Abstract
Introduction: Inflammation contributes to the initiation and progression of atherosclerosis, although the underlying inflammatory pathways are not entirely known. Specifically, the role of the proinflammatory soluble CD40 ligand (sCD40L) on the expression of chronic coronary syndrome (CCS) is not completely understood. We evaluated whether sCD40L expression is associated with the presence of CCS and with the clinical and anatomical severity of CCS. Methods: We prospectively recruited 94 participants, assigned to two groups matched by age and sex, without coronary artery disease (n=26) and with CCS (n=68). Clinical, laboratory and anatomical data were prospectively collected, and serum levels of sCD40L were measured. Results: In patients with CCS, classic cardiovascular risk factors were more prevalent, and the sCD40L levels, leukocyte and neutrophil counts, and neutrophil/lymphocyte ratio, but not the C-reactive protein levels, were significantly higher than those in controls. sCD40L was independently associated with the presence of obstructive coronary artery disease in multivariate analysis. Regarding CCS severity, sCD40L levels showed a significant stepwise increase with increasing angina severity (ANOVA P=0.001). In addition, sCD40L was independently associated with the anatomical severity of coronary artery disease, as assessed by the Gensini score. Among patients with CCS, those with previous coronary artery bypass grafting (n=23) had lower sCD40L levels than patients waiting for revascularization (n=45) [4.3 (2.1) ng/mL vs. 6.8 (3.5) ng/mL, P=0.001]. Conclusions: The expression of the proinflammatory sCD40L was associated with the presence of CCS and reflected the clinical and anatomical severity of CCS. In addition, we describe for the first time the association between prior CABG and reduced sCD40L levels in patients with CCS.
Keywords: Chronic coronary syndrome, coronary artery disease, inflammation, soluble CD40 ligand
Introduction
Atherosclerosis is recognized as an inflammatory disease, although the underlying inflammatory pathways associated with atherosclerotic disease initiation and progression are not completely understood [1-3]. Identifying major inflammatory mediators that participate in atherosclerosis regulation may contribute to not only a better understanding of pathophysiology but also clinical care, since they may potentially be used as diagnostic biomarkers and therapeutic targets, such as in the Canakinumab Antiinflammatory Thrombosis Outcome Study [1-4].
The soluble form of CD40 ligand (sCD40L) is a mediator of vascular inflammation that interacts with the CD40 receptor expressed on different cells involved in atherogenesis, including macrophages, endothelial cells and T-cells [5,6]. Furthermore, we have previously observed that sCD40L expression is associated with vascular function [7,8]. Altogether, the role of sCD40L in vascular inflammation and function supports sCD40L as a mediator of atherosclerosis expression [5-8]. sCD40L has been extensively studied mechanistically and as a potential diagnostic and prognostic marker in patients with acute coronary syndrome [6-10]. Less is known about the role of sCD40L in chronic coronary syndromes (CCSs). CCS, a recently introduced concept that encompasses stable coronary artery disease, has a pathophysiology that is distinct from that of acute coronary syndrome since inflammation mainly regulates the initiation and progression of stable lesions in CCS; therefore, the study of inflammation specifically in CCS deserves special attention [1,2,11]. Some studies reported higher levels of sCD40L in patients with CCS than in controls [12-14], while other studies did not [15-20]. However, most of these studies have limitations regarding the sample size, method used for the exclusion of coronary artery disease, and lack of proper adjustment for confounding variables [12-20]. More importantly, there are few data on the relationship between sCD40L levels and the clinical or anatomical severity of CCS [20].
We aimed to evaluate whether the serum levels of sCD40L are associated with 1) the presence of CCS, and 2) the clinical and anatomical severity of CCS. We hypothesize that sCD40L levels are increased in CCS with obstructive coronary artery disease and that higher sCD40L levels are associated with increased severity of CCS.
Materials and methods
The study protocol was approved by the ethics committees of the involved institutions (Centro Hospitalar Universitário de Lisboa Central, Nr. 245/2015, and NOVA Medical School/Faculdade de Ciências Médicas, Universidade NOVA de Lisboa, Nr. 000176). The investigation conformed to the principles outlined in the Declaration of Helsinki. All participants signed informed consent forms.
Selection of participants
We prospectively recruited 94 participants followed in our center, which were assigned to two groups: 26 individuals without coronary artery disease (controls) and 68 patients with CCS.
Inclusion criteria: 1) Controls were recruited from the outpatient clinic and were eligible if they presented no effort angina; no evidence of coronary artery disease on coronary computed tomography angiography, including calcium score =0 and no soft plaques; and no positive myocardial stress test (the latter was not mandated to be assessed per protocol); and 2) Patients with CCS were recruited from those referred for elective invasive coronary angiography and were eligible in case of documented obstructive coronary artery disease, including luminal stenosis of at least 50% for the left main artery or at least 70% for other epicardial vessels.
Exclusion criteria: Patients with acute atherosclerotic events or coronary artery bypass grafting (CABG) within 12 months; those with any previous percutaneous coronary intervention, heart failure, hemodynamically significant valvular heart disease, hematological disorders, active infection, history of malignancy, chronic kidney disease (stage 4 or 5), or severe hepatic dysfunction; those under 18 years of age; or those unable or unwilling to consent to study participation were excluded. If performed at least 12 months before inclusion, CABG was not an exclusion criterion since the presence and extent of coronary artery lesions, which were the focus of this study, are not affected by the surgical placement of coronary artery bypass grafts [21].
Data collection
Clinical, laboratory and anatomical data were collected prospectively after patient inclusion. A standardized record of clinical and demographic characteristics (including cardiovascular risk factors, ongoing medication, clinical symptoms, and physical data), hematological and biochemical parameters, echocardiographic data, and angiography results was obtained from each participant. For the assessment of the anatomical severity of coronary artery disease, we assessed, for each patient, the number of obstructive lesions (luminal stenosis of at least 50% for the left main artery or at least 70% for other epicardial vessels); the number of vessels with obstructive lesions (the left main artery, left anterior descending artery, circumflex artery and right coronary artery were considered separately, with total score ranging from 0 to 4); and the Gensini score, which was calculated as previously described [22].
Blood sampling and sCD40L measurements
Peripheral blood was collected early in the morning under fasting conditions. Serum was separated by centrifugation (500 g for 10 min) within 15 min of sampling. Aliquots were stored at -80°C; samples were thawed only once. Concentrations of sCD40L were measured in serum by an enzyme-linked immunosorbent assay commercial kit (R&D Systems). Each sample was measured in duplicate. The intra-assay variation among the duplicates for all samples was less than 10%.
Sample size
A sample size of 20 controls and 60 patients with CCS simultaneously provided: 1) 80% power to detect a 20% difference in sCD40L levels between controls and patients (for the first objective), with a standard deviation (SD) of sCD40L levels of 35% [12-14], at a 5% significance level; 2) a number of patients with CCS enough to carry out a comprehensive analysis on the association between sCD40L levels and CCS severity (for the second objective), including a potential multivariate linear regression analysis [23,24]. The recruitment was carried out in a control:patient ratio of 1:3, matching by age and sex. Accounting for potential dropouts, additional participants were recruited. There were no dropouts and a total of 26 controls and 68 patients were included.
Statistical analysis
Discrete data are presented as frequencies (percentages), and continuous variables are presented as the mean (SD) or the median (interquartile range, IQR). Continuous variables were analyzed using Student’s t-test or the Mann-Whitney test when normality was not verified (Shapiro-Wilk test); analysis of variance (ANOVA) was used for comparisons between multiple patient groups, and Bonferroni post hoc correction was used for multiple comparisons. Categorical variables were analyzed using the chi-squared or Fisher’s exact tests. Pearson’s correlation was used to test correlations between continuous variables. Multivariate logistic regression analysis was applied to identify which parameters were independently associated with the presence of obstructive coronary artery disease and (post hoc analysis) with previous CABG. Multivariate linear regression analysis was used to identify the independent predictors of the anatomical severity of coronary artery disease, as assessed by the Gensini score. Outliers were excluded, as appropriate [25]. The level of significance considered was α=0.05. Analyses were conducted with SPSS software, version 26 (IBM).
Results
Clinical and laboratory characteristics of participants
The characteristics of the 26 controls and 68 patients with CCS are presented in Table 1. In patients with CCS, hypertension, dyslipidemia, diabetes, a smoking history (current or past), and the use of antiplatelet and statin therapy were significantly more prevalent. In the CCS group, leukocyte count, neutrophil count, neutrophil/lymphocyte ratio, the percentage of glycosylated hemoglobin, creatinine levels, and sCD40L levels were significantly higher, and high-density lipoprotein (HDL) cholesterol levels were significantly lower, than in the control group. Of note, C-reactive protein levels did not differ between groups.
Table 1.
Clinical, laboratory and anatomical data of controls and patients with chronic coronary syndrome
| Controls | Patients with CCS | p-value | |
|---|---|---|---|
| (n=26) | (n=68) | ||
| Clinical data | |||
| Age, years | 61.3 (9.4) | 64.8 (9.3) | 0.099 |
| Male, n (%) | 23 (88.5) | 61 (89.7) | 1.000 |
| Hypertension, n (%) | 14 (53.8) | 64 (94.1) | <0.001 |
| Dyslipidemia, n (%) | 18 (69.2) | 65 (95.6) | <0.001 |
| Diabetes mellitus, n (%) | 3 (11.5) | 29 (42.6) | 0.007 |
| Smoking history (current or past), n (%) | 6 (23.1) | 37 (54.4) | 0.006 |
| Previous coronary artery bypass grafting, n (%) | 0 (0.0) | 23 (33.8) | <0.001 |
| Left ventricular ejection fraction > 50%, n (%) | 26 (100.0) | 68 (100.0) | - |
| Antiplatelet agent, n (%) | 6 (23.1) | 66 (97.1) | <0.001 |
| Statin therapy, n (%) | 13 (50.0) | 61 (89.7) | <0.001 |
| Laboratory data | |||
| Hemoglobin, g/dL | 13.9 (1.3) | 13.6 (1.6) | 0.380 |
| Leukocyte count, 10^9/L | 6.4 (1.7) | 7.6 (1.8) | 0.012 |
| Neutrophil count, 10^9/L | 3.5 (1.5) | 4.4 (1.5) | 0.010 |
| Lymphocyte count, 10^9/L | 2.0 (0.5) | 2.1 (0.8) | 0.722 |
| Neutrophil/lymphocyte ratio | 1.9 (0.7) | 2.4 (1.1) | 0.029 |
| Platelet count, 10^9/L | 230.1 (58.8) | 222.6 (51.1) | 0.546 |
| Fasting glycaemia, mg/dL | 95.3 (35.1) | 112.7 (52.5) | 0.124 |
| Percentage of glycosylated hemoglobin | 5.8 (1.2) | 6.4 (1.5) | 0.014 |
| Creatinine, mg/dL | 0.8 (0.2) | 1.0 (0.3) | 0.001 |
| Total cholesterol, mg/dL | 186.0 (50.7) | 166.4 (45.9) | 0.082 |
| LDL-cholesterol, mg/dL | 111.3 (44.2) | 100.9 (37.7) | 0.261 |
| HDL-cholesterol, mg/dL | 51.5 (13.1) | 37.6 (9.1) | <0.001 |
| Triglycerides, mg/dL | 133.6 (50.9) | 149.0 (55.8) | 0.234 |
| C-reactive protein, mg/L | 4.1 (2.0) | 3.7 (1.7) | 0.334 |
| sCD40L, ng/mL | 4.0 (1.5) | 5.9 (3.3) | 0.018 |
| Anatomical data | |||
| Nr. of vessels with obstructive disease | 0 (0.0) | 2.9 (0.8) | <0.001 |
| Nr. of obstructive lesions | 0 (0.0) | 4.0 (1.4) | <0.001 |
| Gensini score | 0 (0.0) | 72.9 (42.5) | <0.001 |
Data are expressed as mean (SD), except if otherwise specified. CCS - chronic coronary syndrome; HDL - high-density lipoprotein; LDL - low-density lipoprotein; Nr. - number; sCD40L - soluble CD40 ligand.
Association of sCD40L with clinical and laboratory data
sCD40L showed a weak positive correlation with leukocyte count, neutrophil count, lymphocyte count, and platelet count and a weak negative correlation with HDL-cholesterol levels (Table 2). The sCD40L levels did not differ according to age, sex or other cardiovascular risk factors and showed no correlation with other laboratory parameters, including C-reactive protein levels (Table 2).
Table 2.
Association of soluble CD40 ligand with clinical and laboratory parameters
| sCD40L, ng/mLa | p-value | ||
|---|---|---|---|
| Clinical parameters | |||
| Age, yearsb | r=-0.085 | 0.419 | |
| Sexc | Male | 5.5 (3.2) | 0.876 |
| Female | 5.4 (3.0) | ||
| Hypertensionc | No | 4.9 (2.4) | 0.555 |
| Yes | 5.5 (3.1) | ||
| Dyslipidemiac | No | 5.3 (2.4) | 0.953 |
| Yes | 5.4 (3.1) | ||
| Diabetes mellitusc | No | 5.5 (3.0) | 0.555 |
| Yes | 5.2 (3.1) | ||
| Smoking historyc | No | 5.1 (2.7) | 0.363 |
| Yes | 5.9 (3.3) | ||
| Laboratory parameters | |||
| Hemoglobin, g/dLb | r=0.123 | 0.243 | |
| Leukocyte count, 10^9/Lb | r=0.301 | 0.004 | |
| Neutrophil count, 10^9/Lb | r=0.219 | 0.037 | |
| Lymphocyte count, 10^9/Lb | r=0.292 | 0.005 | |
| Neutrophil/lymphocyte ratiob | r=-0.041 | 0.704 | |
| Platelet count, 10^9/Lb | r=0.223 | 0.035 | |
| Fasting glycaemia, mg/dLb | r=0.114 | 0.284 | |
| Percentage of glycosylated hemoglobinb | r=0.136 | 0.217 | |
| Creatinine, mg/dLb | r=0.118 | 0.258 | |
| Total cholesterol, mg/dLb | r=-0.017 | 0.874 | |
| LDL-cholesterol, mg/dLb | r=0.014 | 0.897 | |
| HDL-cholesterol, mg/dLb | r=-0.222 | 0.036 | |
| Triglycerides, mg/dLb | r=0.039 | 0.717 | |
| C-reactive protein, mg/Lb | r=0.095 | 0.388 |
Soluble CD40 ligand levels are expressed as mean (SD);
Correlations between soluble CD40 ligand levels and continuous variables were tested and the correlation coefficient (r) is presented for each;
soluble CD40 ligand levels were compared between groups for categorical variables.
HDL - high-density lipoprotein; LDL - low-density lipoprotein; sCD40L - soluble CD40 ligand.
sCD40L and the presence of obstructive coronary artery disease
Considering all clinical and laboratory data, sCD40L was an independent predictor of obstructive coronary artery disease in the multivariate logistic regression analysis, in addition to hypertension and HDL-cholesterol levels (Table 3).
Table 3.
Predictors of obstructive stable coronary artery disease by multivariate logistic regression analysis
| Predictors | β | 95% CI | p-value |
|---|---|---|---|
| Hypertension | 0.055 | 0.010 to 0.305 | 0.001 |
| HDL-cholesterol, mg/dL | 0.885 | 0.825 to 0.949 | 0.001 |
| sCD40L, ng/mL | 1.329 | 1.005 to 1.757 | 0.046 |
95% CI - 95% confidence interval; HDL - high-density lipoprotein; sCD40L - soluble CD40 ligand.
sCD40L and the severity of obstructive coronary artery disease
Clinically, the sCD40L levels showed a stepwise increase with increasing severity of angina among patients with obstructive coronary artery disease (ANOVA P=0.001) (Figure 1). Anatomically, there was a weak positive correlation between sCD40L levels and the number of coronary artery vessels with obstructive disease (r=0.285, P=0.006), the number of obstructive coronary artery lesions (r=0.238, P=0.022), and the Gensini score (r=0.279, P=0.007). The levels of sCD40L were higher in higher categories of the Gensini score (ANOVA P=0.026) (Figure 2).
Figure 1.
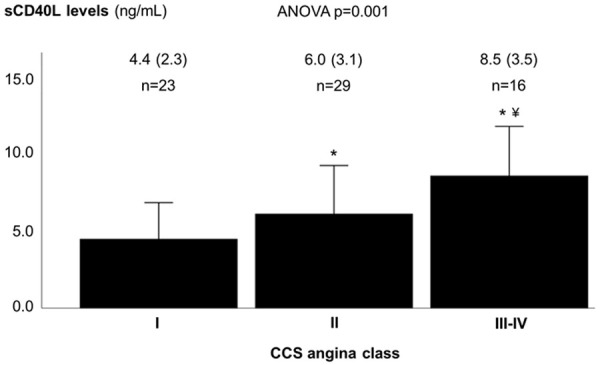
Soluble CD40 ligand levels according to angina class, among patients with obstructive coronary artery disease. Soluble CD40 ligand values are expressed as ng/mL, mean (SD). CCS - Canadian Cardiovascular Society (angina class); sCD40L - soluble CD40 ligand. * p-value <0.05 vs. angina class I; ¥ p-value <0.05 vs. angina class II.
Figure 2.
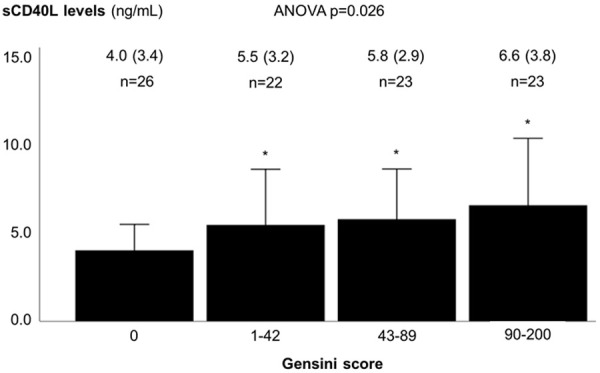
Soluble CD40 ligand levels according to the Gensini score. The first group corresponds to controls (all of which had a Gensini score of zero), and the remaining participants were equally divided into three groups according to the Gensini score. Soluble CD40 ligand values are expressed as ng/mL, mean (SD). sCD40L - soluble CD40 ligand. * p-value <0.05 vs. Gensini score of zero.
The univariate analysis assessing parameters associated with the Gensini score (as a continuous variable) is presented in Table 4. In the multivariate linear regression analysis, sCD40L was an independent predictor of coronary artery disease severity, as assessed by the Gensini score, in addition to neutrophil/lymphocyte ratio, creatinine levels, and HDL-cholesterol levels (Table 5).
Table 4.
Parameters associated with the Gensini score in univariate analysis
| Gensini scorea | p-value | ||
|---|---|---|---|
| Clinical parameters | |||
| Age, yearsb | r=0.201 | 0.053 | |
| Sexc | Male | 53.6 (49.9) | 0.877 |
| Female | 45.3 (39.4) | ||
| Hypertensionc | No | 25.9 (53.4) | 0.002 |
| Yes | 58.2 (46.2) | ||
| Dyslipidemiac | No | 12.4 (32.2) | 0.001 |
| Yes | 58.1 (48.2) | ||
| Diabetes mellitusc | No | 49.1 (51.4) | 0.170 |
| Yes | 59.7 (43.1) | ||
| Smoking historyc | No | 54.5 (53.3) | 0.105 |
| Yes | 48.4 (36.2) | ||
| Laboratory parameters | |||
| Hemoglobin, g/dLb | r=0.066 | 0.530 | |
| Leukocyte count, 10^9/Lb | r=0.294 | 0.005 | |
| Neutrophil count, 10^9/Lb | r=0.265 | 0.011 | |
| Lymphocyte count, 10^9/Lb | r=0.000 | 0.999 | |
| Neutrophil/lymphocyte ratiob | r=0.309 | 0.003 | |
| Platelet count, 10^9/Lb | r=-0.048 | 0.650 | |
| Fasting glycaemia, mg/dLb | r=0.031 | 0.771 | |
| Percentage of glycosylated hemoglobinb | r=0.044 | 0.689 | |
| Creatinine, mg/dLb | r=0.322 | 0.002 | |
| Total cholesterol, mg/dLb | r=-0.149 | 0.156 | |
| LDL-cholesterol, mg/dLb | r=-0.064 | 0.543 | |
| HDL-cholesterol, mg/dLb | r=-0.450 | <0.001 | |
| Triglycerides, mg/dLb | r=0.064 | 0.545 | |
| C-reactive protein, mg/Lb | r=0.066 | 0.549 | |
| sCD40L, ng/mLb | r=0.279 | 0.007 |
The Gensini score is expressed as mean (SD);
Correlations between the Gensini score and continuous variables were tested and the correlation coefficient (r) is presented for each;
The Gensini score was compared between groups for categorical variables.
HDL - high-density lipoprotein; LDL - low-density lipoprotein; sCD40L - soluble CD40 ligand.
Table 5.
Predictors of the Gensini score by multivariate linear regression analysis
| Predictors | β | 95% CI | p-value |
|---|---|---|---|
| Neutrophil/lymphocyte ratio | 12.821 | 4.017 to 21.625 | 0.005 |
| Creatinine, mg/dL | 33.284 | 4.393 to 62.176 | 0.024 |
| HDL-cholesterol, mg/dL | -1.205 | -1.989 to -0.421 | 0.003 |
| sCD40L, ng/mL | 3.177 | 0.276 to 6.078 | 0.032 |
95% CI - 95% confidence interval; HDL - high-density lipoprotein; sCD40L - soluble CD40 ligand.
sCD40L and previous coronary revascularization
Among the 68 patients with CCS, 23 had previously undergone CABG (median 36 months, IQR 14 months to 10 years, before recruitment), and 45 had not been previously revascularized. Patients with prior CABG showed lower levels of sCD40L [4.3 (2.1) ng/mL vs. 6.8 (3.5) ng/mL, P=0.001]. Leukocyte, neutrophil, and platelet counts were also significantly lower in patients with prior CABG (Table 6). There were no other differences between groups, including the extent of native coronary artery disease (Table 6). In the multivariate logistic regression analysis, sCD40L was the only parameter independently associated with previous surgical revascularization (β 0.736, 95% confidence interval 0.597 to 0.908, P=004). There was no correlation between time elapsed from CABG and sCD40L levels (r=-0.263, P=0.225). When patients with prior CABG were classified according to the median time elapsed from CABG (36 months), there were no differences regarding the severity of coronary artery disease (Table 7) or sCD40L levels [5.0 (2.3) ng/mL vs. 3.6 (1.6) ng/mL, if time elapsed from CABG was inferior to or at least 36 months, respectively, p=0.098].
Table 6.
Characteristic of patients with chronic coronary syndrome with and without prior coronary artery bypass grafting
| No previous CABG | Previous CABG | p-value | |
|---|---|---|---|
| (n=45) | (n=23) | ||
| Clinical data | |||
| Age, years | 64.9 (10.2) | 64.7 (7.3) | 0.645 |
| Male, n (%) | 41 (91.1) | 20 (87.0) | 0.681 |
| Hypertension, n (%) | 43 (95.6) | 21 (91.3) | 0.599 |
| Dyslipidemia, n (%) | 42 (93.3) | 23 (100.0) | 0.546 |
| Diabetes mellitus, n (%) | 20 (44.4) | 9 (39.1) | 0.675 |
| Smoking history (current or past), n (%) | 26 (57.8) | 11 (47.8) | 0.436 |
| Left ventricular ejection fraction > 50%, n (%) | 45 (100.0) | 23 (100.0) | - |
| Antiplatelet agent, n (%) | 43 (95.6) | 23 (100.0) | 0.546 |
| Statin therapy, n (%) | 40 (88.9) | 21 (91.3) | 1.000 |
| Laboratory data | |||
| Hemoglobin, g/dL | 13.6 (1.5) | 13.7 (1.8) | 0.780 |
| Leukocyte count, 10^9/L | 8.0 (1.8) | 6.7 (1.6) | 0.003 |
| Neutrophil count, 10^9/L | 4.8 (1.4) | 3.8 (1.7) | 0.001 |
| Lymphocyte count, 10^9/L | 2.2 (0.9) | 1.8 (0.7) | 0.133 |
| Neutrophil/lymphocyte ratio | 2.4 (1.0) | 2.3 (1.1) | 0.457 |
| Platelet count, 10^9/L | 233.6 (49.3) | 199.7 (47.9) | 0.011 |
| Fasting glycaemia, mg/dL | 113.7 (50.1) | 110.5 (58.1) | 0.892 |
| Percentage of glycosylated hemoglobin | 6.6 (1.5) | 6.1 (1.3) | 0.171 |
| Creatinine, mg/dL | 1.1 (0.4) | 1.0 (0.3) | 0.367 |
| Total cholesterol, mg/dL | 170.9 (42.6) | 157.9 (51.6) | 0.274 |
| LDL-cholesterol, mg/dL | 104.4 (35.5) | 94.1 (41.6) | 0.291 |
| HDL-cholesterol, mg/dL | 37.6 (8.9) | 37.6 (9.6) | 0.859 |
| Triglycerides, mg/dL | 155.6 (57.9) | 136.0 (52.3) | 0.169 |
| C-reactive protein, mg/L | 3.6 (1.3) | 3.8 (1.9) | 0.611 |
| sCD40L, ng/mL | 6.8 (3.5) | 4.3 (2.1) | 0.001 |
| Anatomical data | |||
| Nr. of vessels with obstructive disease | 3.0 (0.9) | 2.8 (0.7) | 0.438 |
| Nr. of obstructive lesions | 4.1 (1.2) | 3.8 (1.6) | 0.243 |
| Gensini score | 71.1 (36.2) | 76.3 (53.5) | 0.928 |
Data are expressed as mean (SD), except if otherwise specified. CABG - coronary artery bypass grafting; HDL - high-density lipoprotein; LDL - low-density lipoprotein; Nr. - number; sCD40L - soluble CD40 ligand.
Table 7.
Severity of native coronary artery disease according to time elapsed from coronary artery bypass grafting
| CABG 12 to 36 months before (n=12) | CABG at least 36 months before (n=11) | p-value | |
|---|---|---|---|
| Nr. of vessels with obstructive disease | 2.8 (0.7) | 2.8 (0.8) | 0.961 |
| Nr. of obstructive lesions | 3.8 (1.5) | 3.8 (1.7) | 0.920 |
| Gensini score | 68.8 (49.1) | 84.5 (59.2) | 0.493 |
Data are expressed as mean (SD). CABG - coronary artery bypass grafting; Nr. - number.
Of note, a post hoc multivariate analysis on predictors of obstructive coronary artery disease, excluding patients with CCS and prior CABG (Table 8), yielded similar results to the multivariate analysis including all patients with CCS (Table 3).
Table 8.
Independent predictors of obstructive coronary artery disease excluding patients with prior coronary artery bypass grafting
| Predictors | β | 95% CI | p-value |
|---|---|---|---|
| Hypertension | 0.058 | 0.007 to 0.464 | 0.007 |
| HDL-cholesterol, mg/dL | 0.893 | 0.828 to 0.964 | 0.004 |
| sCD40L, ng/mL | 1.344 | 1.016 to 1.778 | 0.038 |
95% CI - 95% confidence interval; HDL - high-density lipoprotein; sCD40L - soluble CD40 ligand.
Discussion
In this prospective case-control study, three findings stood out: sCD40L levels were higher in patients with CCS; sCD40L levels were associated with the clinical and anatomical severity of coronary artery disease; and prior CABG was associated with reduced sCD40L levels among patients with CCS.
Regarding the increased levels of sCD40L in patients with CCS compared to controls, this association was independent from other clinical variables, such as classic cardiovascular risk factors, and from laboratory measures, such as those reflecting inflammatory activation, including leukocyte, neutrophil and platelet counts and C-reactive protein levels. Some previous studies also reported higher levels of sCD40L in the presence of stable coronary artery disease [12-14], while others did not [15-20]. However, some of these studies have limitations regarding sample size [14,16-19]. On the other hand, in previous studies, the exclusion of coronary artery disease in controls was based on invasive coronary angiography or on clinical assessment without an evaluation of coronary anatomy [12-20]. We used coronary computed tomography angiography to exclude coronary artery atherosclerosis in controls, which may have a higher negative predictive power than invasive coronary angiography [26]; indeed, invasive coronary angiography visualizes only luminal narrowing and may be insensitive to coronary artery lesions with positive remodeling and no compromise of luminal dimensions, which are conversely detectable by coronary computed tomography angiography [26]. In addition, most studies that tested sCD40L as a potential predictor of the presence of obstructive coronary artery disease lack proper adjustment for confounding variables [12,14,16-20]. We conducted a comprehensive multivariate analysis, which confirmed the independent association between sCD40L and coronary artery disease status.
Second, we observed that sCD40L was consistently and independently associated with the severity of coronary artery disease. There are few data available on this subject, and our results provide further insights into the pathophysiology of CCS presentation and severity. Previous studies reported no association between sCD40L levels and the anatomical severity of coronary artery disease, assessed by an angiographic score [12,27] or by coronary artery calcium score [28]. Nevertheless, theses scores do not assign a specific weight for each diseased segment and therefore do not account for the perfused territories supplied by stenoses located in different segments [12,27,28]. We used the Gensini score, which simultaneously measures coronary artery disease burden and considers the myocardium at risk for each lesion/segment [22]. This may explain our positive findings in contrast to those of the aforementioned studies. In fact, of the studies focusing on stable coronary artery disease, one reported increased sCD40L levels in multivessel disease compared to less severe disease and a positive correlation between sCD40L and the Gensini score [29]. Additional data on the relationship between stable coronary artery disease severity and sCD40L levels are scarce. On the other hand, to the best of our knowledge, there are no published data on the association between angina severity, as a measure of the clinical severity of CCS, and sCD40L. Therefore, our results may add to the knowledge on how inflammation may indirectly be reflected in clinical status.
Third, we describe for the first time the variation in sCD40L levels according to coronary revascularization status. In addition to having a causal role in atherosclerosis development, sCD40L might itself be influenced by atherosclerosis and reflect coronary artery disease burden and ischemic stress [30]. Indeed, in our sample, among patients with CCS, those with previous CABG showed lower levels of sCD40L than patients without previous CABG. It is known that severe chronic coronary ischemia promotes the release of reactive oxygen species, cytokines and other inflammatory markers and that revascularization may ameliorate inflammation and vascular function [31,32]. It is possible that the reduction of ischemic stress after bypass grafting may have reduced inflammation without modifying coronary atherosclerosis burden, which translated into lower sCD40L release into peripheral blood [21]. The hypothesis that sCD40L is a surrogate of coronary artery disease burden and myocardial ischemic stress needs to be explored in future studies. The scarce data available on the long-term effects of CABG on inflammation in patients with CCS point to an attenuation of the acute-phase reaction, reflected by a reduction in C-reactive protein levels [33]. Specifically, for sCD40L, the levels were reported to rise immediately after CABG, probably due to endothelial disruption and inflammatory activation related to the surgery itself, followed by a decrease in the first month after surgery [34]. Nevertheless, no published long-term data are available.
There are strengths of this study that should be acknowledged. The prospective nature of the study, the use of coronary computed tomography angiography to exclude coronary artery atherosclerosis in controls and, importantly, the multivariate analysis adjusting for confounders contributed to higher accuracy in the identification of sCD40L as a predictor of obstructive coronary artery disease compared to previous studies. In addition, analyses on the clinical and anatomical severity of CCS, with adjustment for confounding variables, added consistency to the results. To the best of our knowledge, the association between the clinical severity of CCS and sCD40L levels has not yet been reported. Finally, we describe for the first time a reduced expression of sCD40L in patients with prior coronary revascularization among those with obstructive coronary artery disease.
Our study has some limitations. First, this is a single-center study and additional studies from multiple centers are warranted. Second, we did not demonstrate a causal relationship between sCD40L and atherogenesis, which has already been studied [6,35]. Instead, we observed that sCD40L probably mediates the interaction between inflammation and atherosclerotic disease expression, and may also be a surrogate marker. The consistency of our results, considering the multiple results pointing towards the same direction, after adjustment for confounders, reinforces the role of sCD40L as a mediator in this process.
In conclusion, the sCD40L levels were increased in patients with CCS, and sCD40L expression was associated with the clinical and anatomical severity of CCS, while coronary artery revascularization was associated with the attenuation of sCD40L dysregulation. Therefore, sCD40L may participate in the association between inflammation and atherosclerosis development, progression and clinical expression. The results of this study add to knowledge on the pathophysiology of coronary atherosclerosis. In addition, our findings suggest that sCD40L may be a promising complementary noninvasive tool for refining the stratification of coronary atherosclerosis burden and severity, which could contribute to the tailoring of the primary prevention strategies. These fields deserve further research.
Acknowledgements
This study is part of the PhD thesis program of one of the authors (TPdS), supervised (MMC) and co-supervised (PN) by other two, conducted in NOVA Medical School/Faculdade de Ciências Médicas, Universidade NOVA de Lisboa, Lisbon, Portugal. TP acknowledges the support of Fundação para a Ciência e Tecnologia, Portugal (Project UID/BIO/04565/2020), and Programa Operacional Regional de Lisboa 2020 (Project N. 007317). The authors are grateful to Joana Castro, PhD, from Medinres - Medical Information and Research, for her advice in the statistical analysis.
Disclosure of conflict of interest
None.
References
- 1.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Flego D, Liuzzo G, Weyand CM, Crea F. Adaptive immunity dysregulation in acute coronary syndromes: from cellular and molecular basis to clinical implications. J Am Coll Cardiol. 2016;68:2107–2117. doi: 10.1016/j.jacc.2016.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Libby P, Ridker PM, Hansson GK Leducq Transatlantic Network on Atherothrombosis. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 5.André P, Nannizzi-Alaimo L, Prasad SK, Phillips DR. Platelet-derived CD40L: the switch-hitting player of cardiovascular disease. Circulation. 2002;106:896–899. doi: 10.1161/01.cir.0000028962.04520.01. [DOI] [PubMed] [Google Scholar]
- 6.Antoniades C, Bakogiannis C, Tousoulis D, Antonopoulos AS, Stefanadis C. The CD40/CD40 ligand system: linking inflammation with atherothrombosis. J Am Coll Cardiol. 2009;54:669–677. doi: 10.1016/j.jacc.2009.03.076. [DOI] [PubMed] [Google Scholar]
- 7.Napoleão P, Monteiro Mdo C, Cabral LB, Criado MB, Ramos C, Selas M, Viegas-Crespo AM, Saldanha C, Carmo MM, Ferreira RC, Pinheiro T. Changes of soluble CD40 ligand in the progression of acute myocardial infarction associate to endothelial nitric oxide synthase polymorphisms and vascular endothelial growth factor but not to platelet CD62P expression. Transl Res. 2015;166:650–659. doi: 10.1016/j.trsl.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Napoleão P, Cabral LB, Selas M, Freixo C, Monteiro Mdo C, Criado MB, Costa MC, Enguita FJ, Viegas-Crespo AM, Saldanha C, Carmo MM, Ferreira RC, Pinheiro T. Stratification of ST-elevation myocardial infarction patients based on soluble CD40L longitudinal changes. Transl Res. 2016;176:95–104. doi: 10.1016/j.trsl.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Heeschen C, Dimmeler S, Hamm CW, van den Brand MJ, Boersma E, Zeiher AM, Simoons ML CAPTURE Study Investigators. Soluble CD40 ligand in acute coronary syndromes. N Engl J Med. 2003;348:1104–1111. doi: 10.1056/NEJMoa022600. [DOI] [PubMed] [Google Scholar]
- 10.Varo N, de Lemos JA, Libby P, Morrow DA, Murphy SA, Nuzzo R, Gibson CM, Cannon CP, Braunwald E, Schönbeck U. Soluble CD40L: risk prediction after acute coronary syndromes. Circulation. 2003;108:1049–1052. doi: 10.1161/01.CIR.0000088521.04017.13. [DOI] [PubMed] [Google Scholar]
- 11.Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 12.Tayebjee MH, Lip GY, Tan KT, Patel JV, Hughes EA, MacFadyen RJ. Plasma matrix metalloproteinase-9, tissue inhibitor of metalloproteinase-2, and CD40 ligand levels in patients with stable coronary artery disease. Am J Cardiol. 2005;96:339–345. doi: 10.1016/j.amjcard.2005.03.072. [DOI] [PubMed] [Google Scholar]
- 13.Tousoulis D, Antoniades C, Nikolopoulou A, Koniari K, Vasiliadou C, Marinou K, Koumallos N, Papageorgiou N, Stefanadi E, Siasos G, Stefanadis C. Interaction between cytokines and sCD40L in patients with stable and unstable coronary syndromes. Eur J Clin Invest. 2007;37:623–628. doi: 10.1111/j.1365-2362.2007.01834.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Li L, Tan HW, Yu GS, Ma ZY, Zhao YX, Zhang Y. Transcoronary concentration gradient of sCD40L and hsCRP in patients with coronary heart disease. Clin Cardiol. 2007;30:86–91. doi: 10.1002/clc.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rondina MT, Lappé JM, Carlquist JF, Muhlestein JB, Kolek MJ, Horne BD, Pearson RR, Anderson JL. Soluble CD40 ligand as a predictor of coronary artery disease and long-term clinical outcomes in stable patients undergoing coronary angiography. Cardiology. 2008;109:196–201. doi: 10.1159/000106683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan J, Wu Z, Huang Z, Li L, Zhong R, Kong X. Clinical implications of increased expression of CD40L in patients with acute coronary syndromes. Chin Med J (Engl) 2002;115:491–493. [PubMed] [Google Scholar]
- 17.Aukrust P, Müller F, Ueland T, Berget T, Aaser E, Brunsvig A, Solum NO, Forfang K, Frøland SS, Gullestad L. Enhanced levels of soluble and membrane-bound CD40 ligand in patients with unstable angina. Possible reflection of T lymphocyte and platelet involvement in the pathogenesis of acute coronary syndromes. Circulation. 1999;100:614–620. doi: 10.1161/01.cir.100.6.614. [DOI] [PubMed] [Google Scholar]
- 18.Peng DQ, Zhao SP, Li YF, Li J, Zhou HN. Elevated soluble CD40 ligand is related to the endothelial adhesion molecules in patients with acute coronary syndrome. Clin Chim Acta. 2002;319:19–26. doi: 10.1016/s0009-8981(02)00014-1. [DOI] [PubMed] [Google Scholar]
- 19.Garlichs CD, Eskafi S, Raaz D, Schmidt A, Ludwig J, Herrmann M, Klinghammer L, Daniel WG, Schmeisser A. Patients with acute coronary syndromes express enhanced CD40 ligand/CD154 on platelets. Heart. 2001;86:649–655. doi: 10.1136/heart.86.6.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozde C, Korkmaz A, Kundi H, Oflar E, Ungan I, Xankisi V, Nurlu N. Relationship between plasma levels of soluble CD40 ligand and the presence and severity of isolated coronary artery ectasia. Clin Appl Thromb Hemost. 2018;24:379–386. doi: 10.1177/1076029616680476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alexander JH, Smith PK. Coronary-artery bypass grafting. N Engl J Med. 2016;375:e22. doi: 10.1056/NEJMc1608042. [DOI] [PubMed] [Google Scholar]
- 22.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51:606. doi: 10.1016/s0002-9149(83)80105-2. [DOI] [PubMed] [Google Scholar]
- 23.Green SB. How many subjects does it take to do a regression analysis. Multivariate Behav Res. 1991;26:499–510. doi: 10.1207/s15327906mbr2603_7. [DOI] [PubMed] [Google Scholar]
- 24.Harris RJ. In: A primer of multivariate statistics. 2nd edition. Harris RJ, editor. New York: Academic Press Inc.; 1985. [Google Scholar]
- 25.Field A. Assessing the regression model I: diagnostics. In: Field A, editor. Discovering Statistics using SPSS for Windows. London: SAGE Publications; 2000. pp. 122–123. [Google Scholar]
- 26.Chang SM, Bhatti S, Nabi F. Coronary computed tomography angiography. Curr Opin Cardiol. 2011;26:392–402. doi: 10.1097/HCO.0b013e32834938c6. [DOI] [PubMed] [Google Scholar]
- 27.Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, McGoon DC, Murphy ML, Roe BB. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975;51:5–40. doi: 10.1161/01.cir.51.4.5. [DOI] [PubMed] [Google Scholar]
- 28.de Lemos JA, Zirlik A, Schönbeck U, Varo N, Murphy SA, Khera A, McGuire DK, Stanek G, Lo HS, Nuzzo R, Morrow DA, Peshock R, Libby P. Associations between soluble CD40 ligand, atherosclerosis risk factors, and subclinical atherosclerosis: results from the dallas heart study. Arterioscler Thromb Vasc Biol. 2005;25:2192–2196. doi: 10.1161/01.ATV.0000182904.08513.60. [DOI] [PubMed] [Google Scholar]
- 29.Liang Y, Yang C, Zhou Q, Pan W, Zhong W, Ding R, Wang A. Serum monokine induced by gamma interferon is associated with severity of coronary artery disease. Int Heart J. 2017;58:24–29. doi: 10.1536/ihj.15-472. [DOI] [PubMed] [Google Scholar]
- 30.Gergei I, Kälsch T, Scharnagl H, Kleber ME, Zirlik A, März W, Krämer BK, Kälsch AI. Association of soluble CD40L with short-term and long-term cardiovascular and all-cause mortality: the Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Atherosclerosis. 2019;291:127–131. doi: 10.1016/j.atherosclerosis.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Rezende PC, Ribas FF, Serrano CV, Hueb W. Clinical significance of chronic myocardial ischemia in coronary artery disease patients. J Thorac Dis. 2019;11:1005–1015. doi: 10.21037/jtd.2019.02.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehta JL, Li DY. Inflammation in ischemic heart disease: response to tissue injury or a pathogenetic villain? Cardiovasc Res. 1999;43:291–299. doi: 10.1016/s0008-6363(99)00132-7. [DOI] [PubMed] [Google Scholar]
- 33.Santangeli P, Sgueglia GA, Sestito A, Lamendola P, Mariani L, Infusino F, Niccoli G, Crea F, Lanza GA. Different effect of percutaneous and surgical coronary revascularization on cardiac autonomic function and inflammation in patients with stable angina. Int J Cardiol. 2008;127:269–270. doi: 10.1016/j.ijcard.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 34.Wang L, Li Y, Gong X. Changes in inflammatory factors and prognosis of patients complicated with non-alcoholic fatty liver disease undergoing coronary artery bypass grafting. Exp Ther Med. 2018;15:949–953. doi: 10.3892/etm.2017.5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mach F, Schönbeck U, Sukhova GK, Atkinson E, Libby P. Reduction of atherosclerosis in mice by inhibition of CD40 signalling. Nature. 1998;394:200–203. doi: 10.1038/28204. [DOI] [PubMed] [Google Scholar]


