Abstract
Background: Venous thromboembolism (VTE) is a well-established complication of trauma. So far, the factors that are related to early post-traumatic pulmonary embolism (PE) occurrence have been given little attention. Aims: We have conducted this literature review in order to analyze the incidence and the physiopathology of post-traumatic PE among intensive care unit (ICU) trauma patients, analyze the incidence of early post-traumatic PE, and elucidate risk factors associated with post-traumatic PE. Moreover, we aim to study the impact/outcome of post-traumatic PE in the ICU. Methods: We used the PubMed and EMBASE databases and entered the following key words in MeSH research: Deep vein thrombosis (DVT), Post-traumatic Pulmonary embolism, Early pulmonary-embolism, risk factors, and Prognosis. Results: The incidence of PE among trauma patients varies considerably, ranging from 0.35% to 24%. The incidence of early post-traumatic PE varies widely from 10 to 42%. After a traumatic injury, many factors have been found to be responsible for the formation of DVT and PE. In addition to the risk factors of hypercoagulability described by Virchow in his original triad, inflammation acting via endothelial damage may be considered as a fourth factor. The literature review showed that lower limb fractures and age are the most frequent factors associated with PE (particularly in early PE). The heterogeneity among studies limits reliable conclusions regarding the true risk factors for the timing of the occurrence of post-traumatic PE. Fatality from pulmonary embolism (PE) is close to 50% in some series. Moreover, high mortality rates, a high rate of nosocomial infections, and a prolonged stay in an ICU and/or in a hospital were found to be associated with the development of PE. Conclusion: Post-traumatic PE is frequent in ICUs. Inflammation acting via endothelial damage may be considered as a fourth factor in addition to the Virchow’s triad of risk factors for venous thrombosis. Fractures of the lower extremities, obesity, and age happen to be the most frequent factors associated with PE (in particular early PE). PE development was associated with high rates of mortality, nosocomial infections, and a prolonged stay in an ICU and/or in a hospital. Therefore, prevention is warranted.
Keywords: Deep vein thrombosis, post-traumatic pulmonary embolism, early pulmonary-embolism, intensive care unit, prognosis
Introduction
There has been an increase in the diagnosis of incidental PE in all groups of patients, including medical, surgical and trauma patients (range varies between 5-24%) [1-20]. Venous thromboembolism (VTE) is a well-established complication of trauma [1-3]. Traumatic events are nowadays known to increase the risk of VTE and were reported to be responsible for approximately 12% of VTE episodes occurring in the community [4]. Lately, it has been established that a transient hypercoagulable state actually occurs in the first few days following injury [5,6], leading to early (within 3 days after trauma), and even immediate, VTE complications after trauma [7-12].
Moreover, PE is also known to cause significant morbidity and mortality in the ICU [1-3]. For injured patients surviving the first 24 hours, the third most common cause of death was found to be fatal PE [13,14]. This risk is particularly more important and more threatening among trauma patients admitted to an ICU [14].
Despite the fact that prophylactic measures for thromboembolic conditions exist, the most effective ones are associated with an increased risk of bleeding [15,16]. Pharmacological prophylaxis is often delayed in injured patients because of bleeding aggravation, which may lead to an increased risk of VTE. These concepts have significant implications for physicians searching for optimal and safe VTE prophylaxis strategies [15,16].
Until now, the factors that are associated with the early occurrence of post-traumatic PE have been given little consideration. Few studies, to the best of our knowledge, have pinpointed the risk factors responsible for early PE after injury. However, these studies were retrospective and have an intrinsic limitation because the diagnostic tool for DVT and/or PE can be different for patients [7-11]. In addition, except for the study conducted by Darabadi F [12], none of them concerned an ICU population and they were all conducted in level 1 trauma centers.
In view of this dilemma and the paucity of research concerning PE among ICU trauma populations, we have conducted this literature review in order to analyze the incidence of post-traumatic PE and early post-traumatic PE among ICU trauma-patients, to reveal risk factors associated with early occurrence of PE, and to study the impact/outcome of post-traumatic PE in the ICU.
We used the PubMed and EMBASE databases and entered the following key words in MeSH research: deep vein thrombosis, Post-traumatic Pulmonary embolism, early pulmonary-embolism, risk factors, and Prognosis. We did not impose any language restrictions.
The incidence of post-traumatic PE
It is well-established, nowadays, that trauma patients bear a significantly increased risk of VTE events [17-22]. In fact, a number of autopsy studies came to confirm the relationship between trauma and VTE, and to highlight the fact that these events are frequently under-diagnosed [23-25].
The incidence of PE among trauma patients vary considerably, ranging from 0.35% to 24% [1,19,20,26]. As a matter of fact, differences in population characteristics, severity of injury, and screening protocols may account for much of the variability in the reported incidences [19]. Indeed, until recently, screening for asymptomatic pulmonary embolism was impractical. In fact, according to the study by Schultz [28], the incidence of occult PE was much higher than commonly reported. They documented a 24% incidence of asymptomatic PE in 90 moderately-to-severely injured trauma patients, undergoing surveillance using systematic contrast-enhanced helical computed tomography (CT) scanning [26,28]. Finally, one might argue that the increase in PE rates could be related to advances in CT scanning technology, including the use of multi-detector-scanners (64 slice multi-detector CT) which increased the accuracy of PE detection [28].
Timing/incidence of early PE
According to our literature review, there is little written about the timing of post-traumatic PE in the ICU population. Usually, it has been thought that PE occurs most commonly between day 5 and day 7 following the traumatic event and that it is rare earlier than day 4 [29]. This concept resulted mainly from the classical teaching endorsing the belief that post-traumatic PE originates from deep venous thrombosis of the lower extremities and the pelvis [7,8,11,29,30]. In 1856, Virchow identified venous stasis, endothelial vascular damage, and hypercoagulability as the classic triad resulting in the formation of DVT. Moreover, as stasis has been considered the predominant factor in the formation of DVT, and consequently of PE in trauma patients, it has been traditionally thought that these events occur after 5 or even 7 days after injury [7,8,11,29,30].
However, after reviewing the literature, we found an increasing trend in the number of PEs being diagnosed during the very early phase following injury [27,31]. Indeed, the incidence of early post-traumatic PE (diagnosed within 3 days after trauma) varies widely from 10 to 42% [7,8,11-13,17,19-21]. Comparing two time periods [20,27], we found a significant decrease in the mean delay of PE diagnosis (from 11.3 days to 7.7 days) with 24% to 40% of the patients diagnosed with PE within the first four days of ICU hospitalization. This result was confirmed by other studies [12,31].
Most recently, Darabadi [12] found that among the trauma patients with PE, 40.4% had early PE. Our findings were consistent with recent data, suggesting that a significant proportion of post-traumatic PEs actually occurs very early and even immediately after injury [7,8,11,12,29,31].
We believe that PEs may occur over 3 phases. The first is the immediate-PE, which can be detected from the first trauma CT-scan, the second is the early PE, and the third is the late PE. The increase of early diagnosis might be due to a number of reasons:
• First, this earlier diagnosis of PE after an injury does not necessarily indicate that PEs are now occurring earlier than before. This noticeable rise is most likely due to a lower clinical threshold for obtaining non-invasive diagnostic imaging with CT scans, which are more sensitive and specific for the diagnosis of PEs. In other words, the technological advances in diagnostic methods, including the use of multi-detector scanners (64 slice multi-detector CT), may explain the increase of early PEs.
• Second, in some cases, “Late” PE may be a delay in the diagnosis of a previously, earlier-installed pulmonary clot. Consequently, the late-stage PE may only be a delayed diagnosis.
Pathophysiology of post-traumatic PE
For more than a century, the development of thrombosis has been linked to Virchow’s Triad [32]. However, as suggested by Knudson [33], we should consider an updated understanding of the pathophysiology of post-traumatic VTE that recognizes the role of acute inflammation due to the severity of trauma as the fourth contributor to the formation of thrombi. In fact, acute inflammation, with pro-inflammatory cytokines release play a significant role in promoting a pro-coagulant state and in increasing endothelial damage. Therefore, we set to:
• Analyze the four determinants of Post-traumatic PE, namely: Virchow’s Triad (hypercoagulability, stasis, and endothelial injury) and the role of acute inflammation due to the severity of trauma.
• Elucidate the underlying pathophysiology of early PE.
Pathophysiological determinants of post-traumatic PE
Hypercoagulability
Pro-coagulopathy of trauma
Physiologically, the coagulation process is regulated to ensure thrombus formation without an overshoot of fibrin generation. Once coagulation is achieved, plasmin-mediated fibrinolysis ensues [34].
The high incidence of VTE after trauma suggests that the usual regulatory pathways can fail, and several mechanisms seem to act together to produce a hypercoagulable state in the acute phase following traumatic events [17]. In fact, during an injury, a massive liberation of tissue factor (TF) represents the central initiator of cell-based coagulation [35]. Normally, TF (factor III) is not expressed in direct contact with the blood but is up-regulated in response to hypoxia, vascular injury, and circulating inflammatory cytokines [36]. In addition, the down-regulation of thrombomodulin could logically be a mechanism of procoagulopathy [34]. Furthermore, after trauma, the levels of natural anticoagulant agents, such as activated C protein (APC), protein S, and antithrombin III (ATIII), are reduced, contributing to thromboembolic events [34,37,38].
Endothelial injury and the role of acute inflammation
The vascular endothelium represents a key element in the protection against thrombus formation and in maintaining blood fluidity. Following trauma, the endothelial injury is accompanied by the expression of adhesive molecules with procoagulant activities, leading to the development of thrombi [39].
Nevertheless, the likelihood that inflammation of the vessel wall could cause thrombosis on undamaged veinsexists [40]. In fact, this may trigger the coagulation system through the induction of TF [40]. Indeed, severe injuries strongly stimulate the production of cytokines, leading to excessive systemic inflammation [41,42]. Tissue injury activates the intrinsic immune system and causes the activation of local platelets, tissue macrophages, neutrophils, and mast cells [43-46]. As a result of this cellular activation, there is an increased release of cytokines [40]. In the first 2 to 3 days following injury, tumor necrosis factor (TNF), interleukin (IL)-1, and IL-6 and IL-8 concentrations increase [46,47]. By inducing tissue factor expression, activated neutrophils and pro-inflammatory cytokines play a significant role in promoting a pro-coagulant state and in increasing endothelial damage [40,47-50]. Furthermore, we hypothesize that “local” inflammation may contribute to the high incidence of PE related to trauma [51]. The local activation of venous endothelium results in the release of granules called Weibel-Palade bodies, containing both Von-Willebrand factor and membrane-bound P-selectin [51]. Both of these proteins have the capacity to initiate thrombus formation [51]. Similarly, Knudson [33] postulated in his study that the local inflammatory process initiated by chest trauma could lead to pulmonary thrombosis and might explain why chest injury is associated with PE, but not significantly with DVT.
Reduced blood flow and stasis
Stasis is a major contributor to thrombotic formation. By increasing the residence time in the large vessels, the natural mechanisms controlling coagulation through interaction with the anticoagulants become impaired and the risk to develop thrombi increases [52]. In fact, reduced blood flow allows the accumulation of procoagulant factors that may overcome the local anticoagulant pathways and induce thrombosis. Stasis may, thus, explain the increased rate of VTE associated with hospitalization, surgery, and paralysis [53]. In humans, the most likely site of thrombus initiation is the valve pocket sinus due to its vertical blood flow and low oxygen tension [54]. In trauma patients, reduced blood flow results from immobilization, sedation, surgical procedures, and also vein compression by hematoma.
Pathophysiology of early PE
Traditionally, it was commonly believed that post-traumatic PE originates from deep venous thrombosis of the lower extremities and pelvis [29]. However, PE and DVT have been known not to coexist, and the origination of PE from DVT of peripheral veins has little evidence [55]. This last hypothesis has received insufficient attention and was rarely discussed in the literature [55]. Moreover, several recent studies showed that an increased proportion of post-traumatic PEs was being diagnosed very early, and even immediately, after injury [9,12,55]. The most recent study by Gelbard [9] showed that more than half of the early PEs occurred within the first 24 hours following trauma. Based on these reports, we hypothesize that PEs diagnosed “early” after trauma may be related to a different underlying pathophysiology. However, demonstrating this would be extremely difficult. Two main explanations for early pulmonary embolism in trauma exist.
The first possibility is the presence of an undiagnosed thrombophilia, congenital or acquired after trauma. Recently, Shreiber [56] reported that a hypercoagulable state is most prevalent in the first 4 days after injury. The second possibility was advanced by Velmahos [55] who showed that the majority of patients (70 to 84%) sustaining an early post-traumatic PE were not diagnosed with DVT, suggesting that pulmonary clots may occur “de novo” within the lungs [7,9]. Indeed, Knudson [33] also found a significantly increased risk of PE with severe chest trauma and suggested localized inflammation, occult vascular injury and the low flow state that occur after chest injuries, as a possible etiology for “in situ” formation of pulmonary thrombosis. Moreover, the role of post-traumatic adrenergic response leading to vascular endothelial inflammation with circulating adhesion molecules production, leading to regional thrombosis and rapid occlusion, may play a role in the formation of pulmonary thrombosis [57]. A third hypothesis was advocated by Brakenridge [11], who found that only the presence of long-bone fracture is an independent risk factor of early PE. He suggested that there was an unknown underlying molecular mechanism associated with fractures contributing to the early occurrence of PE.
We conclude that there are many factors contributing to the formation of DVT and PE after traumatic injury. In addition to the risk factors for hypercoagulability originally described by Virchow, we suggest in this specific condition that a fourth factor should also be considered: the role of inflammation acting via endothelial damage. There are 4 possible explanations for the early occurrence of PE along with the lack of association between PE and DVT:
• Rapidly developing post-traumatic hypercoagulable disorders.
• Clots formed in the lower extremity veins embolize completely to the pulmonary circulation.
• Errors in DVT screening and undetected upper extremity DVTs.
• The appearance of clots in the pulmonary circulation is “de novo” and they are not generated from peripheral DVTs.
The role of major chest trauma in stimulating local inflammation leading directly to the formation of a PE by direct inflammation of pulmonary vessels requires further investigations in the future. Further studies are required on this subject. Figure 1 summarizes the Pathophysiology of post-traumatic PE and early post-traumatic PE.
Figure 1.
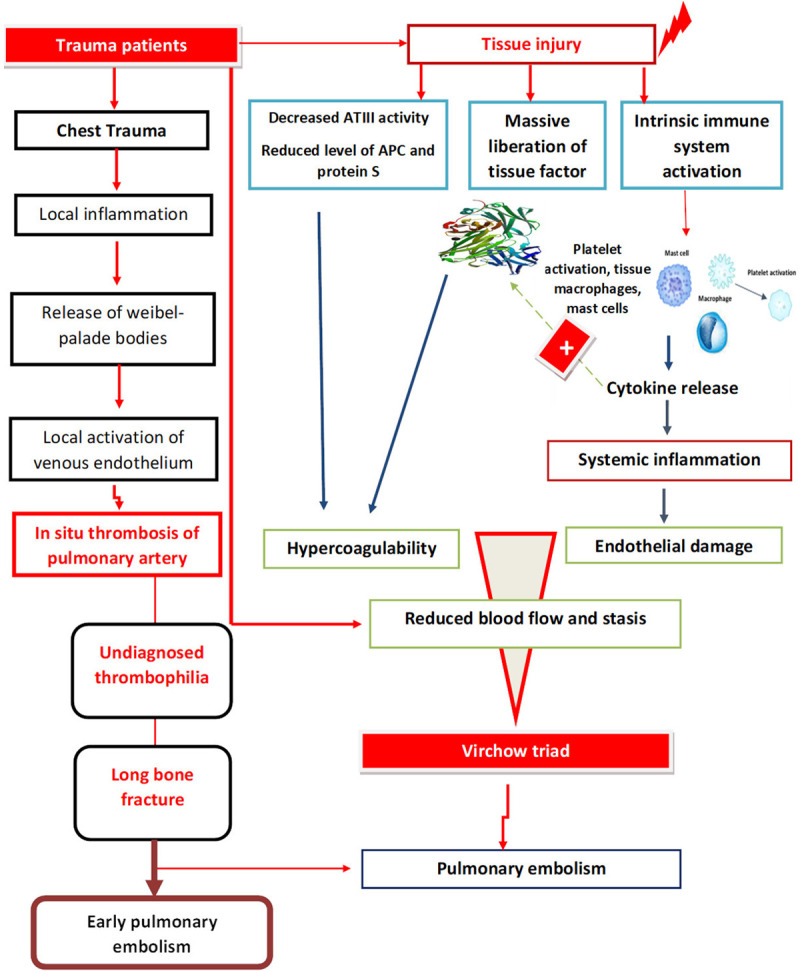
Pathophysiology of post-traumatic PE and Early post-traumatic PE.
Predictive factors of post-traumatic PE and risk factors of early occurrence: Recent literature has shown that a significant proportion of PEs among trauma patients may occur very early after injury [7-12]. However, there is little written about early post-traumatic PE peculiarities in the ICU, where diagnosis and treatment are more complicated. On the other hand, while the incidence of DVT and PE is generally different, in most cases, risk factors are determined as a single entity for VTE. We hypothesize that PE and DVT may not always be related. In addition, we believe that “early” and “late” PEs are separate clinical entities, with a different underlying pathophysiology. Therefore, we set to identify specific risk factors associated with the timing of occurrence of post-traumatic PE. We also believe that a better understanding of these risk factors may help to risk-stratify patients and guide the timing of pharmacologic prophylaxis. Reviewing the literature, lower limb fractures and age are the most frequent factors associated with early PE [7-12]. The heterogeneity among studies limits reliable conclusions regarding the true risk factors for the timing of occurrence of post-traumatic PE.
Age
As established in the literature, the aging process was proven to be an important risk factor in the development of VTEs [12,20]. The incidence of VTE was found to be 2 to 7 times higher in patients above 55 years of age [58]. The influence of age on the incidence of VTE is likely to be multifactorial [59]. This may be related to anatomic changes in the veins and more pronounced stasis in the venous valve pockets [60]. Furthermore, higher levels of thrombin-activation, and an increase in Weibel-Palade bodies (P-selectin surface) expression from vein walls and platelets were found among older patients [60,61]. However, fewer studies analyzed the impact of the age on the timing of early occurrence of post-traumatic PE. Recently, in a study by Darabadi [12], one of the main determinants of early occurrence of PE was older age with an OR of 1.24.
Obesity
Studies have shown that obesity could be a risk factor for arterial and venous thrombosis with an odds ratio ranging from 1.7 to 2.2 for VTE [62]. Likewise, in a recent work, the Reasons for Geographic and Racial Differences (REGARDS) cohort has shown that an association between Body Mass index (BMI) and VTE exists [63]. Also, in some studies, it was suggested that inflammation may be a possible mechanism which is fundamental to the relationship between obesity and VTE risk [19,62,63].
Injury characteristics
Regarding the injury characteristics, various studies have pointed toward different predictors for the timing of occurrence of PE in trauma patients. Certain injury patterns - such as spinal cord injuries, traumatic brain injuries, lower extremity fractures, and severe chest trauma - have been identified as predictive risk factors for the development of early or late PE [7,8,11,12]. It has been thought that orthopedic and neurologic injuries, in particular, due to extended periods of immobility, predispose patients to stasis and lead to VTE events. This purely mechanical explanation was consistent with the late occurrence of PE.
The study by Brakenridge [11] was the first to elucidate factors associated with the timing of post-traumatic PE, with long-bone extremity fractures identified as the only independent risk factor for early PE. On the other hand, the late PE group had a higher injury severity score (ISS), severe head injury, and/or severe chest injury. Most recently, Darabadi [12] identified higher ISS and the presence of long-bone fractures of the lower extremities as two independent determinants of early occurrence of PE.
Hemodynamic state
Although not all acute PEs present with right ventricular (RV) impairment (25% overall), PE can lead to right ventricular dysfunction, which predisposes patients to hemodynamic instability and cardiogenic shock (not specific to trauma). If present, it would help to risk-stratify patients. The hemodynamic changes are due to the severity of PE or late presentation of a large PE than a different pathophysiology. In their study, Gelbard [9] showed that early post-traumatic PEs were not more associated with RV dysfunction.
Sepsis
The risk of VTE might be affected by systemic infections with their resultant inflammation and hypercoagulable state [65]. Likewise, a hypercoagulable state can be activated by an infection, and this can induce platelet activation tissue factor production, leading to thrombotic complications. In addition, indirect factors related to the septic state, such as hypoxemia and hypotension, can represent triggers for thrombosis initiation [66]. There are, however, few data on the risk of thrombosis associated with infection [65,67]. Donze [68] showed the presence of a significant association between sepsis and the risk of arterial and venous thrombosis following major non-trauma surgeries in a large multicenter surgical cohort. Improved prevention of thromboembolic events among ICU trauma patients can be achieved by a better assessment of the impact of infection and its severity on post-traumatic thrombotic complications.
Hypoxemia
Hypoxemia in the post-traumatic context could be an aggravating factor, thus, increasing the risk of post-traumatic VTE. Indeed, hypoxia followed by re-oxygenation is known to be harmful to tissues and could be a risk factor of thrombus development in veins (although this relationship has not been directly proven). Animal experimentations [69] showed that exposure to 6% oxygen for 24 hours followed by 1-3 hours of re-oxygenation led to a significantly increased thrombosis prevalence in a mouse model of DVT. Moreover, the thrombi extension from the test group significantly exceeded those in the control group.
The initial use of transfusions
Blood transfusion is a common event in the acute phase following trauma [70-73]. Transfused blood can disrupt the balance of coagulation factors, modulating the inflammatory cascade [70]. Since inflammation and coagulation are tightly coupled, we believe that blood transfusions might be associated with the development of venous thromboembolic events. The pro-inflammatory and immunomodulatory nature of blood transfusion may promote a hypercoagulable state [71]. In CRASH-2 study, Perel [72] found an evident association between red blood cell (RBC) transfusions and the risk of fatal and non-fatal vascular occlusive events (including PE, DVT, stroke and myocardial infarction) with an odds ratio (OR) of 2.58. However, less focus was attributed to the association between the initial use of transfusions and the timing of occurrence of PE after trauma. According to the research performed by Coleman [8], late PEs were more likely to occur in patients who had blood transfusion within the first 24 hours in univariate analysis. However, multivariate analysis showed that transfusions were not independently associated with the timing of PE.
Surgical measures within 72 hours
Even though operative procedures are known to be a risk factor for thromboembolic events, there has been little written about the interval between surgery and the occurrence of a PE [29]. The use of radioactive-labeled fibrinogen [74] showed that patients undergoing surgery for a major injury had evidence of clotting in leg veins and that, interestingly, all of the patients who subsequently developed clinical complications had increased radioactivity in their leg veins during surgery. So, one might conclude that the clotting starts on the operating table and proceeds from there.
Use of tranexamic acid (TXA) in head trauma
TXA is an interesting treatment in hemorrhagic shock. Its efficiency in head trauma is still debated and controversial. Its use was associated with a higher rate of pulmonary embolism in the treated group in one study [75]. However, this result was not confirmed by other studies [76,77].
In summary, many factors were associated with post-traumatic PE. Factors associated with early and late development of PE are summarized in Table 1.
Table 1.
Pulmonary embolism’s risk factors in trauma patients
| Risk factors | Study | Results |
|---|---|---|
| Elder Age | Bahloul M [20 ] | NT |
| Cambien B [61] | NT | |
| Darabadi [12] | Increased risk of Early PE | |
| Obesity | Howard VJ [63] | NT |
| Lentz SR [62] | NT | |
| Blokhin IO [64] | NT | |
| Severe ISS | Brakenridge [11] | Increased risk of Late PE |
| Coleman JJ [8] | Increased risk of Late PE | |
| Benns M [7] | Increased risk of Late PE | |
| Darabadi [12] | Increased risk of Early PE | |
| Brain injury | Brakenridge [11] | Increased risk of Late PE |
| Coleman JJ [8] | Increased risk of Late PE | |
| Benns M [7] | Increased risk of Late PE | |
| Spinal cord injury | Coleman [8] | Increased risk of Late PE |
| Long bone fractures | Brakenridge [11] | Increased risk of Early PE |
| Coleman JJ [8] | Increased risk of Early PE | |
| Benns M [7] | Increased risk of Early PE | |
| Darabadi [12] | Increased risk of Early PE | |
| AIS | Coleman [8] | Increased risk of Early PE |
| Severe chest injury | Brakenridge [11] | Increased risk of Late PE |
| Hemodynamic instability | Gelbard [9] | NT |
| Sepsis | Donze [68] | NT |
| Hypoxemia | Levine [67] | NT |
| Brill A [69] | NT | |
| Transfusion | Coleman [8] | Increased risk of Late PE |
| Surgical intervention < 72 h | Blaisdell FW [74] | NT |
| Use of TXA | Chakroun-Walha [75] | NT |
PE: pulmonary embolism; NT: no timing precision; TXA: Tranexamic Acid.
Impact on outcome
Mortality rates
Pulmonary embolism (PE) is well-known to cause significant morbidity and mortality in the ICU [1-3]. Fatality from pulmonary embolism (PE) was found to be the third most common cause of death in injured patients that survive the first 24 hours [13,14], with a percentage close to 50% in some series [78]. Furthermore, post-traumatic PE has been reported to increase mortality rates among major trauma patients, up to 10 times [79,80]. This risk is particularly more notable and more threatening among trauma patients admitted to the intensive care unit [14]. Age, severity of injuries, severity scores, and co-morbidities, according to our literature review, were important risk factors of fatal PE [78-80].
Length of stay (LOS)
Previous studies showed that PE might prolong the duration of ICU stay and delay weaning from mechanical ventilation [18,81-86]. In his prospective study, Skrifvars [18] showed that patients who developed VTE complications had significantly longer ICU and hospital stays (P < 0.001) with significantly longer median time of mechanical ventilation (P < 0.001), in comparison with patients who did not develop VTE complications. The comparison between the PE (+) group and the PE (-) group showed that the development of PE was associated with a high mortality rate, a high rate of nosocomial infections and a high length of stay in ICU and/or in hospital [83-86].
Conclusion
Post-traumatic PE is frequent in ICUs. In addition to risk factors for hypercoagulability, as originally described by Virchow, the role of acute inflammation (acting via promoting a pro-coagulant state and in increasing endothelial damage) should be considered. Lower limb fractures, obesity, and age are the most frequent factors associated with PE (in particular, early PE). The development of PE was associated with high mortality rates, a high rate of nosocomial infections and a prolonged stay in an ICU and/or in a hospital. Prevention is immensely warranted.
Acknowledgements
All authors thank Professor Chokri Khalaf for his help in the redaction of this manuscript.
Disclosure of conflict of interest
None.
Abbreviations
- ICU
intensive care unit
- VTE
Venous thromboembolism
- PE
Pulmonary embolism
- DVT
Deep vein thrombosis
- IVC
Inferior vena cava
- TF
Tissue factor
- APC
Activated C protein
- TNF
Tumor necrosis factor
- BMI
Body Mass Index
- ISS
Injury Severity Score
- TXA
Tranexamic Acid
- ATIII
Antithrombin III
- RBC
Red Blood Cell
References
- 1.Shuster R, Mathew J, Olaussen A, Gantner D, Varma D, Koukounaras J, Fitzgerald MC, Cameron PA, Mitra B. Variables associated with pulmonary thromboembolism in injured patients: a systematic review. Injury. 2018;49:1–7. doi: 10.1016/j.injury.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 2.Ruskin KJ. Deep vein thrombosis and venous thromboembolism in trauma. Curr Opin Anaesthesiol. 2018;3:215–218. doi: 10.1097/ACO.0000000000000567. [DOI] [PubMed] [Google Scholar]
- 3.Lichte P, Kobbe P, Almahmoud K, Pfeifer R, Andruszkow H, Hildebrand F, Lefering R, Pape HC Trauma register DGU. Post-traumatic thrombo-embolic complications in polytrauma patients. Orthop. 2015;39:947–954. doi: 10.1007/s00264-015-2698-6. [DOI] [PubMed] [Google Scholar]
- 4.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000;160:809–815. doi: 10.1001/archinte.160.6.809. [DOI] [PubMed] [Google Scholar]
- 5.Liou DZ, Shafi H, Bloom MB, Chung R, Ley EJ, Salim A, Tcherniantchouk O, Margulies DR. Defining early trauma-induced coagulopathy using thromboelastography. Am Surg. 2014;80:994–998. [PubMed] [Google Scholar]
- 6.Shaz BH, Winkler AM, James AB, Hillyer CD, MacLeod JB. Pathophysiology of early trauma-induced coagulopathy: emerging evidence for hemodilution and coagulation factor depletion. J Trauma. 2011;70:1401–1407. doi: 10.1097/TA.0b013e31821266e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benns M, Reilly P, Kim P. Early pulmonary embolism after injury: a different clinical entity? Injury. 2014;45:241–244. doi: 10.1016/j.injury.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 8.Coleman JJ, Zarzaur BL, Katona CW, Plummer ZJ, Johnson LS, Fecher A, O’Rear JM, Feliciano DV, Rozycki GS. Factors associated with pulmonary embolism within 72 hours of admission after trauma: a multicenter study. J Am Coll Surg. 2015;220:731–736. doi: 10.1016/j.jamcollsurg.2014.12.032. [DOI] [PubMed] [Google Scholar]
- 9.Gelbard RB, Karamanos E, Farhoomand A, Keeling WB, McDaniel MC, Wyrzykowski AD, Shafii MS, Rajani RR. Immediate post-traumatic pulmonary embolism is not associated with right ventricular dysfunction. Am J Surg. 2016;212:769–774. doi: 10.1016/j.amjsurg.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 10.Menaker J, Stein DM, Scalea TM. Incidence of early pulmonary embolism after injury. J Trauma. 2007;63:620–4. doi: 10.1097/TA.0b013e31812f60aa. [DOI] [PubMed] [Google Scholar]
- 11.Brakenridge SC, Toomay SM, Sheng JL, Gentilello LM, Shafi S. Predictors of early versus late timing of pulmonary embolus after traumatic injury. Am J Surg. 2011;201:209–215. doi: 10.1016/j.amjsurg.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darabadi FK, JafariZare MA, Goodarzi ZT, Namdar P. Prevalence and main determinants of early post-traumatic thromboembolism in patients requiring ICU admission. Eur J Trauma Emerg Surg. 2018;44:133–136. doi: 10.1007/s00068-017-0770-1. [DOI] [PubMed] [Google Scholar]
- 13.Bahloul M, Dlela M, Bouchaala K, Triki A, Chelly H, Ben Hamida C, Haddar S, Bouaziz M. Early post-traumatic pulmonary embolism in intensive care unit: incidence, risks factors, and impact outcome. Am J Cardiovasc Dis. 2020;10:207–218. [PMC free article] [PubMed] [Google Scholar]
- 14.Bahloul M, Regaieg K, Chtara K, Turki O, Baccouch N, Chaari A, Bouaziz M. Post-traumatic thromboembolic complications: incidence, risk factors, pathophysiology and prevention. Ann Cardiol Angeiol. 2017;66:92–101. doi: 10.1016/j.ancard.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Schaible EV, Thal SC. Anticoagulation in patients with traumatic brain injury. Curr Opin Anaesthesiol. 2013;26:529–534. doi: 10.1097/01.aco.0000432519.16586.6b. [DOI] [PubMed] [Google Scholar]
- 16.Scales DC, Riva-Cambrin J, Wells D, Athaide V, Granton JT, Detsky AS. Prophylactic anticoagulation to prevent venous thromboembolism in traumatic intracranial hemorrhage: a decision analysis. Crit Care. 2010;14:R72. doi: 10.1186/cc8980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holley AD, Reade MC. The ‘procoagulopathy’ of trauma: too much, too late? Curr Opin Crit Care. 2013;19:578–586. doi: 10.1097/MCC.0000000000000032. [DOI] [PubMed] [Google Scholar]
- 18.Skrifvars MB, Bailey M, Presneill J, French C, Nichol A, Little L, Duranteau J, Huet O, Haddad S, Arabi Y, McArthur C, Cooper DJ, Bellomo R EPO-TBI investigators and the ANZICS Clinical Trials Group. Venous thromboembolic events in critically ill traumatic brain injury patients. Intensive Care Med. 2017;43:419–428. doi: 10.1007/s00134-016-4655-2. [DOI] [PubMed] [Google Scholar]
- 19.Ball J. Venous thromboembolism in critically Ill patients requires significant reconsideration. Crit Care Med. 2020;48:934–935. doi: 10.1097/CCM.0000000000004324. [DOI] [PubMed] [Google Scholar]
- 20.Bahloul M, Chaari A, Dammak H, Medhioub F, Abid L, Ksibi H, Haddar S, Kallel H, Chelly H, Hamida CB, Bouaziz M. Post-traumatic pulmonary embolism in the intensive care unit. Ann Thorac Med. 2011;6:199–206. doi: 10.4103/1817-1737.84773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffman H, Jalal MS, Chin LS. The risk factors, outcomes, and costs associated with venous thromboembolism after traumatic brain injury: a nationwide analysis. Brain Inj. 2019;33:1671–1678. doi: 10.1080/02699052.2019.1667536. [DOI] [PubMed] [Google Scholar]
- 22.McCartney JS. Pulmonary embolism following trauma. Am J Pathol. 1934;10:709–710. [Google Scholar]
- 23.Sevitt S, Gallagher N. Venous thrombosis and pulmonary embolism. A clinico-pathological study in injured and burned patients. Br J Surg. 1961;48:475–489. doi: 10.1002/bjs.18004821103. [DOI] [PubMed] [Google Scholar]
- 24.Fitts WT, Lehr HB, Bitner RL, Spelman JW. An analysis of 950 fatal injuries. Surgery. 1964;56:663–8. [PubMed] [Google Scholar]
- 25.Coon WW. Risk factors in pulmonary embolism. Surg Gynecol Obstet. 1976;143:385–390. [PubMed] [Google Scholar]
- 26.Knudson MM, Ikossi DG, Khaw L, Morabito D, Speetzen LS. Thromboembolism after trauma: an analysis of 1602 episodes from the American College of Surgeons National Trauma Data Bank. Ann Surg. 2004;240:490–498. doi: 10.1097/01.sla.0000137138.40116.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bahloul M, Chelly H, Regaieg K, Rekik N, Bellil S, Chaari A, Bouaziz W, Chabchoub I, Haddar S, Ben Hamida C, Bouaziz M. Pulmonary embolism following severe traumatic brain injury: incidence, risk factors and impact outcome. Intensive Care Med. 2017;43:1433–1435. doi: 10.1007/s00134-017-4815-z. [DOI] [PubMed] [Google Scholar]
- 28.Schultz DJ, Brasel KJ, Washington L, Goodman LR, Quickel RR, Lipchik RJ, Clever T, Weigelt J. Incidence of asymptomatic pulmonary embolism in moderately to severely injured trauma patients. J Trauma. 2004;56:727–733. doi: 10.1097/01.ta.0000119687.23542.ec. [DOI] [PubMed] [Google Scholar]
- 29.Owings JT. Timing of the occurrence of pulmonary embolism in trauma patients. Arch Surg. 1997;132:862. doi: 10.1001/archsurg.1997.01430320064010. [DOI] [PubMed] [Google Scholar]
- 30.Sing RF, Camp SM, Heniford BT, Rutherford EJ, Dix S, Reilly PM, Holmes JH, Haut E, Hayanga H. Timing of pulmonary emboli after trauma: implications for retrievable vena cava filters. J Trauma. 2006;60:732–735. doi: 10.1097/01.ta.0000210285.22571.66. [DOI] [PubMed] [Google Scholar]
- 31.Menaker J, Stein DM, Scalea TM. Pulmonary embolism after injury: more common than we think? J Trauma. 2009;67:1244–1249. doi: 10.1097/TA.0b013e31818c173a. [DOI] [PubMed] [Google Scholar]
- 32.Kyrle PA, Eichinger S. Deep vein thrombosis. Lancet. 2005;365:1163–1174. doi: 10.1016/S0140-6736(05)71880-8. [DOI] [PubMed] [Google Scholar]
- 33.Knudson MM, Gomez D, Haas B, Cohen MJ, Nathens AB. Three thousand seven hundred thirty-eight posttraumatic pulmonary emboli: a new look at an old disease. Ann Surg. 2011;254:625–632. doi: 10.1097/SLA.0b013e3182300209. [DOI] [PubMed] [Google Scholar]
- 34.Campbell JE, Brummel-Ziedins KE, Butenas S, Mann KG. Cellular regulation of blood coagulation: a model for venous stasis. Blood. 2010;116:6082–6091. doi: 10.1182/blood-2010-01-266395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Owens AP, Mackman N. Tissue factor and thrombosis: the clot starts here. Thromb Haemost. 2010;104:432–439. doi: 10.1160/TH09-11-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manly DA, Boles J, Mackman N. Role of Tissue factor in venous thrombosis. Annu Rev Physiol. 2011;73:515–525. doi: 10.1146/annurev-physiol-042210-121137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maeda S, Takahashi S, Sato M. Serum thrombomodulin as a newly identified biomarker for postoperative lung injury: a prospective observational study. Tohoku J Exp Med. 2012;228:135–141. doi: 10.1620/tjem.228.135. [DOI] [PubMed] [Google Scholar]
- 38.Bone RC. Modulators of coagulation. A critical appraisal of their role in sepsis. Arch Intern Med. 1992;152:1381–1389. doi: 10.1001/archinte.152.7.1381. [DOI] [PubMed] [Google Scholar]
- 39.Wu KK, Thiagarajan P. Role of endothelium in thrombosis and hemostasis. Annu Rev Med. 1996;47:315–331. doi: 10.1146/annurev.med.47.1.315. [DOI] [PubMed] [Google Scholar]
- 40.Branchford BR, Carpenter SL. The role of inflammation in venous thromboembolism. Front Pediatr. 2018;23:142. doi: 10.3389/fped.2018.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bahloul M, Dlela M, KhlafBouaziz N, Turki O, Chelly H, Bouaziz M. Early post-traumatic pulmonary-embolism in patients requiring ICU admission: more complicated than we think! J Thorac Dis. 2018;10:S3850–S3854. doi: 10.21037/jtd.2018.09.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frank J, Maier M, Koenig J, Rose S, Bouma M, Buurman WA, Marzi I. Circulating inflammatory and metabolic parameters to predict organ failure after muliple trauma. Eur J Trauma. 2002;28:333–339. [Google Scholar]
- 43.Koh TJ, Dipietro LA. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med. 2011;13:e23. doi: 10.1017/S1462399411001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barbul A, Breslin RJ, Woodyard JP, Wasserkrug HL, Efron G. The effect of in vivo T helper and T suppressor lymphocyte depletion on wound healing. Ann Surg. 1989;209:479–483. doi: 10.1097/00000658-198904000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Odland G, Ross R. Human wound repair. I. Epidermal regeneration. J Cell Biol. 1968;39:135–151. doi: 10.1083/jcb.39.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yao YM, Redl H, Bahrami S, Schlag G. The inflammatory basis of trauma/shock-associated multiple organ failure. Inflamm Res. 1998;47:201–210. doi: 10.1007/s000110050318. [DOI] [PubMed] [Google Scholar]
- 47.Gołąbek-Dropiewska K, Pawłowska J, Witkowski J, Lasek J, Marks W, Stasiak M, Jaskólski D, Kawecka A, Łuczkiewicz P, Baczkowski B. Analysis of selected pro- and anti-inflammatory cytokines in patients with multiple injuries in the early period after trauma. Cent Eur J Immunol. 2018;43:42–49. doi: 10.5114/ceji.2018.74872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wakefield TW, Myers DD, Henke PK. Mechanisms of venous thrombosis and resolution. Arterioscler Thromb Vasc Biol. 2008;28:387–391. doi: 10.1161/ATVBAHA.108.162289. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Golden JB, Fritz Y, Zhang X, Diaconu D, Camhi MI, Gao H, Dawes SM, Xing X, Ganesh SK, Gudjonsson JE, Simon DI, McCormick TS, Ward NL. Interleukin 6 regulates psoriasiform inflammation-associated thrombosis. JCI Insight. 2016;1:e89384. doi: 10.1172/jci.insight.89384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Savchenko AS, Martinod K, Seidman MA, Wong SL, Borissoff JI, Piazza G, Libby P, Goldhaber SZ, Mitchell RN, Wagner DD. Neutrophil extracellular traps form predominantly during the organizing stage of human venous thromboembolism development. J Thromb Haemost. 2014;12:860–870. doi: 10.1111/jth.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Myers D. Pathophysiology of venous thrombosis. Phlebol J Venous Dis. 2015;30:7–13. doi: 10.1177/0268355515569424. [DOI] [PubMed] [Google Scholar]
- 52.Esmon CT. Basic mechanisms and pathogenesis of venous thrombosis. Blood Rev. 2009;23:225–229. doi: 10.1016/j.blre.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mackman N. New insights into the mechanisms of venous thrombosis. J Clin Invest. 2012;122:2331–2336. doi: 10.1172/JCI60229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bovill EG, Van der Vliet A. Venous valvular stasis-associated hypoxia and thrombosis: what is the link? Annu Rev Physiol. 2011;73:527–545. doi: 10.1146/annurev-physiol-012110-142305. [DOI] [PubMed] [Google Scholar]
- 55.Velmahos GC, Spaniolas K, Tabbara M, Abujudeh HH, Moya M, Gervasini A, Alam HB. Pulmonary embolism and deep venous thrombosis in trauma: are they related? Arch Surg. 2009;144:928–32. doi: 10.1001/archsurg.2009.97. [DOI] [PubMed] [Google Scholar]
- 56.Schreiber MA, Differding J, Thorborg P, Mayberry JC, Mullins RJ. Hypercoagulability is most prevalent early after injury and in female patients. J Trauma. 2005;58:475–481. doi: 10.1097/01.ta.0000153938.77777.26. [DOI] [PubMed] [Google Scholar]
- 57.Morris JA, Norris PR, Waitman LR, Ozdas A, Guillamondegui OD, Jenkins JM. Adrenal insufficiency, heart rate variability, and complex biologic systems: a study of 1,871 critically Ill trauma patients. J Am Coll Surg. 2007;204:885–892. doi: 10.1016/j.jamcollsurg.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 58.Nordström M, Lindblad B, Bergqvist D, Kjellström T. A prospective study of the incidence of deep-vein thrombosis within a defined urban population. J Intern Med. 1992;232:155–160. doi: 10.1111/j.1365-2796.1992.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 59.Anderson FA, Wheeler HB, Goldberg RJ, Hosmer DW, Forcier A. The prevalence of risk factors for venous thromboembolism among hospital patients. Arch Intern Med. 1992;152:1660–1664. [PubMed] [Google Scholar]
- 60.Gibbs NM. Venous thrombosis of the lower limbs with particular reference to bed-rest. Br J Surg. 1957;45:209–236. doi: 10.1002/bjs.18004519102. [DOI] [PubMed] [Google Scholar]
- 61.Cambien B, Wagner DD. A new role in hemostasis for the adhesion receptor P-selectin. Trends Mol Med. 2004;10:179–186. doi: 10.1016/j.molmed.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 62.Lentz SR. Thrombosis in the setting of obesity or inflammatory bowel disease. Blood. 2016;128:2388–2394. doi: 10.1182/blood-2016-05-716720. [DOI] [PubMed] [Google Scholar]
- 63.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 64.Blokhin IO, Lentz SR. Mechanisms of thrombosis in obesity. Curr Opin Hematol. 2013;20:437–444. doi: 10.1097/MOH.0b013e3283634443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bahloul M, Bradai S, Turki O, Bouchaala K, Bouaziz NK, Chelly H, Haddar S, Bouaziz M. Thromboembolic complications in patients with septic shock requiring invasive mechanical ventilation: incidence, risk factors, and outcomes. J Anaesthesiol Clin Pharmacol. 2020;36:135–137. doi: 10.4103/joacp.JOACP_163_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vallance P, Collier J, Bhagat K. Infection, inflammation, and infarction: does acute endothelial dysfunction provide a link? Lancet. 1997;349:1391–1392. doi: 10.1016/S0140-6736(96)09424-X. [DOI] [PubMed] [Google Scholar]
- 67.Levine R, LeClerc J, Bailey J, Monberg MJ, Sarwat S. Venous and arterial thromboembolism in severe sepsis. Thromb Haemost. 2008;99:892–898. doi: 10.1160/TH08-01-0004. [DOI] [PubMed] [Google Scholar]
- 68.Donzé JD, Ridker PM, Finlayson SR, Bates DW. Impact of sepsis on risk of postoperative arterial and venous thromboses: large prospective cohort study. BMJ. 2014;349:g5334–g5334. doi: 10.1136/bmj.g5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brill A, Suidan GL, Wagner DD. Hypoxia, such as encountered at high altitude, promotes deep vein thrombosis in mice. J Thromb Haemost. 2013;11:1773–1775. doi: 10.1111/jth.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xenos ES, Vargas HD, Davenport DL. Association of blood transfusion and venous thromboembolism after colorectal cancer resection. Thromb Res. 2012;129:568–572. doi: 10.1016/j.thromres.2011.07.047. [DOI] [PubMed] [Google Scholar]
- 71.Zallen G, Moore EE, Ciesla DJ, Brown M, Biffl WL, Silliman CC. Stored red blood cells selectively activate human neutrophils to release IL-8 and secretory PLA2. Shock. 2000;13:29–33. doi: 10.1097/00024382-200013010-00006. [DOI] [PubMed] [Google Scholar]
- 72.Perel P, Clayton T, Altman DG, Croft P, Douglas I, Hemingway H, Hingorani A, Morley KI, Riley R, Timmis A, Van der Windt D, Roberts I PROGRESS Partnership. Red blood cell transfusion and mortality in trauma patients: risk-stratified analysis of an observational study. PLoS Med. 2014;11:e1001664. doi: 10.1371/journal.pmed.1001664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zander AL, Olson EJ, Van Gent JM, Bandle J, Calvo RY, Shackford SR, Peck KA, Sise CB, Sise MJ, King BS. Does resuscitation with plasma increase the risk of venous thromboembolism? J Trauma. 2015;78:39–44. doi: 10.1097/TA.0000000000000480. [DOI] [PubMed] [Google Scholar]
- 74.Blaisdell FW, Graziano CJ. Assessment of clotting by the determination of fibrinogen catabolism. Am J Surg. 1978;135:436–443. doi: 10.1016/0002-9610(78)90081-8. [DOI] [PubMed] [Google Scholar]
- 75.Chakroun-Walha O, Samet A, Jerbi M, Nasri A, Talbi A, Kanoun H, Souissi B, Chtara K, Bouaziz M, Ksibi H, Rekik N. Benefits of the tranexamic acid in head trauma with no extracranial bleeding: a prospective follow-up of 180 patients. Eur J Trauma Emerg Surg. 2019;45:719–726. doi: 10.1007/s00068-018-0974-z. [DOI] [PubMed] [Google Scholar]
- 76.Walker PF, Bozzay JD, Johnston LR, Elster EA, Rodriguez CJ, Bradley MJ. Outcomes of tranexamic acid administration in military trauma patients with intracranial hemorrhage: a cohort study. BMC Emerg Med. 2020;20:39. doi: 10.1186/s12873-020-00335-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mahmood A, Needham K, Shakur-Still H, Davies D, Belli A, Jamaluddin SF, Harris T, Mohamed FL, Leech C, Lotfi H, Moss P, Hopkins P, Wong D, Kendall J, Boyle A, Wilson M, Darwent M, Robert I. Tranexamic acid in traumatic brain injury: an explanatory study nested within the CRASH-3 trial. Eur J Trauma Emerg Surg. 2020 doi: 10.1007/s00068-020-01316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rogers FB, Cipolle MD, Velmahos G, Rozycki G, Luchette FA. Practice management guidelines for the prevention of venous thromboembolism in trauma patients: the EAST practice management guidelines work group. J Trauma. 2002;53:142–164. doi: 10.1097/00005373-200207000-00032. [DOI] [PubMed] [Google Scholar]
- 79.Tuttle-Newhall JE, Rutledge R, Hultman CS, Fakh SM. Statewide, population-based, time-series analysis of the frequency and outcome of pulmonary embolus in 318,554 trauma patients. J Trauma. 1997;42:90–99. doi: 10.1097/00005373-199701000-00016. [DOI] [PubMed] [Google Scholar]
- 80.Ho KM, Burrell M, Rao S, Baker R. Incidence and risk factors for fatal pulmonary embolism after major trauma: a nested cohort. Br J Anaesth. 2010;105:596–602. doi: 10.1093/bja/aeq254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bahloul M, Chaari A, Kallel H, Abid L, Hamida CB, Dammak H, Rekik N, Mnif J, Chelly H, Bouaziz M. Pulmonary embolism in intensive care unit: predictive factors, clinical manifestations and outcome. Ann Thorac Med. 2010;5:97–103. doi: 10.4103/1817-1737.62473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kasper W, Konstantinides S, Geibel A, Olschewski M, Heinrich F, Grosser KD, Rauber K, Iversen S, Redecker M, Kienast J. Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry. J Am Coll Cardiol. 1997;30:1165–1171. doi: 10.1016/s0735-1097(97)00319-7. [DOI] [PubMed] [Google Scholar]
- 83.Arnoult E, Wiramus S, Textoris J, Craighero F, Ragonnet B, Hammad E, Chaumoître K, Martin C, Leone M. Occult pulmonary embolism in intensive care unit patients undergoing chest computed tomography scan: incidence and effect on outcomes. J Cardiothorac Vasc Anesth. 2013;27:474–478. doi: 10.1053/j.jvca.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 84.Cook DJ, Donadini MP. Pulmonary embolism in medical-surgical critically Ill patients. Hematol Oncol Clin North Am. 2010;24:677–682. doi: 10.1016/j.hoc.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 85.Lavorini F, Di Bello V, De Rimini ML, Lucignani G, Marconi L, Palareti G, Pesavento R, Prisco D, Santini M, Sverzellati N, Palla A, Pistolesi M. Diagnosis and treatment of pulmonary embolism: a multidisciplinary approach. Multidiscip Respir Med. 2013;8:75. doi: 10.1186/2049-6958-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Paffrath T, Wafaisade A, Lefering R, Simanski C, Bouillon B, Spanholtz T, Wutzler S, Maegele M Trauma Registry of DGU. Venous thromboembolism after severe trauma: incidence, risk factors and outcome. Injury. 2010;41:97–101. doi: 10.1016/j.injury.2009.06.010. [DOI] [PubMed] [Google Scholar]


