Abstract
Anticoagulation-related nephropathy (ARN) is a clinical entity that has significant morbidity and mortality consequences/burden but has not been well described. Consequently, ARN has been underdiagnosed and sub-optimally managed. ARN has been reported with warfarin use especially in the setting of supratherapeutic international normalized ratio (INR) but the association is far less established with the use of direct-acting oral anticoagulants (DOAC). Accelerated progression to CKD and ultimately ESRD has been reported in patients with ARN. With the expanding indications for DOAC use, there is growing concern about ARN in the setting of DOAC use and its attendant clinical and socioeconomic burden. In this review, we highlight precautionary measures to aid prompt diagnosis of ARN and suggest possible therapeutic strategies.
Keywords: Anticoagulation, nephropathy, DOAC
Introduction
Anticoagulation-related nephropathy (ARN) is an understudied renal complication of anticoagulant therapy. It is characterized by acute kidney injury (AKI,) defined as an increase in baseline serum creatinine ≥ 0.3 mg/d, without any alternate etiology, in the setting of a supra-therapeutic International Normalized Ratio (INR) greater than 3.0 [1-5]. ARN usually occurs in the first two months of starting anticoagulant therapy. The true incidence of ARN is not known as there are no prospective studies on this unique form of renal injury, however retrospective studies have estimated the prevalence to range from 16% to 37% of patients on anticoagulation in the setting of usage of warfarin [4,5].
The pathophysiologic mechanism of ARN is complex and involves multiple factors that include excessive glomerular proliferation, glomerular hypertension causing hematuria, and a complex interaction of heme molecules that result in oxidative stress and activation of inflammatory cascade in the renal tubular epithelium and surrounding stroma [1-5].
The use of anticoagulation is ubiquitous in medicine for various indications including atrial fibrillation and the prophylaxis and treatment of venous thromboembolism. It is estimated that more than two million patients are treated with oral anticoagulants annually in the United States [6]. The burden of renal disease on the American health system in terms of morbidity, mortality and health costs is profound. A recent analysis found that end stage renal disease treatment alone costs the Medicare program $35 billion per year [7]. An awareness of ARN is thus important to the internist as both anticoagulation and renal damage co-exist in ways that were previously unrecognized and under-diagnosed, and it is likely that the accompanying morbidity and mortality could have been prevented with early diagnosis. The expanding indications for the usage of direct-acting oral anticoagulants has also prompted consideration of ARN as a complication of DOAC use.
Pathophysiology
ARN has mainly been studied retrospectively in relation to warfarin [1-5]. Kidney biopsy findings indicate that the kidney injury may be the result of severe glomerular hematuria leading to extensive tubular obstruction by red cell casts [2,3]. The underlying molecular mechanism is theorized to be due to warfarin-induced thrombin depletion which may also lead to increases in endothelial pressure that leads to renal damage [8-10]. Some recent studies have also suggested alternate pathways involving a reduction in protein C and abnormal endothelial protein C receptor signaling [11,12].
The strongest risk factor for ARN is pre-existing chronic kidney disease (CKD) [1-5]. Other risk factors include supratherapeutic INR in coumadin therapy, older age, diabetes mellitus and hypertension as shown in Table 1 [1-5]. Patients with ARN have an accelerated progression of CKD and a retrospective study of more than 15,000 patients on warfarin showed a 65% increase in mortality in patients with ARN [2].
Table 1.
Risk factors for Anticoagulation-Related Nephropathy (ARN)
| Risk Factors for ARN |
|---|
| Pre-existing Chronic Kidney Disease |
| Hypertension |
| Diabetes Mellitus |
| Age > 65 years |
| Supra-therapeutic INR on coumadin therapy |
Direct-acting oral anticoagulants (DOACs) such as dabigatran, rivaroxaban and apixaban are gradually replacing warfarin in the clinical setting. A few animal models and sporadic case reports have shown that these DOACs can lead to deterioration of kidney function, but this has not been studied epidemiologically [13-16]. The clinical trials that compared the DOACs to warfarin did not specifically study ARN and did not repeat creatinine and urinalysis testing in the first two to three months of therapy to establish possible ARN diagnosis and hence the condition was underdiagnosed in those trials [17-24]. A recent study of a retrospective cohort in Taiwan suggests a lower rate of ARN in patients on Dabigatran compared to coumadin [25]. The safety data from patients randomized to apixaban in ARISTOTLE trial showed that the observed worsening of glomerular filteration rate was more than 20% in 16,869 patients [26]. This observation adds to the growing evidence that DOACs may cause clinically significant renal injury. Considering the increasing number of patients requiring anticoagulation and specifically DOACs, the incidence of ARN is expected to increase and may result to a worsened morbidity burden and survival decline in affected patients. If undiagnosed, ARN may also accelerate progression to end stage renal disease, especially in patients with pre-existing renal disease, and pose huge economic consequences. We suggest an age-matched prospective study stratified across different GFR categories to establish a strong association between DOACs and ARN. However, achieving an ideal study design may be challenging due to the confounding variables. Also, because certain DOACs are contra-indicated when GFR drops below 30 mls/min the chances of a perfectly stratified data/analysis are low.
Diagnosis
The clinical diagnosis of ARN is usually one of exclusion [1]. ARN should be suspected when a patient develops an AKI in the first few months of starting anticoagulation therapy, and a urinalysis with hematuria. It is important that other causes of AKI be excluded beginning with a well taken history (to exclude dehydration, hypotension or recent use of nephrotoxic agents) and a physical examination (ie. signs of volume contraction) and imaging (such as a renal ultrasound to exclude obstruction). A definitive diagnosis can be established with a kidney biopsy, however bleeding risks make this an unlikely choice. Wheeler, et al, suggest a clinical diagnostic algorithm that can help internists in early recognition of ARN (Figure 1). They also recommend a monthly monitoring of INR and kidney function in the first three months of commencement of anticoagulation, with regular quarterly or biannual monitoring after the first three months for those with and without CKD respectively [1].
Figure 1.
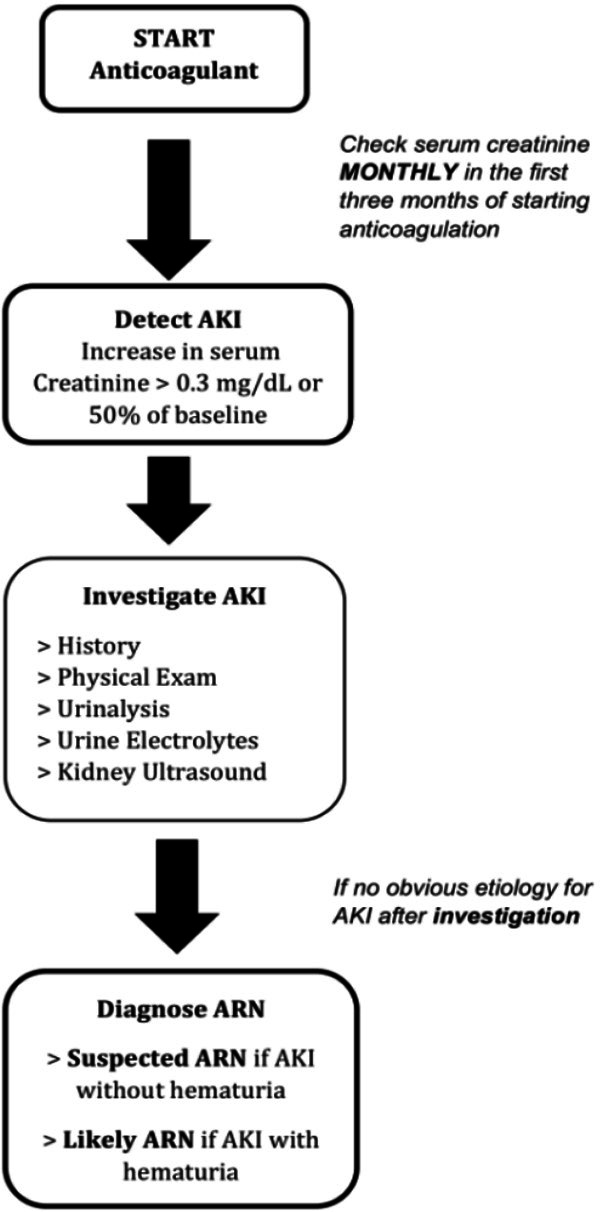
Clinical Diagnostic Algorithm for Suspected ARN (adapted from Wheeler et al [1]).
Management
The cornerstone of management of ARN is early detection of the AKI, hence monitoring kidney function is vital. Treatment should involve correcting supratherapeutic INR for patients on coumadin, switching anticoagulation and precautionary measures such as adequate hydration and avoiding nephrotoxic agents such as non-steroidal anti-inflammatory drugs and contrast agents. Revisiting the need for continued anticoagulation is crucial when considering discontinuation of anticoagulation therapy to avoid further renal deterioration.
Prognosis
In most patients with ARN, the kidney function recovers spontaneously, however it should be noted that in the seminal study of nine patients of biopsy proven ARN, five patients did not regain previous kidney function [3]. Patients with ARN have an accelerated progression of CKD and increase in mortality which is evident even after the first year [2,5]. In a cohort of 4006 patients, Brodsky et al showed that the 5 year Kaplan-Meier survival rate was significantly lower in patients with ARN than those without ARN (58% vs. 73%, P < 0.001), with 1-year survival of 68.9% in ARN vs. 81.1% in no-ARN group, P=0.049 [5].
Conclusion
Anticoagulant-related nephropathy (ARN) is a relatively new and underdiagnosed complication of anticoagulant therapy with the potential to accelerate chronic kidney disease and lead to increased morbidity and mortality. Carefully monitoring kidney function in the first few months of starting anticoagulation holds the key to early diagnosis and potentially averting irreversible kidney injury.
Disclosure of conflict of interest
None.
References
- 1.Wheeler DS, Giugliano RP, Rangaswami J. Anticoagulation-related nephropathy. J Thromb Haemost. 2016;14:461–467. doi: 10.1111/jth.13229. [DOI] [PubMed] [Google Scholar]
- 2.Brodsky SV, Nadasdy T, Rovin BH, Satoskar AA, Nadasdy GM, Wu HM, Bhatt UY, Hebert LA. Warfarin-related nephropathy occurs in patients with and without chronic kidney disease and is associated with an increased mortality rate. Kidney Int. 2011;80:181–189. doi: 10.1038/ki.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brodsky SV, Satoskar A, Chen J, Nadasdy G, Eagen JW, Hamirani M, Hebert L, Calomeni E, Nadasdy T. Acute kidney injury during warfarin therapy associated with obstructive tubular red blood cell casts: a report of 9 cases. Am J Kidney Dis. 2009;54:1121–1126. doi: 10.1053/j.ajkd.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 4.An JN, Ahn SY, Yoon CH, Youn TJ, Han MK, Kim S, Chin HJ, Na KY, Chae DW. The occurrence of warfarin-related nephropathy and effects on renal and patient outcomes in korean patients. PLoS One. 2013;8:e57661. doi: 10.1371/journal.pone.0057661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brodsky SV, Collins M, Park E, Rovin BH, Satoskar AA, Nadasdy G, Wu H, Bhatt U, Nadasdy T, Hebert LA. Warfarin therapy that results in an international normalization ratio above the therapeutic range is associated with accelerated progression of chronic kidney disease. Nephron Clin Pract. 2010;115:c142–c146. doi: 10.1159/000312877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirley K, Qato DM, Kornfield R, Stafford RS, Alexander GC. National trends in oral anticoagulant use in the United States, 2007 to 2011. Circ Cardiovasc Qual Outcomes. 2012;5:615–621. doi: 10.1161/CIRCOUTCOMES.112.967299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Ishani A, Johansen K, Kasiske BL, Kutner N, Liu J, St Peter W, Guo H, Hu Y, Kats A, Li S, Li S, Maloney J, Roberts T, Skeans M, Snyder J, Solid C, Thompson B, Weinhandl E, Xiong H, Yusuf A, Zaun D, Arko C, Chen SC, Daniels F, Ebben J, Frazier E, Johnson R, Sheets D, Wang X, Forrest B, Berrini D, Constantini E, Everson S, Eggers P, Agodoa L. US renal data system 2013 annual data report. Am J Kidney Dis. 2014;63:A7. doi: 10.1053/j.ajkd.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 9.Minami T, Sugiyama A, Wu SQ, Abid R, Kodama T, Aird WC. Thrombin and phenotypic modulation of the endothelium. Arterioscler Thromb Vasc Biol. 2004;24:41–53. doi: 10.1161/01.ATV.0000099880.09014.7D. [DOI] [PubMed] [Google Scholar]
- 10.Darrow AL, Fung-Leung WP, Ye RD, Santulli RJ, Cheung WM, Derian CK, Burns CL, Damiano BP, Zhou L, Keenan CM, Peterson PA, Andrade-Gordon P. Biological consequences of thrombin receptor deficiency in mice. Thromb Haemost. 1996;76:860–866. [PubMed] [Google Scholar]
- 11.Christiaans SC, Wagener BM, Esmon CT, Pittet JF. Protein C and acute inflammation: a clinical and biological perspective. Am J Physiol Lung Cell Mol Physiol. 2013;305:L455–L466. doi: 10.1152/ajplung.00093.2013. [DOI] [PubMed] [Google Scholar]
- 12.Madhusudhan T, Wang H, Straub BK, Gröne E, Zhou Q, Shahzad K, Müller-Krebs S, Schwenger V, Gerlitz B, Grinnell BW, Griffin JH, Reiser J, Gröne HJ, Esmon CT, Nawroth PP, Isermann B. Cytoprotective signaling by activated protein C requires protease-activated receptor-3 in podocytes. Blood. 2012;119:874–883. doi: 10.1182/blood-2011-07-365973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ware KM, Vance JC, Muni N, Hebert LA, Satoskar AA, Nadasdy G, Ivanov I, Nadasdy T, Rovin BH, Brodsky SV. Oral warfarin and the thrombin inhibitor dabigatran increase blood pressure in rats: hidden danger of anticoagulants? Am J Hypertens. 2015;28:182–189. doi: 10.1093/ajh/hpu129. [DOI] [PubMed] [Google Scholar]
- 14.Ryan M, Ware K, Qamri Z, Satoskar A, Wu H, Nadasdy G, Rovin B, Hebert L, Nadasdy T, Brodsky SV. Warfarin-related nephropathy is the tip of the iceberg: direct thrombin inhibitor dabigatran induces glomerular hemorrhage with acute kidney injury in rats. Nephrol Dial Transplant. 2014;29:2228–2234. doi: 10.1093/ndt/gft380. [DOI] [PubMed] [Google Scholar]
- 15.Moeckel GW, Luciano RL, Brewster UC. Warfarin-related nephropathy in a patient with mild IgA nephropathy on dabigatran and aspirin. Clin Kidney J. 2013;6:507–509. doi: 10.1093/ckj/sft076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stöllberger C, Finsterer J. A probable life-saving switch from apixaban to phenprocoumon. Heart Surg Forum. 2015;18:186. doi: 10.1532/hsf.1268. [DOI] [PubMed] [Google Scholar]
- 17.ROCKET AF Study Investigators. Rivaroxaban-once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in Atrial Fibrillation: rationale and design of the ROCKET AF study. Am Heart J. 2010;159:340–347. e341. doi: 10.1016/j.ahj.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 18.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 19.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 20.Böhm M, Ezekowitz MD, Connolly SJ, Eikelboom JW, Hohnloser SH, Reilly PA, Schumacher H, Brueckmann M, Schirmer SH, Kratz MT, Yusuf S, Diener HC, Hijazi Z, Wallentin L. Changes in renal function in patients with atrial fibrillation. J Am Coll Cardiol. 2015;65:2481–2493. doi: 10.1016/j.jacc.2015.03.577. [DOI] [PubMed] [Google Scholar]
- 21.Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, Kakkar AK, Bandel TJ, Beckmann H, Muehlhofer E, Misselwitz F, Geerts W. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358:2765–2775. doi: 10.1056/NEJMoa0800374. [DOI] [PubMed] [Google Scholar]
- 22.Caldeira D, Gonçalves N, Pinto FJ, Costa J, Ferreira JJ. Risk of renal failure with the non-vitamin K antagonist oral anticoagulants: systematic review and meta-analysis. Pharmacoepidemiol Drug Saf. 2015;24:757–764. doi: 10.1002/pds.3791. [DOI] [PubMed] [Google Scholar]
- 23.Brodsky S, Eikelboom J, Hebert LA. Anticoagulant-related nephropathy. J Am Soc Nephrol. 2018;29:2787–2793. doi: 10.1681/ASN.2018070741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Aquino Moura KB, Behrens PMP, Pirolli R, Sauer A, Melamed D, Veronese FV, da Silva ALFA. Anticoagulant-related nephropathy: systematic review and meta-analysis. Clin Kidney J. 2019;12:400–407. doi: 10.1093/ckj/sfy133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan YH, Yeh YH, See LC, Wang CL, Chang SH, Lee HF, Wu LS, Tu HT, Kuo CT. Acute kidney injury in asians with atrial fibrillation treated with dabigatran or warfarin. J Am Coll Cardiol. 2016;68:2272–2283. doi: 10.1016/j.jacc.2016.08.063. [DOI] [PubMed] [Google Scholar]
- 26.Hijazi Z, Hohnloser SH, Andersson U, Alexander JH, Hanna M, Keltai M, Parkhomenko A, López-Sendón JL, Lopes RD, Siegbahn A, Granger CB, Wallentin L. Efficacy and safety of apixaban compared with warfarin in patients with atrial fibrillation in relation to renal function over time: insights from the ARISTOTLE randomized clinical trial. JAMA Cardiol. 2016;1:451. doi: 10.1001/jamacardio.2016.1170. [DOI] [PubMed] [Google Scholar]


