Abstract
A few months ago a new coronavirus was identified in Cina officially named by the WHO as COVID-19. The thousands of patients who died showed pneumonia and alveolar damage, but actually, according to several authors in addition to the acute respiratory distress syndrome the virus can give rise to multiorgan failure. In fact, many people died equally despite being intubated and treated for respiratory failure. In this review, we especially wanted to describe the virus effects on the cardiovascular system, probably the leading cause of death of thousands of deceased patients. Therefore, mortality is indirectly induced by the virus through vascular inflammation and cardiovascular damage and patients with severe COVID-19 infection showed significantly increased levels of cardiac troponin I and inflammatory cytokines. The main activation of the signal pathways for the production of inflammatory cytokines are the toll-like receptors that recognize the presence of viral nucleic acids and the ACE-2 receptors, that the virus uses to infect the cells. The binding to ACE-2 also allows to promote high levels of angiotensin II by promoting high levels of blood pressure. High levels of IL-6, IL-1B and IL-8 have been associated with plaque instability and increased thrombotic risk. Furthermore IL-6 is involved in the stimulation of matrix-degrading enzymes such as matrix metalloproteinases, and may contribute to the development of acute coronary syndrome. In addition, TNF-α, IL-1 and IL-6 present in patients with severe COVID-19 are associated with coagulation activation and thrombin generation resulting in disseminated intravascular coagulation or thrombotic microangiopathy. Considering these pathological effects of the virus, anti-inflammatory and anticoagulant treatments are to be considered to avoid cardiovascular events. In this regard, heparin, in addition to its anticoagulant characteristics, has been shown to have good control over inflammation and to be a good anti-viral drug.
Keywords: COVID-19, pneumonia, cardiovascular disease, toll-like receptors, ACE-2 receptors, cytokines, plaque instability, disseminated intravascular coagulation, histone deacetylases, heparin therapy
Introduction
Coronaviruses consist of 26-32 kilobase single-stranded RNA genomes, enveloped in a capsid made up of two phospholipid layers, covered with the spiked proteins and the hemagglutinin HE.
Coronaviruses belong to a family that has been divided genetically and serologically into 4 classes, α, β, γ, and δ. Generally, in humans, the infections concern the α and β classes and it involves the upper respiratory tract giving rise to fever, headache, and cough although in some cases lower respiratory tract infections occur.
A new coronavirus was identified in Cina in 2019 officially named by the WHO as COVID-19, which has spread rapidly throughout China in the past five months [1-5] and it spread worldwide.
COVID-19 has been identified in swabs performed on the throat and nose of patients who suffer or are suspected of the disease and can cause mild or highly acute respiratory syndrome, which in many cases need to use artificial respiration. At the moment its mode of action is still unclear and the most valid strategy to defeat it is the lockdown.
The thousands of patients who died referred to pneumonia and alveolar damage. Many patients have been intubated to improve breathing, but death may not be caused solely by lung failure. According to several authors, in addition to the acute respiratory distress syndrome, the virus can give rise to multiorgan failure [6].
However, some aspect of its mechanisms is beginning to be brought to light by researchers.
The disease is reported to affect more easily older people who have other comorbidities as cardiovascular diseases and a weaker immune system [1,7]. As SARS and MERS-CoV coronavirus infection [8,9] also COVID-19 induces an highly increment of inflammatory cytokines such as interleukin (IL)-1b, IL-6 [10], and the C-reactive protein (CRP) [11], that are associated with the damage to the lung alveoli and cardiovascular tissues [1,7,12].
Therefore, mortality is indirectly induced by the virus through the damage caused by inflammation.
Here, we will look into the mechanisms involved between COVID-19 infections, vascular inflammation, and cardiovascular risk.
Toll-like receptor
Eukaryotic toll-like receptors (TLRs) have been identified as the primary immune receptors that distinguish between different pathogens and activate a rapid innate immune response [13]. TLRs are expressed in macrophages and other cell types [13]. A subset of TLRs, TLR3, TLR7/8, and TLR9, is involved in antiviral responses by triggering the production of proinflammatory cytokines such as IL-6, NF-kB and type I interferons (IFNs). The start of TLR-induced signal transmission occurs following their dimerization and interaction of cytoplasmic molecules called adapters such as the MyD88 or TRIF adapter [14,15] (Figure 1). Innate immunity is needed in a precise regulation to eliminate the virus, otherwise will result in immunopathology as it happens in patients with critical COVID-19 condition, where the virus particles invade the respiratory mucosa firstly and infect other cells, triggering a series of immune responses and the production of cytokine storm in the body [16].
Figure 1.
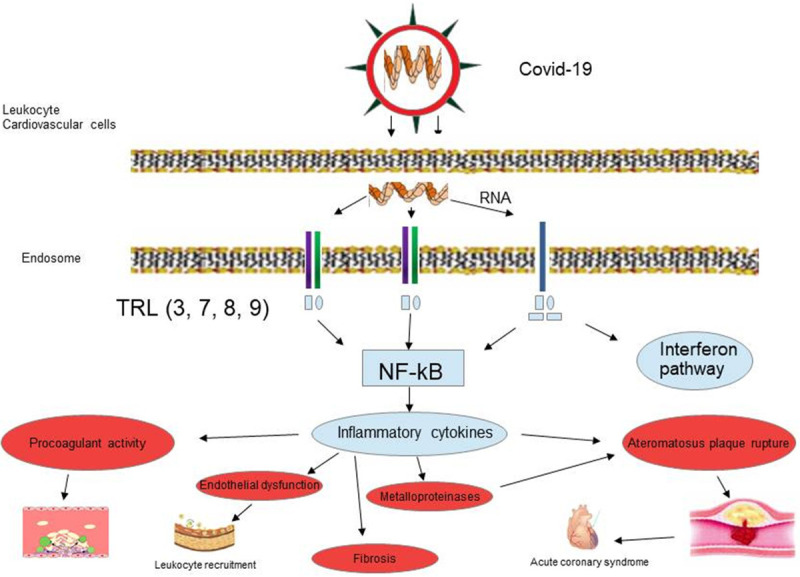
The virus through TLR receptors can trigger the signal transmission path which determines the production of interferons and NF-kB. The biochemical cascade that induces vascular inflammation, promotes plaque rupture, disseminated intravascular coagulation, endothelial dysfunction, fibrosis and acute coronary syndrome.
In fact, the binding of COVID-19 to the TLR induces a vicious cycle of inflammation which is responsible not only for lung inflammation, fever, and fibrosis, but also for causing multiple injuries to the liver, heart, and kidneys [17]. Several studies have established that the hyper inflammatory response induced by COVID-19 is a major cause of disease severity and death, in particular in patients with comorbidities such as cardiovascular diseases, where a link between TLR signaling in cardiovascular diseases and production of pro-inflammatory cytokines has been reported [18,19].
ACE-2 receptors
Several evidences describe Angiotensin-Converting Enzyme 2 (ACE2) as a receptor used by SARS-CoV-2 to infect the cell via the virus spike protein [20,21].
ACE-2 is a type I transmembrane metallocarboxypeptidase enzyme, known to be a key player in the Renin-Angiotensin system (RAS) and a target for the treatment of hypertension [22]. The major substrate for ACE-2 is Angiotensin II [23]. Angiotensin II, binding angiotensin receptor 1 (AT2R1) is responsible for vasoconstriction and causes hypertension. ACE-2 degrades Angiotensin II, thereby, negatively regulating RAS [24] and exhibiting a protective function in the cardiovascular system [25].
In the presence of COVID-19, ACE2 provides an entry door for the virus contributing to develop lung injury and hyper-inflammation [26,27]. Since ACE2 is also highly expressed in the heart [28,29], it has been rationally hypothesized that COVID-19 induced cardiac injury by ACE2 [30,31]; however, in a recent pathological study a patient with COVID-19 did not show substantial myocardial damage [32] suggesting that COVID-19 not directly impairs the heart.
Other studies suggest that ACE2 could be protective in several models of lung injury, including lung injury by COVID-19 [33]. ACE2 acting as a counter-regulatory mechanism of the classical RAS system, besides to block the pro-hypertensive features of angiotensin II [34], blocks angiotensin II ability to activate the production of proinflammatory cytokines [35].
In presence of a downregulation of ACE2, as observed in old subjects with comorbidities affecting the angiotensin system, such as hypertension or diabetes, it has been hypothesized that COVID19 could remove the brakes from angiotensin II, inducing the local activation of immune cells and an uncontrolled inflammatory response (Figure 2) [27,36]. Therefore the treatment with the well-known category of anti-hypertensive medications ACE inhibitors and Angiotensin receptor blockers (ARB) could be helpful in patients with COVID-19 and these comorbidities. The study by Meng et al. [37] in a hypertensive Chinese population with COVID-19 under treatment with ACEi/ARB drugs displayed an attenuated inflammatory response and, during hospitalization, 12 patients from the non-ACEi/ARB group were considered severe and one patient died versus ACEi/ARB group with 4 severe patients and no deaths. However further studies will be needed to clarify if the beneficial action of ACE2 by Angiotensin II inhibition prevails on its deleterious action favouring the entry of the virus into the cell.
Figure 2.
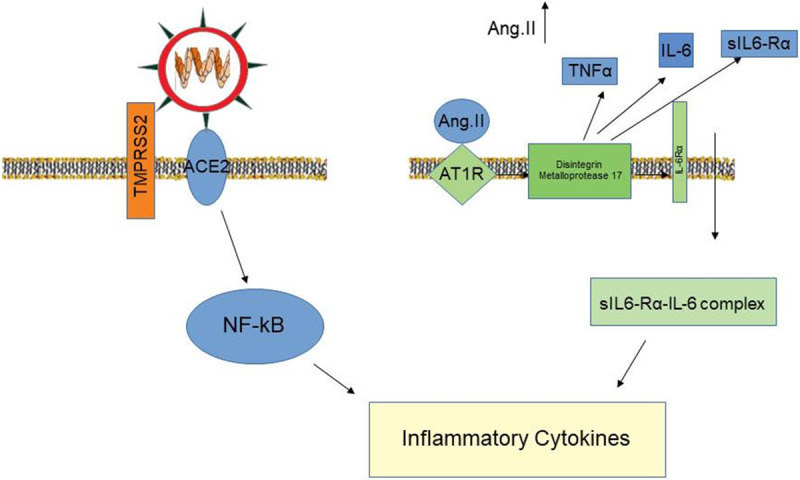
The virus by binding to ACE-2 receptors directly determines the production of inflammatory cytokines. Indirectly this link favors the increase in Angiotensin II levels inducing the production of various cytokines and high blood pressure.
COVID-19 infection and cardiovascular risk
Inflammatory cytokines and plaque instability
Some studies highlighted the association between infections and vascular inflammation in atherosclerosis risk [38,39].
The relationship between cytokines, migration of monocytes in the intima and development of atheroma is now well known. The atheroma recruits T-cells, mast cells, other inflammatory cells to the intima [40] and the production of several factors [41] that degrade collagen, resulting in thinning of the fibrous cap and its instability [42,43]. IL 6 and IL 8 cytokine levels have been found to correlate with atherosclerotic plaque instability [44].
The occlusion of the vessel lumen, which comes from atherosclerotic plaque rupture/erosion initiating thrombus formation, is a common condition in the pathogenesis of significant clinical forms of atherosclerosis, such as sudden death, myocardial infarction, and stroke [45].
Previous studies have highlighted that patients with sepsis secondary to infection in the intensive care unit showed significantly increased levels of IL-6, which well correlated with Acute Physiology and Chronic Health Evaluation II score and overall mortality [46]. Acute infections, especially systematic respiratory tract infections, are associated with a transient increased risk for myocardial infarction, especially during the first 3 days of infection [47]. Various infections have been associated with inflammatory cytokines, as observed in patients affected by rheumatoid arthritis, where increased levels of IL-6 in serum and synovial fluid [48] correlate with the inflammatory condition of these diseases, with an increased risk of mortality attributed largely to cardiovascular events [49].
Recent studies demonstrated the statistically significant association between cardiac injury and mortality in hospitalized patients with COVID-19 in Wuhan, China [30].
Plasma concentrations of IL-6 and C-reactive protein (CRP) appear to reflect the intensity of occult plaque inflammation and the vulnerability to rupture [50].
High IL-6 concentrations were reported in samples from patients with unstable angina [51,52], confirming IL-6 importance in the pathophysiology of AMI syndrome [53].
CRP and IL-6 resulted as independent predictors of future clinical events such as myocardial infarction, unstable angina, stroke, and peripheral vascular disease in symptomatic and in apparently asymptomatic patients [38,52]. Furthermore, since IL-6 is a strong independent marker of increased mortality in unstable CAD, it can identify patients who could benefit from a strategy of early invasive management.
IL-6 is also involved in the stimulation of matrix-degrading enzymes such as matrix metalloproteinases (MMPs) [54] and may contribute to the development of acute coronary syndrome [55]. When macrophages, vascular smooth muscle cells (VSMCs), and other cells overexpressing activated forms of MMPs accumulate locally, they may destroy the extracellular matrix (ECM) in atheroma and thereby destabilize and rupture the plaque [56].
Studies in animal models [57] and human atherosclerotic arteries [58] have evidenced that also interleukin-1B (IL-1B) is associated with the development of atherosclerosis. IL-1B, produced and secreted in particular by innate immune cells such as monocytes, macrophages, and dendritic cells upon activation, is a key pro-inflammatory cytokine that induces the production of other cytokines, adhesion molecules, and metalloproteinases [59,60]. In the arterial wall, IL-1B influences the inflammatory processes that lead to the development of atherosclerotic plaques and acute coronary syndrome [40,61].
IL-1 has been considered as a crucial cytokine for hosts to defend against a broad range of pathogens. The classical IL-1 cytokines are IL-1A and IL-1B [62]. The IL-1 cytokines, particularly IL-1B, have been studied for their role in the antifungal host response against Aspergillus fumigatus infection. Recent studies showed the association of serum IL-1B level with the disease activity of chronic pulmonary aspergillosis [63].
Recent literature showed that cardiac troponin I (cTnI) values are significantly increased in patients with severe COVID-19 infection compared to those with milder forms of the disease [64]. This shows that it is important to measure cardiac damage biomarkers immediately after hospitalization for COVID-19 infection, as well as longitudinal monitoring during hospital stay, identifying a subset of patients with possible cardiac injury. Also, in this case, we should investigate the use of antiviral, anti-inflammatory and antithrombotics drugs to avoid heart damage.
Thrombosis
Many patients with severe COVID-19 have coagulation abnormalities and a substantial percentage of patients with severe COVID-19 develop venous and arterial thromboembolic complications [65,66]. The increased concentrations of pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and IL-1 and IL-6 present in these patients induce tissue factor expression on mononuclear cells, with subsequently coagulation activation and thrombin generation.
Coronavirus infections are also associated with a remarkable activation of the fibrinolytic system, an important factor in lethality. A massive release of plasminogen activators by inflammation-induced endothelial cell injury could explain the high concentrations of D-dimer and fibrin degradation products observed in patients with severe COVID-19 [65].
Thrombotic microangiopathy is typically caused by pathologically enhanced platelet-vessel wall interaction due to ultra-large von Willebrand factor multimers released from perturbed endothelial cells, normally cleaved by a disintegrin and metalloprotease (ADAMTS13). Since a significant reduction of ADAMTS13 has been observed in severe inflammation, we can speculate that ADAMTS13 could represent an important factor also in COVID-19 infection. The coagulation changes associated with COVID-19 suggest the presence of a hypercoagulable state that might increase the risk of thromboembolic complications [65]. Recent findings reported a 35-45% incidence of thromboembolic complications in patients with COVID-19 [67]. Moreover, patients with increased concentrations of D-dimer (6 times the upper limit of normal) and treated with heparin have a lower mortality than those not treated [66].
However, mortality also exists in healthy elderly subjects. In older subjects in the presence of COVID-19, even without previous diseases, the possible presence of non-significant atheromatous plaques could be sufficient to induce platelet aggregations and thrombi inducing cardiac ischemic events.
A highly significant positive association between age and IL-6 levels was observed by our group in a healthy general population (unpublished data) (Figure 3). In conclusion, in older subjects, the presence of a high level of IL-6 due to the age plus that produced by COVID-19 is certainly more harmful than in young people.
Figure 3.
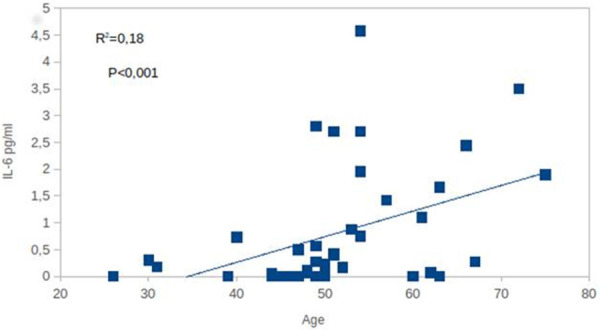
Association between age and plasma IL-6 levels in a general population of healthy subjects (our unpublished data). According to us, the presence of the virus in elderly subjects, that have already high basal IL-6 levels, is dangerous because it promotes a higher IL.
Treatments
Given the need to neutralize the devastating effects of the COVID-19 at the vascular level, strategies target cytokine production, toll-like receptors, and ACE2 receptors are needed.
Inhibitors against these targets have been considered as promising therapeutics for COVID-19 infection and are under examination for clinical trials [68] (see ClinicalTrials.gov website).
It has been shown that inhibition of a group of enzymes with histone deacetylase activity modulates the immune response triggered by several pathogens and toll-like receptor (TLR) ligands in macrophages [69,70]. These histone deacetylase enzymes (HDACs) have an important cellular role in mediating different biochemical and epigenetic processes involved in gene expression by eliminating acetyl groups from histone or non-histone proteins [71]. Valproic acid (VPA) is a well-known drug that increase in cerebral levels of gamma amino butyric acid (GABA), an inhibitory neurotransmitter [72]. It has been reported that VPA such as trichostatin A (TSA) and vorinostat inhibitors of HDAC activity of several inflammatory diseases showed a modulatory effect in the expression of inflammatory cytokines in in vivo models [71,72]. Recent findings showed that HDAC inhibition with VPA during infection with dengue virus exerted an important regulatory effect in the production of inflammatory cytokines, representing a significant advance in the design of novel therapeutic viral infections treatments [73].
Therapies targeting ACE2 with blocking agents may be detrimental, with the risk of worsening hypertension, while boosting ACE2 activity could represent a good treatment of COVID-19 [74], as previously described for SARS [75].
The upregulation of proinflammatory cytokines observed in COVID-19 patients that induces a severe inflammation leads to a marked increase of D-dimers and the fibrinolysis [76].
Heparin may also be helpful in microvascular dysfunction in cardiac failure because it has been postulated that ischemic hypoxia in the subendocardial layer can make it lose its natural anticoagulant properties [77]. According to some authors the introduction of heparin to block thrombin could reduce the inflammatory response in COVID-19 patients [78]. One of the properties of heparin is its anti-inflammatory activity. As many authors have described, heparin can bind to cytokines, inhibit chemotaxis and leukocyte recruitment, neutralize the complement factor C5a [79,80].
Activation of the coagulation system is relevant in the pathogenesis of COVID-19 [81]. Treatment with heparin may thus help to mitigate this coagulopathy. A demonstration of this evidence resulted from a study where treatment with low molecular weight heparin within 7 days of the appearance of acute respiratory distress syndrome greatly reduces the risk of mortality [82]. Another increasingly recognized complication of COVID 19 is the endothelial dysfunction well known to contribute to cardiac effects [81].
Heparin therapy is also important for its recognized antiviral activity. Thanks to its polyanionic nature capable of binding numerous proteins thus acting as an effective inhibitor of viral binding to cells [83].
Conclusions
The scientific community for a few months did not understand the pathological effect triggered by this virus. The diagnosis of pneumonia prevailed for many days over the real pathological picture that was occurring. This mistake added to the delays in understanding that the world was facing a new virus has caused thousands of deaths. The even more tragic factor is that despite the treatments to improve the breathing of the patients it was not noticed that the people died anyway. Finally, today we are aware that the treatments to be carried out promptly in case of COVID-19 infection are mainly related to the reduction of inflammatory cytokines induced in large quantities by the virus and in the prevention of disseminated coagulation, factors that induce important damage to the cardiovascular system.
Acknowledgements
The authors are grateful to Lucrecia Mota Garcia for her English editing support.
Disclosure of conflict of interest
None.
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng JL, Huang C, Zhang GJ, Liu DW, Li P, Lu CY, Li J. An update on the epidemiological characteristics of novel coronavirus pneumonia (COVID-19). Special expert group for control of the epidemic of novel coronavirus pneumonia of the Chinese preventive. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:327–331. doi: 10.3760/cma.j.cn112147-20200222-00148. [DOI] [PubMed] [Google Scholar]
- 3.Yang Y, Peng F, Wang R, Guan K, Jiang T, Xu G, Sun J, Chang C. The deadly coronaviruses: The 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. J Autoimmun. 2020;109:102434. doi: 10.1016/j.jaut.2020.102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Wang Y, Chen Y, Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol. 2020;92:568–576. doi: 10.1002/jmv.25748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conti P, Ronconi G, Caraffa A, Gallenga CE, Ross R, Frydas I, Kritas SK. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by COVID-19: anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34:327–331. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 7.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong CK, Lam CW, Wu AK, Ip WK, Lee NL, Chan IH, Lit LC, Hui DS, Chan MH, Chung SS, Sung JJ. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahallawi WH, Khabour OF, Zhang Q, Makhdoum HM, Suliman BA. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine. 2018;104:8–13. doi: 10.1016/j.cyto.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu B, Li M, Zhou Z, Guan X, Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun. 2020;111:102452. doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han H, Ma Q, Li C, Liu R, Zhao L, Wang W, Zhang P, Liu X, Gao G, Liu F, Jiang Y, Cheng X, Zhu C, Xia Y. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020;9:1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. Cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020;55:105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adamczak DM. Role of Toll-like receptors and vitamin D in cardiovascular diseases. Int J Mol Sci. 2017;18:2252. doi: 10.3390/ijms18112252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawai T, Akira S. Toll-like receptor downstream signalling. Arthritis Res Ther. 2005;7:12–9. doi: 10.1186/ar1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boehme KW, Compton T. Innate sensing of viruses by toll-like receptors. J Virol. 2004;78:7867–7873. doi: 10.1128/JVI.78.15.7867-7873.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, Tan KS, Wang DY, Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7:11–21. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng G, Zheng KI, Yan QQ, Rios RS, Targher G, Byrne CD, Poucke SV, Liu WY, Zheng MH. COVID-19 and liver dysfunction: current insights and emergent therapeutic strategies. J Clin Transl Hepatol. 2020;8:18–24. doi: 10.14218/JCTH.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boukhris M, Hillani A, Moroni F, Annabi MS, Addad F, Ribeiro MH, Mansour S, Zhao X, Ybarra LF, Abbate A, Vilca LM, Azzalini L. Cardiovascular implications of the COVID-19 pandemic: a global perspective. Can J Cardiol. 2020;36:1068–1080. doi: 10.1016/j.cjca.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang Y, Chen T, Mui D, Ferrari V, Jagasia D, Scherrer-Crosbie M, Chen Y, Han Y. Cardiovascular manifestations and treatment considerations in covid-19. Heart. 2020;106:1132–1141. doi: 10.1136/heartjnl-2020-317056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riordan JF. Angiotensin-I-converting enzyme and its relatives. Genome Biol. 2003;4:225. doi: 10.1186/gb-2003-4-8-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Int Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofmann H, Pyrc K, van der Hoek L, Geier M, Berkhout B, Pöhlmann S. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. PNAS. 2005;102:7988–7993. doi: 10.1073/pnas.0409465102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ksiazek TG, Erdman D, Goldsmith C. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 24.Tikellis C, Thomas MC. Angiotensin-Converting Enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int J Pept. 2012;2012:1–8. doi: 10.1155/2012/256294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kubaa K, Imai Y, Ohto-Nakanishi T, Penning JM. Trilogy of ACE2: a peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol Ther. 2010;128:119–128. doi: 10.1016/j.pharmthera.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verdecchia P, Cavallini C, Spanevello A, Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. 2020;76:14–20. doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rivellese F, Prediletto E. ACE2 at the centre of COVID-19 from paucisymptomatic infections to severe pneumonia. Autoimmun Rev. 2020;19:1–4. doi: 10.1016/j.autrev.2020.102536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimabukuro-Vornhagen A, Gödel P, Subklewe M, Stemmler HJ, Schlößer HA, Schlaak M, Kochanek M, Böll B, von Bergwelt-Baildon MS. Cytokine release syndrome. J Immunother Cancer. 2018;6:56–70. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia S, Liu M, Wang C, Xu W, Lan Q, Feng S, Qi F, Bao L, Du L, Liu S, Qin C, Sun F, Shi Z, Zhu Y, Jiang S, Lu L. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30:343–355. doi: 10.1038/s41422-020-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu Z, Lei Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Zhou W, Yang L, You R. Physiological and pathological regulation of ACE2, the SARS-CoV-2 receptor. Pharmacol Res. 2020;157:104833. doi: 10.1016/j.phrs.2020.104833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, Mohan K, Raizada MK, Grant MB, Oudit GY. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sodhi CP, Wohlford-Lenane C, Yamaguchi Y, Prindle T, Fulton WB, Wang S, McCray PB, Chappell M, Hackam DJ, Jia H. Attenuation of pulmonary ACE2 activity impairs inactivation of des-Arg9 bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration. Am J Physiol Lung Cell Mol Physiol. 2018;314:L17–L31. doi: 10.1152/ajplung.00498.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Te Riet L, van Esch JH, Roks AJ, van den Meiracker AH, Danser AH. Hypertension: renin-angiotensin-aldosterone system alterations. Circ Res. 2015;116:960–975. doi: 10.1161/CIRCRESAHA.116.303587. [DOI] [PubMed] [Google Scholar]
- 37.Meng J, Xiao G, Zhang J, He X, Ou M, Bi J, Yang R, Di W, Wang Z, Li Z, Gao H, Liu L, Zhang G. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microb Infect. 2020;9:757–760. doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arshinov AB, Maslova IG. Role of the infection and inflammation in atherosclerosis development. Angiol Sosud Khirurg Angiol and Vasc Surg. 2010;17:35–41. [PubMed] [Google Scholar]
- 39.Kozarov E, Huber K, Wojta J. Infection-associated biomarkers of inflammation in atherosclerosis. Curr Pharmaceutical Design. 2015;21:1776–1782. doi: 10.2174/1381612821666141129173343. [DOI] [PubMed] [Google Scholar]
- 40.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 41.Riley E, Dasari V, Frishman WH, Sperber K. Vaccines in development to prevent and treat atherosclerotic disease. Cardiol Rev. 2008;16:288–300. doi: 10.1097/CRD.0b013e3181885933. [DOI] [PubMed] [Google Scholar]
- 42.Libby P, Geng YJ, Aikawa M, Schoenbeck U, Mach F, Clinton SK, Sukhova GK, Lee RT. Macrophages and atherosclerotic plaque stability. Curr Opin Lipidol. 1996;7:330–335. doi: 10.1097/00041433-199610000-00012. [DOI] [PubMed] [Google Scholar]
- 43.Libby P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation. 2001;104:365–372. doi: 10.1161/01.cir.104.3.365. [DOI] [PubMed] [Google Scholar]
- 44.Ragino IuI, Cherniavskiĭ AM, Polonskaia IaV, Volkov AM, Kashtanova EV, Tsymbal SIu, Polovnikova EM. Inflammatory-destructive biomarkers of atherosclerotic plaques instability. Study of arterial wall and blood. Kardiologiia. 2012;52:37–41. [PubMed] [Google Scholar]
- 45.Saha D, S S, Sergeeva EG, Ionova ZI, Gorbach AV. Tissue factor and atherothrombosis. Curr Pharm Des. 2015;21:1152–7. doi: 10.2174/1381612820666141013154946. [DOI] [PubMed] [Google Scholar]
- 46.Damas P, Ledoux D, Nys M, Vrindts Y, De Groote D, Franchimont P, Lamy M. Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Ann Surg. 1992;215:356–362. doi: 10.1097/00000658-199204000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 48.Houssiau FA, Devogelaer JP, Van Damme J, de Deuxchaisnes CN, Van Snick J. Interleukin-6 in synovial fluid and serum of patients with rheumatoid arthritis and other inflammatory arthritides. Arthritis Rheum. 1988;31:784–788. doi: 10.1002/art.1780310614. [DOI] [PubMed] [Google Scholar]
- 49.Gabriel SE. Cardiovascular morbidity and mortality in rheumatoid arthritis. Am J Med. 2008;121:S9–S14. doi: 10.1016/j.amjmed.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lindmark E, Diderholm E, Wallentin L, Siegbahn A. Relationship between interleukin 6 and mortality in patients with unstable coronary artery disease: effects of an early invasive or non invasive strategy. JAMA. 2001;286:2107–2113. doi: 10.1001/jama.286.17.2107. [DOI] [PubMed] [Google Scholar]
- 51.Biasucci LM, Vitelli A, Liuzzo G, Altamura S, Caligiuri G, Monaco C, Rebuzzi AG, Ciliberto G, Maseri A. Elevated levels of interleukin-6 in unstable angina. Circulation. 1996;94:874–877. doi: 10.1161/01.cir.94.5.874. [DOI] [PubMed] [Google Scholar]
- 52.Lubrano V, Cocci F, Battaglia D, Papa A, Marraccini P, Zucchelli GC. Usefulness of High-Sensitivity IL-6 measurement for clinical characterization of patients with coronary artery disease. J Clin Lab Anal. 2005;19:110–114. doi: 10.1002/jcla.20061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferroni P, Rosa A, Di Franco M, Palmirotta R, Guadagni F, Davì G, Bertazzoni G, Basili S. Prognostic significance of interleukin-6 measurement in the diagnosis of acute myocardial infarction in the emergency. Clin Chim Acta. 2007;381:151–156. doi: 10.1016/j.cca.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 54.Kishimoto T, Akira S, Narazaki M, Taga T. Interleukin-6 family of cytokines and gp130. Blood. 1995;86:1243–1254. [PubMed] [Google Scholar]
- 55.Galis ZS, Muszynski M, Sukhova GK, Simon-Morrissey E, Unemori EN, Lark MW, Amento E, Libby P. Cytokine-stimulated smooth muscle cells stimulate a complement of enzymes required for extracellular matrix digestion. Circ Res. 1994;75:181–189. doi: 10.1161/01.res.75.1.181. [DOI] [PubMed] [Google Scholar]
- 56.Dollery CM, McEwan JR, Henney AM. Matrix metalloproteinase and cardiovascular disease. Circ Res. 1995;77:863–868. doi: 10.1161/01.res.77.5.863. [DOI] [PubMed] [Google Scholar]
- 57.Kirii H, Niwa T, Yasuhiro Y, Wada H, Saito K, Iwakura Y, Asano M, Moriwaki H, Seishima M. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:656–660. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 58.Olofsson PS, Sheikine Y, Jatta K, Ghaderi M, Samnegård A, Eriksson P, Sirsjö A. A functional interleukin-1 receptor antagonist polymorphism influences atherosclerosis development. The interleukin-1beta: interleukin-1 receptor antagonist balance in atherosclerosis. Circ J. 2009;73:1531–1536. doi: 10.1253/circj.cj-08-1150. [DOI] [PubMed] [Google Scholar]
- 59.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 60.Dinarello CA. A clinical perspective of IL-1beta as the gatekeeper of inflammation. Eur J Immunol. 2009;4:1203–1217. doi: 10.1002/eji.201141550. [DOI] [PubMed] [Google Scholar]
- 61.Ridker PM, Morrow DA. C-reactive protein, inflammation, and coronary risk. Cardiol Clin. 2003;2:315–325. doi: 10.1016/s0733-8651(03)00079-1. [DOI] [PubMed] [Google Scholar]
- 62.Dinarello CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev. 2018;28:8–27. doi: 10.1111/imr.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhan M, Xu B, Zhao L, Li B, Xu L, Sun Q, Zhang J, Zhang Z, Chu H. The serum level of IL-1B correlates with the activity of chronic pulmonary aspergillosis. Can Respir J. 2018;2018:8740491. doi: 10.1155/2018/8740491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;63:390–391. doi: 10.1016/j.pcad.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Levi M, Thachil T, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coaugulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Teodori L, Sestili P, Madiai V, Fraternale D, Bruno M, Rocchi L, Ramakrishna S, Albertini MC. Pathways and microRNAs bioinformatics analyses identifying possible existing therapeutics for COVID-19 treatment. Research Square. 2020 doi: 10.3389/fphar.2020.582003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gupta KD, Shakespear MR, Lyer A, Fairlie DP, Sweet MJ. Histone deacetylases in monocyte/macrophage development, activation and metabolism: refining HDAC targets for inflammatory and infectious diseases. Clin Transl Immunol. 2016;5:e62. doi: 10.1038/cti.2015.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roger T, Lugrin J, Le Roy D, Goy G, Mombelli M, Koessler T, Ding XC, Chanson AL, Reymond MK, Miconnet I, Schrenzel J, François P, Calandra T. Histone deacetylase inhibitors impair innate immune responses to toll-like receptor agonists and to infection. Blood. 2011;117:1205–1217. doi: 10.1182/blood-2010-05-284711. [DOI] [PubMed] [Google Scholar]
- 71.Hull EE, Montgomery MR, Leyva KJ. Hdac inhibitors as epigenetic regulators of the immune system: impacts on cancer therapy and inflammatory diseases. Biomed Res Int. 2016;2016:8797206. doi: 10.1155/2016/8797206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Owens MJ, Nemeroff CB. Pharmacology of valproate. Psychopharmacol Bull. 2003;37:17–24. [PubMed] [Google Scholar]
- 73.Félix G, Delgado D, Cárdenas P, Castellanos JE. Valproic acid downregulates cytokine expression in human macrophages infected with dengue virus. Diseases. 2018;6:59. doi: 10.3390/diseases6030059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lei C, Fu W, Qian K, Li T, Zhang S, Ding M, Hu S. Potent neutralization of 2019 novel coronavirus by recombinant ACE2-Ig. Nat Commun. 2020;11:2070. doi: 10.1038/s41467-020-16048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moore MJ, Dorfman T, Li W, Wong SK, Li Y, Kuhn JH, Coderre J, Vasilieva N, Han Z, Greenough TC, Farzan M, Choe H. Retroviruses pseudo typed with the severe acute respiratory syndrome coronavirus spike protein efficiently infect cells expressing angiotensin-converting enzyme 2. J Virol. 2004;78:10628–10635. doi: 10.1128/JVI.78.19.10628-10635.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Idell S. Coagulation, fibrinolysis, and fibrin deposition in acute lung injury. Crit Care Med. 2003;31:S213–S220. doi: 10.1097/01.CCM.0000057846.21303.AB. [DOI] [PubMed] [Google Scholar]
- 77.Wojnicz R, Nowak J, Szyguła-Jurkiewicz B, Wilczek K, Lekston A, Trzeciak P, Nowalany-Kozielska E, Zembala M, Wodniecki J, Poloński L. Adjunctive therapy with low-molecular-weight heparin in patients with chronic heart failure secondary to dilated cardiomyopathy: one-year follow-up results of the randomized trial. Am Heart J. 2006;152:713–719. doi: 10.1016/j.ahj.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 78.Thachil J. The versatile heparin in COVID-19. J Thromb Haemost. 2020;18:1020–1022. doi: 10.1111/jth.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gaertner F, Massberg S. Blood coagulation in immunothrombosis. At the frontline of intravascular immunity. Semin Immunol. 2016;28:561–569. doi: 10.1016/j.smim.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 80.Young E. The anti-inflammatory effects of heparin and related compounds. Thromb Res. 2008;122:743–752. doi: 10.1016/j.thromres.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 81.Xiong TY, Redwood S, Prendergast B, Chen M. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur Heart J. 2020;41:1798–1800. doi: 10.1093/eurheartj/ehaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li J, Li Y, Yang B, Wang H, Li L. Low-molecular-weight heparin treatment for acute lung injury/acute respiratory distress syndrome: a meta-analysis of randomized controlled trials. Int J Clin Exp Med. 2018;11:414–422. [Google Scholar]
- 83.Shukla D, Spear PG. Herpesviruses and heparan sulfate: an intimaterelationship in aid of viral entry. J Clin Invest. 2001;108:503–510. doi: 10.1172/JCI13799. [DOI] [PMC free article] [PubMed] [Google Scholar]


