Abstract
Background: As an established procedure for patients with aortic valve stenosis and a high surgical risk profile, transcatheter aortic valve replacement (TAVR) can be associated with conductance abnormalities. However, data regarding the impact of pre-existing left bundle branch block (LBBB) on post-TAVR outcome is scarce. Objectives: We conducted this meta-analysis to pool available data in the literature on the impact of pre-existing LBBB on the clinical outcomes of patients undergoing TAVR. Methods: We queried Medline/PubMed, Scopus, and Cochrane Library to identify comparative studies of patients with and without a pre-existing LBBB undergoing TAVR for aortic stenosis. Risk ratio (RR) and the corresponding 95% confidence interval (95% CI) were estimated to measure the effect of pre-existing LBBB on developing post-procedure stroke, permanent pacemaker implantation (PPM), or moderate/severe aortic regurgitation (AR). Results: Data of three clinical trials encompassing 4,668 patients undergoing TAVR were included in this meta-analysis. Patients with pre-existing LBBB prior to TAVR had an increased risk of developing moderate/severe AR (RR = 1.04 [0.79-1.37]; P = 0.77), stroke (RR = 1.72 [0.61-4.85]; P = 0.31), and a need for PPM implantation (RR = 4.43 [0.43-45.64]; P = 0.21) following TAVR. Conclusion: Preexisting LBBB seems to increase the risk of developing stroke, aortic regurgitation, and the need for a permanent pacemaker implantation. However, due to scarcity of data and high heterogeneity among the current studies, further clinical trials are warranted.
Keywords: Left bundle branch block, transcatheter aortic valve replacement, aortic stenosis, aortic regurgitation, stroke, permanent pacemaker implantation
Introduction
Transcatheter aortic valve replacement (TAVR) is widely used as an alternative option to surgical valve replacement in patients with aortic stenosis (AS) and an intermediate-to-high risk for surgery [1-4]. However, conduction abnormalities are reported following TAVR of which left bundle branch block (LBBB) remains relatively common [5-8]. This might be due to the compression of the aortic valve prosthesis on the membranous septum as well as the inter-leaflet triangle between the right and non-coronary cusps, which can cause damage to the nearby left bundle branch and result in a baseline conduction deficit [5,6,8].
Although post-procedural LBBB has been linked to poor clinical outcomes such as an increased risk of atrioventricular block, permanent pacemaker implantation, and heart failure [8-11], pre-existing LBBB prior to TAVR and its impact on post-procedural outcome has not been studied in great depth and requires further elaboration.
To address this paucity of knowledge, we aimed to pool the existing data and systematically evaluate the impact of pre-existing LBBB on post-procedural clinical outcomes of patients with aortic stenosis who underwent TAVR.
Materials and methods
Study design
This systematic review and meta-analysis was designed in accordance with the Preferred Reporting Items for Systematic review and Meta-Analyses (PRISMA) [12]. Literature search was performed from inception until January 2019. Two independent reviewers performed the literature review, screened the title/abstract of the retrieved articles, and accessed the full texts of relevant studies. Any discrepancy was resolved by discussion with the third investigator. Endnote Reference Library (Version X8.1; Clarivate Analytics, Philadelphia, Pennsylvania) was used to manage literature review and screening of the records.
Search strategy
An electronic search was performed using the online databases Scopus, MEDLINE/PubMed, and Cochrane Library. The following key terms were used: “Transcatheter aortic valve implantation”, “TAVI”, “Transcatheter aortic valve replacement”, “TAVR”, “Percutaneous aortic valve implantation”, “Percutaneous aortic valve replacement”, “left bundle branch block”, and “conduction abnormality”.
Study selection
The inclusion criteria were comparative studies in English language that sought to determine the effect of pre-existing LBBB on the outcomes of TAVR. Articles in languages other than English, animal experiments, and case studies were excluded. Studies were included in meta-analysis only if data on variables of interest was extractable.
Outcomes measures
The primary endpoint of this meta-analysis was to compare the clinical outcome of TAVR between patients with and without a pre-existing LBBB. Data was abstracted on the following variables: characteristics of included studies, demographics of TAVR patients, and cardiovascular outcome following the procedure.
Statistical analysis
Pooled risk ratio (RR) and the corresponding 95% confidence interval (CI) were calculated using STATA 11.0, and the results were depicted as a forest plot. The quality assessment for the risk of bias of the included studies was done by the Newcastle-Ottawa scale (NOS) as shown in Table 1. A score of > 7 was considered to have a low risk of bias and an excellent methodological domain. Generic invariance random-effects model was used to perform the meta-analysis. Heterogeneity across the studies was evaluated by Higgins I2 method using chi-squared (Q) statistic and degrees of freedom (df) as follows:
Table 1.
Risk of bias assessment of nonrandomized studies included in the meta-analysis using the Newcastle-Ottawa
| Study | Yr | Selection | Comparability | Outcomes | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||
| Cohort representability | Selection of non-exposed cohort | Ascertainment of exposure | Outcome not present at baseline | Comparability of cohorts for important factors | Comparability of cohorts for other variables | Assessment of outcome | Follow-up long-enough for outcome | Adequacy of follow-up | Overall risk of bias | ||
| Fischer | 2018 | * | * | * | * | * | * | * | * | Low | |
| Urena | 2014 | * | * | * | * | * | * | * | Low | ||
| Dizon | 2015 | * | * | * | * | * | * | * | Low | ||
| Toviabrodie | 2017 | * | * | * | * | * | * | * | * | Low | |
| Doshi | 2018 | * | * | * | * | * | * | * | Low | ||
| Bacik | 2018 | * | * | * | * | * | Moderate | ||||
Yr: Year; *: Availability of variables.
I2 = (Q - df)/Q * 100%
An I2 = 25%-50% was considered mild in heterogeneity, 50%-75% as moderate, and > 75% as severe. A statistical significance was set at p-value < 0.05. The risk of publication bias was calculated using egger’s test and the standardized effect size was demonstrated by the Funnel Plot.
Results
Literature review
The initial search yielded 592 articles of which six studies had compared the clinical outcomes of the patients with and without a preexisting LBBB after the TAVR [13-18]. However, only three clinical trials encompassing 4,668 patients undergoing TAVR reported data on the variables of interest and were included in this systematic review [15,17,18]. The results of our literature search are depicted in the PRISMA flow chart (Figure 1).
Figure 1.
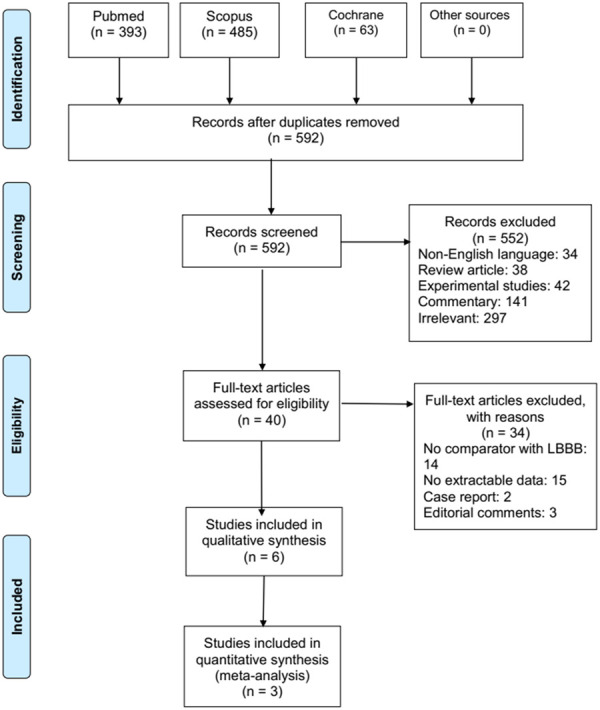
PRISMA flowchart of search strategy and study selection.
Study population
The weighted average of the patients’ age was 82.9±7.8 years, and 50.2% were male. Moderate/severe AR and stroke, and PPM implantation were evaluated in 2137 patients [17,18] and 4233 patients [15,17], respectively (Table 2).
Table 2.
Characteristics of included studies and patient population
| Study | Study type | Mean age, years | Gender (Male) | Intervention | Control | Outcome |
|---|---|---|---|---|---|---|
| Urena/2014 | Clinical trial | 81 ± 8 | 217 (49.9%) | TAVR in LBBB patients | TAVR in patients with no LBBB | Moderate-to-severe AR; Stroke |
| Dizon/2015 | Clinical trial | 84.5 ± 7.2 | 1324 (52.3%) | TAVR in LBBB patients | TAVR in patients with no LBBB | PPM implantation |
| Fischer/2018 | Clinical trial | 81.1 ± 8 | 805 (52.7%) | TAVR in LBBB patients | TAVR in patients with no LBBB | Moderate-to-severe AR; Stroke; PPM implantation |
| Toviabrodie/2017 | Clinical trial | 82 | 47 (58%) | TAVR in LBBB patients | TAVR in patients with no LBBB | N/A |
| Doshi/2018 | Clinical trial | N/A | 3929 (47.9%) | TAVR in LBBB patients | TAVR in patients with no LBBB | N/A |
| Bacik/2018 | Clinical trial | 77.1 ± 5.7 | 69 (59.5%) | TAVR in LBBB patients | TAVR in patients with no LBBB | N/A |
yrs: years; TAVR: Transcatheter aortic valve replacement; LBBB: Left bundle branch block; AR: Aortic regurgitation; PPM: Permanent pacemaker; N/A: Not applicable.
Outcome measures
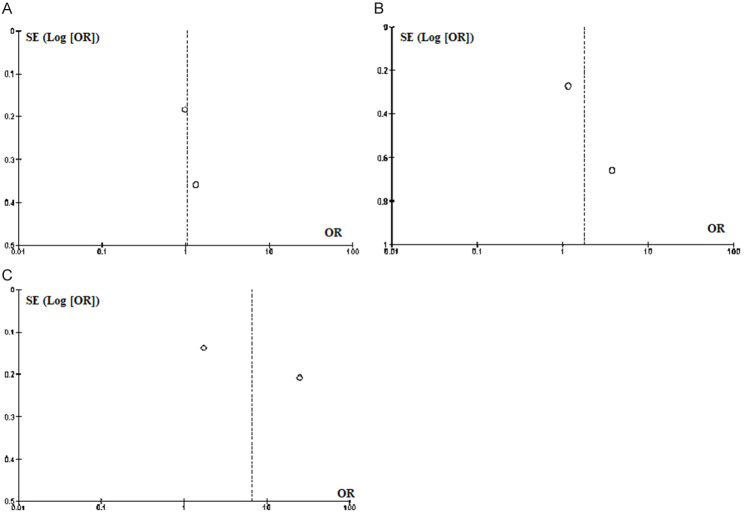
Table 3 shows the pooled RR for the outcomes of interest among patients included in this meta-analysis. As it shows, patients with pre-existing LBBB prior to TAVR had an increased risk of 4% for developing moderate/severe AR (RR = 1.04 [0.79-1.37]; P = 0.775; I2 = 0.0%). Furthermore, preexisting LBBB in patients who underwent TAVR did increase the risk of stroke by 72% (RR = 1.72 [0.61-4.85]; P = 0.307; I2 = 61.8%). Lastly, patients with preexisting LBBB had roughly an increased risk of 4% for requiring PPM implantation (RR = 4.43 [0.43-45.64]; P = 0.212; I2 = 99.1%) following TAVR. However, these findings did not achieve a statistical significance (P>0.05) and the heterogeneity among the studies was significantly high (Figure 2). The risk of publication bias for the studied variables has been depicted in Figure 3.
Table 3.
Pooled-Variance Analyses for the outcomes of Post-Transcatheter Aortic Valve Regurgitation (TAVR) in patients with preexisting Left Bundle Branch Block (LBBB)
| Outcome measure | Included studies into pooled analysis | Number of patients | RR, 95% CI | P value |
|---|---|---|---|---|
| Moderate/sever AR | Urena 2014 and Fischer 2018 | 3839 | 1.04 (0.79-1.37); I2 = 0.0% | 0.77 |
| Stroke | Urena 2014 and Fischer 2018 | 3839 | 1.72 (0.61-4.85); I2 = 61.8% | 0.31 |
| PPM implantation | Dizon 2015 and Fischer 2018 | 5935 | 4.43 (0.43-45.64); I2 = 99.1% | 0.21 |
TAVR: Transcatheter aortic valve replacement; LBBB: Left bundle branch block; RR: Risk ratio; CI: Confidence interval; I2 = Heterogeneity.
Figure 2.
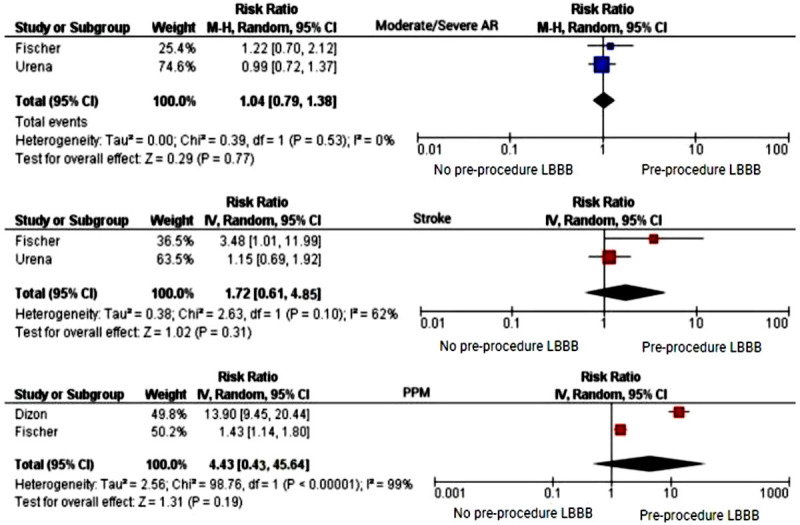
Forest plot comparing the odds of moderate/severe aortic regurgitation (AR), stroke, and permanent pacemaker (PPM) implantation between patients with and without a preexisting left bundle branch block (LBBB).
Figure 3.
Risk of publication bias according to the analyzed variables; A: Moderate/Severe AR; B: Stroke; C: Pacemaker.
Discussion
In this meta-analysis of clinical trials, patients who had pre-existing LBBB showed comparable outcome with those without LBBB following TAVR including the risk of developing stroke, moderate/severe AR, and need for a PPM implantation. These findings are in line with the study by Bhardwaj et al., who also showed that patients with pre-existing AR and LBBB undergoing TAVR did not have a significant risk for developing moderate to severe AR [14]. Additionally, the study conducted by Fischer et al. did not show an association between post-procedural AR and LBBB [17], be it before or after TAVR. However, a recent study has suggested that pre-procedural abnormalities in the electrocardiography of patients undergoing TAVR can identify those with a higher chance of developing post-procedural complications [19].
Our pooled analysis showed no statistically significant association between pre-existing LBBB and developing the risk of post-TAVR PPM implantation. However, the analysis detected a four times increased risk of PPM implantation in patients with a pre-existing LBBB undergoing TAVR as compared to those with no LBBB. This is partially in accordance with the study conducted by Fischer et al., which found that the occurrence of PPM implantation was higher in the patients with pre-existing LBBB (21.1%) compared with those with no presence of LBBB (14.8%). The discrepancy may originate from variation in the valves implanted by different studies, ventricular outflow calcium scores, concomitant right bundle branch block, and the depth of prosthesis implantation.
Finally, our results did not indicate a significant association between patients with pre-existing LBBB and incident stroke. Although, post-procedural stroke has occurred with a steady rate after TAVR [20-22], its association with LBBB has not been elucidated in this context yet.
To the extent of our knowledge, this is the first meta-analysis to assess the effect of a pre-existing LBBB on post-procedural outcomes of TAVR. However, our study is not without limitations. First, the astute of evidence was based on the non-randomized nature of the included clinical trials, especially in the assessment of moderate/severe AR and stroke; therefore, the certainty of the estimates is modest. Second, the number of studies included for each variable may not be sufficient to rely upon. Third, the post-procedural outcomes, such as myocardial infarction, 30-day death, and bleeding could not be assessed due to a lack of data within the included studies.
Conclusion
Our meta-analysis of comparative studies did not find any association between a pre-existing LBBB and developing poor cardiovascular outcomes following TAVR. However, due to the limited number of studies and high heterogeneity, further clinical trials with larger sample size are warranted.
Disclosure of conflict of interest
None.
References
- 1.Puri R, Chamandi C, Rodriguez-Gabella T, Rodes-Cabau J. Future of transcatheter aortic valve implantation - evolving clinical indications. Nat Rev Cardiol. 2018;15:57–65. doi: 10.1038/nrcardio.2017.116. [DOI] [PubMed] [Google Scholar]
- 2.Kheiri B, Osman M, Abubakar H, Subahi A, Chahine A, Ahmed S, Bachuwa G, Alkotob ML, Hassan M, Bhatt DL. Transcatheter versus surgical aortic valve replacement in low-risk surgical patients: a meta-analysis of randomized clinical trials. Cardiovasc Revasc Med. 2019;20:838–842. doi: 10.1016/j.carrev.2018.12.023. [DOI] [PubMed] [Google Scholar]
- 3.Witberg G, Lador A, Yahav D, Kornowski R. Transcatheter versus surgical aortic valve replacement in patients at low surgical risk: a meta-analysis of randomized trials and propensity score matched observational studies. Catheter Cardiovasc Interv. 2018;92:408–416. doi: 10.1002/ccd.27518. [DOI] [PubMed] [Google Scholar]
- 4.Elmaraezy A, Ismail A, Abushouk AI, Eltoomy M, Saad S, Negida A, Abdelaty OM, Abdallah AR, Aboelfotoh AM, Hassan HM, Elmaraezy AG, Morsi M, Althaher F, Althaher M, AlSafadi AM. Efficacy and safety of transcatheter aortic valve replacement in aortic stenosis patients at low to moderate surgical risk: a comprehensive meta-analysis. BMC Cardiovasc Disord. 2017;17:234. doi: 10.1186/s12872-017-0668-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alperi Garcia A, Muntane-Carol G, Junquera L, Del Val D, Faroux L, Philippon F, Rodes-Cabau J. Can we reduce conduction disturbances following transcatheter aortic valve replacement? Expert Rev Med Devices. 2020;17:309–322. doi: 10.1080/17434440.2020.1741349. [DOI] [PubMed] [Google Scholar]
- 6.Judson GL, Agrawal H, Mahadevan VS. Conduction system abnormalities after transcatheter aortic valve replacement: mechanism, prediction, and management. Interv Cardiol Clin. 2019;8:403–409. doi: 10.1016/j.iccl.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Leong D, Sovari AA, Ehdaie A, Chakravarty T, Liu Q, Jilaihawi H, Makkar R, Wang X, Cingolani E, Shehata M. Permanent-temporary pacemakers in the management of patients with conduction abnormalities after transcatheter aortic valve replacement. J Interv Card Electrophysiol. 2018;52:111–116. doi: 10.1007/s10840-018-0345-z. [DOI] [PubMed] [Google Scholar]
- 8.Auffret V, Puri R, Urena M, Chamandi C, Rodriguez-Gabella T, Philippon F, Rodes-Cabau J. Conduction disturbances after transcatheter aortic valve replacement: current status and future perspectives. Circulation. 2017;136:1049–1069. doi: 10.1161/CIRCULATIONAHA.117.028352. [DOI] [PubMed] [Google Scholar]
- 9.Maan A, Refaat MM, Heist EK, Passeri J, Inglessis I, Ptaszek L, Vlahakes G, Ruskin JN, Palacios I, Sundt T, Mansour M. Incidence and predictors of pacemaker implantation in patients undergoing transcatheter aortic valve replacement. Pacing Clin Electrophysiol. 2015;38:878–886. doi: 10.1111/pace.12653. [DOI] [PubMed] [Google Scholar]
- 10.Mangieri A, Montalto C, Pagnesi M, Lanzillo G, Demir O, Testa L, Colombo A, Latib A. TAVI and post procedural cardiac conduction abnormalities. Front Cardiovasc Med. 2018;5:85. doi: 10.3389/fcvm.2018.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mack MJ, Brennan JM, Brindis R, Carroll J, Edwards F, Grover F, Shahian D, Tuzcu EM, Peterson ED, Rumsfeld JS, Hewitt K, Shewan C, Michaels J, Christensen B, Christian A, O’Brien S, Holmes D STS/ACC TVT Registry. Outcomes following transcatheter aortic valve replacement in the United States. JAMA. 2013;310:2069–77. doi: 10.1001/jama.2013.282043. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. 2009;3:e123–130. [PMC free article] [PubMed] [Google Scholar]
- 13.Bacik P, Poliacikova P, Kaliska G. Who needs a permanent pacemaker after transcatheter aortic valve implantation? Bratisl Lek Listy. 2018;119:560–565. doi: 10.4149/BLL_2018_101. [DOI] [PubMed] [Google Scholar]
- 14.Bhardwaj A, Ramanan T, Sawant AC, Sinibaldi E, Pham M, Khan S, Qureshi R, Agrawal N, Khalil C, Hansen R, Baldo S, Colern G, Corbelli J, Pershad A, Beck H, Iyer V. Quality of life outcomes in transcatheter aortic valve replacement patients requiring pacemaker implantation. J Arrhythm. 2018;34:441–449. doi: 10.1002/joa3.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dizon JM, Nazif TM, Hess PL, Biviano A, Garan H, Douglas PS, Kapadia S, Babaliaros V, Herrmann HC, Szeto WY, Jilaihawi H, Fearon WF, Tuzcu EM, Pichard AD, Makkar R, Williams M, Hahn RT, Xu K, Smith CR, Leon MB, Kodali SK, Office PP. Chronic pacing and adverse outcomes after transcatheter aortic valve implantation. Heart. 2015;101:1665–1671. doi: 10.1136/heartjnl-2015-307666. [DOI] [PubMed] [Google Scholar]
- 16.Doshi R, Decter DH, Meraj P. Incidence of arrhythmias and impact of permanent pacemaker implantation in hospitalizations with transcatheter aortic valve replacement. Clin Cardiol. 2018;41:640–645. doi: 10.1002/clc.22943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer Q, Himbert D, Webb JG, Eltchaninoff H, Munoz-Garcia AJ, Tamburino C, Nombela-Franco L, Nietlispach F, Moris C, Ruel M, Dager AE, Serra V, Cheema AN, Amat-Santos IJ, de Brito FS, Ribeiro H, Abizaid A, Sarmento-Leite R, Dumont E, Barbanti M, Durand E, Alonso Briales JH, Bouleti C, Imme S, Maisano F, Del Valle R, Miguel Benitez L, Garcia Del Blanco B, Cote M, Philippon F, Urena M, Rodes-Cabau J. Impact of preexisting left bundle branch block in transcatheter aortic valve replacement recipients. Circ Cardiovasc Interv. 2018;11:e006927. doi: 10.1161/CIRCINTERVENTIONS.118.006927. [DOI] [PubMed] [Google Scholar]
- 18.Urena M, Hayek S, Cheema AN, Serra V, Amat-Santos IJ, Nombela-Franco L, Ribeiro HB, Allende R, Paradis JM, Dumont E, Thourani VH, Babaliaros V, Francisco Pascual J, Cortes C, Del Blanco BG, Philippon F, Lerakis S, Rodes-Cabau J. Arrhythmia burden in elderly patients with severe aortic stenosis as determined by continuous electrocardiographic recording: toward a better understanding of arrhythmic events after transcatheter aortic valve replacement. Circulation. 2015;131:469–477. doi: 10.1161/CIRCULATIONAHA.114.011929. [DOI] [PubMed] [Google Scholar]
- 19.Coisne A, Ninni S, Pontana F, Aghezzaf S, Janvier F, Mouton S, Ridon H, Ortmans S, Seunes C, Wautier M, Coppin A, Madika AL, Boutie B, Koussa M, Bical A, Vincentelli A, Juthier F, Loobuyck V, Sudre A, Marchetta S, Martinez C, Staels B, Lancellotti P, Modine T, Montaigne D. Clinical significance of electrocardiographic markers of myocardial damage prior to aortic valve replacement. Int J Cardiol. 2020;307:130–135. doi: 10.1016/j.ijcard.2020.01.073. [DOI] [PubMed] [Google Scholar]
- 20.Abdelgawad AME, Hussein MA, Naeim H, Abuelatta R, Alghamdy S. A comparative study of TAVR versus SAVR in moderate and high-risk surgical patients: hospital outcome and midterm results. Heart Surg Forum. 2019;22:E331–E339. doi: 10.1532/hsf.2243. [DOI] [PubMed] [Google Scholar]
- 21.Jimenez Diaz VA, Lozano I, Tello Montoliu A, Baz Alonso JA, Iniguez Romo A. Is there a link between stroke, anticoagulation, and platelet reactivity?: the multifactorial stroke mechanism following TAVR. JACC Cardiovasc Interv. 2019;12:2560–2561. doi: 10.1016/j.jcin.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 22.De Backer O, Butt JH, Wong YH, Torp-Pedersen C, Terkelsen CJ, Nissen H, Fosbol EL, Kober L, Sondergaard L. Early and late risk of ischemic stroke after TAVR as compared to a nationwide background population. Clin Res Cardiol. 2020;109:791–801. doi: 10.1007/s00392-019-01565-0. [DOI] [PubMed] [Google Scholar]