (7.3–57.3) |
| 4 | 34.5 ± 9.5 (17.9–56.3) | 30.6 ± 18.6 (6.2–92.9) |
| 5 | 35.6 ± 11.3 (17.2–61.1) | 32.2 ± 22.5 (4.9–120.6) |
| 6 | 33.1 ± 10.1 (19.4–56.3) | 33.3 ± 19.7 (6.2–95.3) |
Data are based on all cohort samples collected and on 3 daily pre-feed samples, from months 1 to 6 of lactation. Values are mean ± SD (range), n = 20.
An analysis of 3 daily pre-feed samples from each month resulted in variations through the day, from morning (28.6 ± 19.8 g/L) to afternoon (33.8 ± 21.4 g/L) to evening (35.1 ± 20.5 g/L). The total lipid concentrations were significantly different between morning samples and afternoon or evening samples (P = 0.014 and P = 0.001, respectively), but not between afternoon and evening samples (Supplemental Table 1). The total lipid concentrations were not significantly different between left (32.2 ± 6.7 g/L) and right (32.8 ± 4.6 g/L) breast samples (Supplemental Table 1).
Sampling protocol lipid concentrations
At 3 months, the mean lipid concentration obtained using the true sampling protocol was 38.5 ± 9.2 g/L, ranging from 21.6–53.5 g/L. Significant differences in concentrations were observed between sampling protocols 1/pre/am, 1/pre/am/L, 2/pre/am/LR, 3/pre/daily, 1/pre/drained, and the true protocol (Table 4).
TABLE 4.
Total lipid concentration
| Sampling protocol | Concentration, g/L1 | Mean difference, g/L2 | P value3 |
|---|---|---|---|
| 1/pre/am | 25.6 ± 18.1 | −12.9 (−20.4 to −5.4) | 0.001 |
| 1/pre/am/L | 27.6 ± 20.1 | −11.0 (−19.8 to −2.1) | 0.016 |
| 1/pre/am/R | 29.7 ± 19.6 | −8.8 (−17.5 to 0) | 0.05 |
| 2/pre/am/LR | 28.7 ± 14.9 | −9.8 (−16.4 to −3.1) | 0.005 |
| 3/pre/daily | 31.2 ± 13.2 | −7.3 (−12.5 to −2.2) | 0.006 |
| 3/pre/daily/L4 | 33.7 ± 12.5 | −4.8 (−10.1 to 0.6) | 0.08 |
| 3/pre/daily/R4 | 34.7 ± 14.0 | −3.8 (−10.2 to 2.7) | 0.25 |
| 6/pre/daily/LR4 | 34.2 ± 10.3 | −4.3 (−9.0 to 0.4) | 0.07 |
| 1/pre/drained | 59.4 ± 19.9 | 19.9 (11.1–28.6) | <0.001 |
| 2/prepost/second5 | 42.0 ± 13.5 | 3.5 (−0.9 to 7.8) | 0.12 |
| 6/prepost/daily5 | 39.7 ± 9.30 | 1.2 (−0.5 to 2.9) | 0.16 |
Data are for each sampling protocol, compared to the true protocol concentration. Abbreviations: am, first feed of the day; daily, mean of samples taken from morning, afternoon, and evening; drained, sample from the most drained breast (highest lipid concentration) at any time through the day; L, left breast; pre, pre-feed sample; post, post-feed sample; R, right breast; second, second feed of the day.
Values are mean ± SD.
Values are mean difference (95% CI).
Bonferroni-adjusted.
Not conducted at 3-month lactation.
Conducted only at 3-month lactation. n = 20.
True total lipid intake
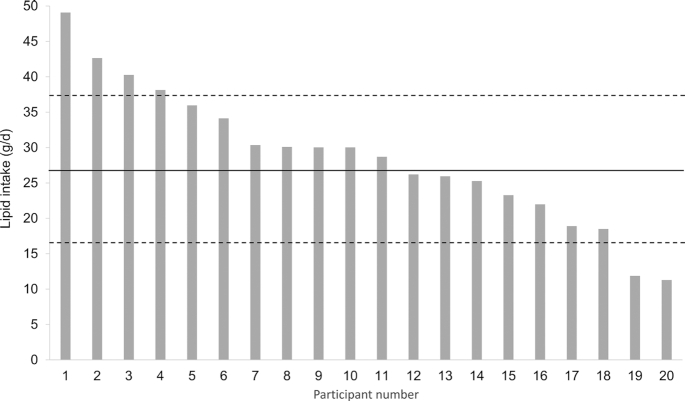
The mean true daily lipid intake for this cohort of infants was 28.6 ± 9.6 g/d, ranging from 11.3–49.1 g/d (Figure 1).
FIGURE 1.
True infant lipid intake (g/d) measured in exclusively breastfeeding cohort of 20 mother-infant dyads. Mean (–) and standard deviation (- -) are indicated.
Total lipid intake from alternate sampling protocols
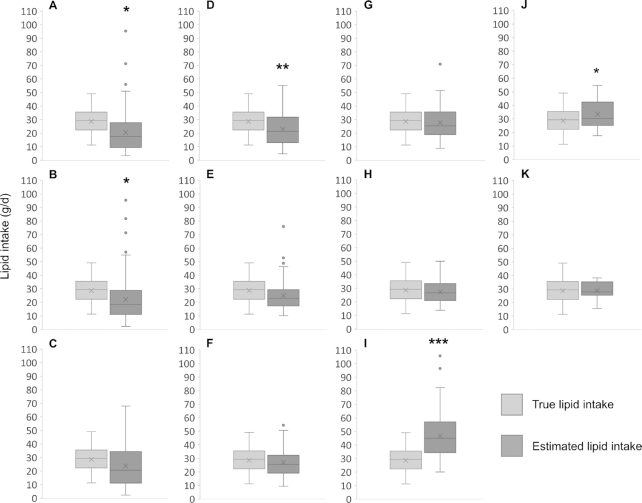
When intake was calculated using an 800 mL/d intake volume, estimates were significantly different from the true intake for the 1/pre/am, 1/pre/am/L, 2/pre/am/LR, 1/pre/drained, and 2/prepost/second sampling protocols (Table 5). When intake was calculated using the true intake volume, all sampling protocols estimated the intake to be significantly different from the true intake, with the exception of the 3/pre/daily/R, 2/prepost/second, and 6/prepost/daily protocols (Table 4; Figure 2). The 6/prepost/daily sampling protocol was the most accurate when either 800 mL/d or the true intake volume were used.
TABLE 5.
Daily lipid intake
| Sampling protocol | Using 800 mL/d, g/d1 | Mean difference, g/d2 | P value3 | Using true volume intake, g/d1 | Mean difference, g/d2 | P value3 |
|---|---|---|---|---|---|---|
| 1/pre/am | 20.5 ± 14.5 | 8.2 (2.0, 14.4) | 0.010 | 18.0 ± 12.2 | 10.6 (5.6, 15.6) | <0.001 |
| 1/pre/am/L | 22.0 ± 16.0 | 6.6 (−0.7, 13.9) | 0.048 | 19.7 ± 14.5 | 9.0 (2.7, 15.3) | 0.006 |
| 1/pre/am/R | 23.8 ± 15.7 | 4.8 (−2.3, 12.0) | 0.18 | 21.3 ± 14.8 | 7.3 (0.8, 13.9) | 0.029 |
| 2/pre/am/LR | 21.0 ± 11.9 | 7.6 (2.6, 12.6) | 0.003 | 20.6 ± 11.1 | −9.8 (−17.5, −2.2) | 0.012 |
| 3/pre/daily | 24.9 ± 10.5 | 3.7 (−0.7, 8.1) | 0.10 | 22.5 ± 10.3 | 6.1 (2.4, 9.8) | 0.001 |
| 3/pre/daily/L4 | 27.0 ± 10.0 | 2.3 (−2.9, 6.2) | 0.47 | 24.3 ± 10.0 | 4.4 (0.5, 8.3) | 0.029 |
| 3/pre/daily/R4 | 27.8 ± 11.2 | 0.8 (−4.5, 6.2) | 0.76 | 25.2 ± 11.7 | 3.4 (−1.6, 8.5) | 0.18 |
| 6/pre/daily/LR4 | 27.4 ± 8.2 | 1.3 (−2.8, 5.3) | 0.54 | 24.7 ± 8.9 | 3.9 (0.4, 7.4) | 0.027 |
| 1/pre/drained | 46.7 ± 15.9 | −18.1 (−25.2, −10.9) | <0.001 | 42.2 ± 16.8 | −13.6 (−20.2, −7.1) | <0.001 |
| 2/prepost/second5 | 33.6 ± 10.8 | −4.9 (−9.5, −0.3) | 0.037 | 30.8 ± 12.7 | −2.2 (−5.6, 1.3) | 0.21 |
| 6/prepost/daily5 | 28.7 ± 6.7 | −0.1 (−3.7, 3.5) | 0.97 | 28.9 ± 9.6 | −0.3 (−1.9, 1.3) | 0.70 |
Data are for sampling protocols compared to the true protocol, calculated with 800 mL/d intake and the true infant volume intake. Abbreviations: am, first feed of the day; daily, mean of samples taken from morning, afternoon, and evening; drained, sample from the most drained breast (highest lipid concentration) at any time through the day; L, left breast; pre, pre-feed sample; post, post-feed sample; R, right breast; second, second feed of the day.
Values are mean ± SD.
Values are mean difference with (95% CI).
Bonferroni-adjusted.
Not conducted at 3-month lactation.
Conducted only at 3-month lactation. n = 20.
FIGURE 2.
Daily lipid intake (g/d) comparison for each sampling protocol, estimated using 800 mL/d intake volume compared to the true infant lipid intake, measured by 24-hour sampling and test weighing. (A) 1/pre/am; (B) 1/pre/am/L; (C) 1/pre/am/R; (D) 2/pre/am/LR; (E) 3/pre/daily; (F) 3/pre/daily/L; (G) 3/pre/daily/R; (H) 6/pre/daily/LR; (I) 1/pre/drained; (J) 2/prepost/second; and (K) 6/prepost/daily. The numbers indicate how many samples were taken, with data shown as the mean for 2, 3, or 6 samples. Data are shown as median, lower and upper quartile, minimum and maximum, mean (x), and outliers (●). Asterisks indicate difference from true lipid intake: *P < 0.05; **P < 0.01; ***P < 0.001. Abbreviations: am, first feed of the day; daily, mean of samples taken from morning, afternoon, and evening; drained, sample from the most drained breast (highest lipid concentration) at any time through the day; L, left breast; pre, pre-feed sample; post, post-feed sample; R, right breast; second, second feed of the day.
Discussion
The results from this study provide evidence that HM sampling protocols have a critical effect on the calculation of infant daily lipid intake, with mean underestimates greater than 8 grams per day, depending on protocol. This translates to an underestimate of over 300 kJ/d energy intake, which is a considerable error considering a 1-month-old infant has a required energy intake of approximately 1800–2000 kJ/d. Currently, the most accurate method of measuring the infant intake is test-weighing the infant before and after every feed, and expressing pre- and post-feed samples from each feed over a 24-hour period (2). This method accounts for variation in milk lipids with respect to the volume of milk removed from the breast for each feed. We calculated the true infant lipid intake in this cohort of infants to be 28.6 ± 9.8 g/d, similar to that measured by Kent et al. (9) with test weighing (32.0 ± 7.7 g/d).
Large differences in the lipid concentration of HM samples, and thus intake estimates, occur at different times due to the highly variable lipid content in the milk. Changes in lipid content are dependent on breast fullness, such that pre-feed milk samples are lower in total lipids than post-feed samples. Significant concentration differences at different times are commonly reported in the literature; however, it is likely that many of these are due to the random effects of HM sampling (8). When all samples were considered (including post-feed samples in month 3) there were no differences in concentrations between months 1 and 6. However, when the concentrations of only 3 daily pre-feed samples were compared for each month, the month 3 samples were significantly different to all other months (with the exception of month 4). This is a likely indication that pre-feed samples collected at home by the mother may be mistimed (between feeds or post-feed), in contrast to more detailed 24-hour test weighing and sampling. It has been proposed that these changes are a result of the milk fat globule membrane adsorbing to the ducts and alveoli of the mammary gland, releasing milk fat globules as milk is removed (12).
Morning samples tend to be lower in total milk lipids, due to the longer overnight intervals between feeds allowing the breast to fill with more milk (2). Indeed, we showed that the single sample collected in the morning (1/pre/am sample) had the lowest lipid content (mean 25.6 g/L) and intake estimate (mean 20.5 g/d); when an 800 mL/d intake was assumed, the 1/pre/am sample also produced the largest mean intake underestimate (of 8.2 g/d), compared to the true intake.
Lactating women typically produce different volumes of milk from each breast, raising the possibility that the lipid content from each breast may be different (9). Sampling protocols often request samples from a specific breast, for sampling consistency (13). We found the lipid concentrations did not differ between breasts, and significant intake underestimates occurred with 1 pre-feed sample, a left breast sample, or a pooled left and right breast sample. A single morning sample from the right breast estimated intake within 4.8 g/d of the true intake, representing an energy underestimate of over 180 kJ/d despite not being significantly different to the true intake (Table 5). Other HM sampling protocols require women not to feed or pump from the breast for at least 2 hours before sampling. This is to ensure filling of the breast with newly synthesized milk and allow the collection of a large sample volume; however, as aforementioned, increased fullness of the breast is associated with a lower lipid content (3). This is the likely reason that the 3/pre/daily samples remained unrepresentative, with underestimates of 3.7 g/d or 140 kJ/d, as the breast would have refilled, and the lipid content would be the lowest. The addition of more pre-feed samples brought the intake estimates significantly closer to the true intake and 3/pre/daily, 3/pre/daily/L, 3/pre/daily/R, and 6/pre/daily/LR sampling protocols, allowing for some of the lipid variability that occurs with changing breast fullness, but was still at least 0.8 g/d different to the true intake (at least 30 kJ/d).
Due to increasing lipid content as milk fat globules are displaced during removal of milk from the breast, it was expected that the inclusion of post-feed samples would substantially improve the intake estimate. Despite using samples from a “drained” breast with a higher lipid content, the intake estimate was different to the true intake, significantly overestimating the lipid intake by over 18 g/d (over 670 kJ/d). This sampling protocol also had the highest lipid concentration and largest SD (59.4 ± 19.9 g/L), and is a protocol that is difficult to standardize due to the differing rates of synthesis and degrees of breast emptying between different women. Pumping the entire breast for the second feed of the day has also been used in existing studies, to obtain a representative sample and, often, to enable multiple analyses of different components (14). To simulate this protocol, we pooled the pre- and post-feed samples of the second feed of the day from the full 24-hour sample set at 3 months, which resulted in the lipid intake being significantly higher than the true intake, by 4.9 g/d (185 kJ/d). Finally, collecting pre- and post-feed samples at 3 time points through the day (6/prepost/daily) gave the closest estimate to the true intake, overestimating by only 0.1 g/d (Table 5). This difference represents an overestimate of less than 4 kJ energy, and is therefore the most accurate estimation of true infant lipid intake.
It is clear from this study that different sampling protocols result in large discrepancies for both concentrations and intake estimates, compared to pre- and post-feed sampling across a 24-hour period. This is not only due to the sampling protocols, but also due to the use of a constant mean volume of 800 mL/d intake for the estimation of total lipid intake. The major advantage of collecting 24-hour pre- and post-feed infant weights is that this protocol uses the actual volume ingested, as intake volume differs widely between infants (419–946 mL/d in this study). The use of 800 mL/d in calculations of intake estimates resulted in large overestimates when the individual intake was considerably lower than 800 mL/d and large underestimates when the individual intake was considerably higher than 800 mL/d. We have previously demonstrated the impact of this finding in a case study of a breastfed infant who was small for gestational age, where the HM lipid concentrations were within normal range but the volume intake was well below average (419 mL/d). If 800 mL was assumed to be the daily intake volume, the lipid intake—and therefore the energy intake—would have been grossly overestimated for this infant (estimated 41.3 g/d vs. true 21.6 g/d) and not identified as the primary reason for the infant's poor growth (15).
In our comparison of intake estimates using the actual infant intake volume with each sampling protocol, results were inconsistent with the 800 mL/d intake assumption. Sampling protocols 3/pre/daily/R and 6/prepost/daily remained significantly close to the true intake, and only the 2/prepost/second sampling protocol with the true volume had a significantly improved intake estimate. This was surprising, as we expected that using the true intake volume would improve the estimate, but due to the possible mistiming of daily pre-feed samples, 800 mL/d with the 3/pre/daily, 3/pre/daily/L, 3/pre/daily/R, and 6/pre/daily/LR sampling protocols allowed for some of the lipid variation associated with differing breast fullness and produced estimates representative of the true infant lipid intake (despite mean underestimates between 30 and 140 kJ/d energy).
A limitation of this study is that 24-hour sampling was carried out only at 3 months, in order to measure infant lipid intake. Ideally, 24-hour sampling carried out every month would be the most robust study design; however, this technique is burdensome for mothers as a repeated measure, and existing literature suggests that there is little variation in 24-hour lipid intake throughout lactation. Using this rationale, we deemed established lactation intake at 3 months to be representative of the true lipid intake of the breastfed infant throughout lactation, and this was the reference by which we assessed methods, irrespective of collection month (2). Due to this healthy cohort and the extensive sampling carried out (1026 samples), with samples taken through the day, over 6 months of exclusive breastfeeding, and for a full 24-hour period in month 3, we were able to explore many different sampling protocols with a sample size of n = 20.
Based on this data, if 24-hour sampling and infant test-weighing is not possible, the most reliable alternative sampling protocol is matched pre- and post-feed samples through the day (6/prepost/daily). As this protocol had a mean overestimate of only 0.1 or 0.3 g/d, using both 800 mL/d and the true volume intake, the discrepancy from the true intake was consistently low and will have negligible effects on the translation of infant lipid intake research.
In conclusion, a systematic comparison of different HM sampling methods for the estimation of infant lipid intake showed that collecting 6 pre- and post-feed samples across a day provides results that are most comparable to the true infant lipid intake as measured by 24-hour test weighing and sampling. In contrast, all other alternative protocols considerably over- or underestimated the infant lipid intake. The results of this study indicate that careful consideration should be given to HM sampling when interpreting the results of lipid measurements. When either method is not feasible, consideration of the discrepancies that are introduced by different sampling protocols is essential, especially in translational research where estimations of lipid intake are related to infant outcomes.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—ADG and DTG: designed the study; ADG and MCLG: collected the data; ADG: performed all laboratory experiments, completed the data analysis; and wrote the manuscript; and all authors: were involved in data interpretation, critically reviewed the manuscript, and read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Conflicts of interest: ADG is supported by a Medela AG Postgraduate Scholarship. BSM receives a Career Development Award from the National Health and Medical Research Council of Australia. MEW receives funding from the National Health and Medical Research Council of Australia. DTG is funded by an unrestricted research grant from Medela AG. MCLG and KM, no conflicts of interest.
Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviation used: HM, human milk.
Contributor Information
Alexandra D George, School of Molecular Sciences, The University of Western Australia, Perth, Australia.
Melvin C L Gay, School of Molecular Sciences, The University of Western Australia, Perth, Australia.
Kevin Murray, School of Population and Global Health, The University of Western Australia, Perth, Australia.
Beverly S Muhlhausler, School of Agriculture, Food and Wine, The University of Adelaide, Adelaide, Australia; Commonwealth Scientific and Industrial Research Organisation, Adelaide, Australia.
Mary E Wlodek, Department of Physiology, School of Biomedical Sciences, Faculty of Medicine, Dentistry and Health Sciences, The University of Melbourne, Victoria, Australia.
Donna T Geddes, School of Molecular Sciences, The University of Western Australia, Perth, Australia.
References
- 1. Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin N Am. 2013;60(1):49–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mitoulas LR, Kent JC, Cox DB, Owens RA, Sherriff JL, Hartmann PE. Variation in fat, lactose and protein in human milk over 24 h and throughout the first year of lactation. Br J Nutr. 2002;88(1):29–37. [DOI] [PubMed] [Google Scholar]
- 3. Hytten FE. Clinical and chemical studies in human lactation. Br Med J. 1954;1(4855):175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gridneva Z, Kugananthan S, Hepworth AR, Tie WJ, Lai CT, Ward LC, Hartmann PE, Geddes DT. Effect of human milk appetite hormones, macronutrients, and infant characteristics on gastric emptying and breastfeeding patterns of term fully breastfed infants. Nutrients. 2017;9(1):15 doi:10.3390/nu9010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gardner AS, Rahman IA, Lai CT, Hepworth A, Trengove N, Hartmann PE, Geddes DT. Changes in fatty acid composition of human milk in response to cold-like symptoms in the lactating mother and infant. Nutrients. 2017;9(9):1034 doi: 10.3390/nu9091034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jackson DA, Imong SM, Silprasert A, Ruckphaopunt S, Woolridge MW, Baum JD, Amatayakul K. Circadian variation in fat concentration of breast-milk in a rural northern Thai population. Br J Nutr. 1988;59(3):349–63. [DOI] [PubMed] [Google Scholar]
- 7. Stellwagen LM, Vaucher YE, Chan CS, Montminy TD, Kim JH. Pooling expressed breastmilk to provide a consistent feeding composition for premature infants. Breastfeed Med. 2013;8:205–9. [DOI] [PubMed] [Google Scholar]
- 8. Leghi GE, Middleton PF, Netting MJ, Wlodek ME, Geddes DT, Muhlhausler BS. A systematic review of collection and analysis of human milk for macronutrient composition. J Nutr. 2020;150:1652–70. [DOI] [PubMed] [Google Scholar]
- 9. Kent JC, Mitoulas LR, Cregan MD, Ramsay DT, Doherty DA, Hartmann PE. Volume and frequency of breastfeedings and fat content of breast milk throughout the day. Pediatrics. 2006;117(3):e387–95. [DOI] [PubMed] [Google Scholar]
- 10. European Food Safety Authority Dietetic Products, Nutrition, and Allergies Panel Scientific opinion on nutrient requirements and dietary intakes of infants and young children in the European Union. EFSA J. 2013;11(10):3408. [Google Scholar]
- 11. Lucas A, Gibbs JA, Lyster RL, Baum JD. Creamatocrit: simple clinical technique for estimating fat concentration and energy value of human milk. Br Med J. 1978;1(6119):1018–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Atwood CS, Hartmann PE. Collection of fore and hind milk from the sow and the changes in milk composition during suckling. J Dairy Res. 1992;59(3):287–98. [DOI] [PubMed] [Google Scholar]
- 13. Stafford J, Villalpando S, Urquieta Aguila B. Circadian variation and changes after a meal in volume and lipid production of human milk from rural Mexican women. Ann Nutr Metab. 1994;38(4):232–7. [DOI] [PubMed] [Google Scholar]
- 14. Dewey KG, Lonnerdal B. Milk and nutrient intake of breast-fed infants from 1 to 6 months: relation to growth and fatness. J Pediatr Gastr Nutr. 1983;2(3):497–506. [DOI] [PubMed] [Google Scholar]
- 15. George AD, Gay MCL, Wlodek ME, Geddes DT. Breastfeeding a small for gestational age infant, complicated by maternal gestational diabetes: a case report. BMC Pregnancy Childb. 2019;19(1):210 doi: 10.1186/s12884-019-2366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.