Abstract
In December 2019, an unprecedented outbreak of pneumonia cases associated with acute respiratory distress syndrome (ARDS) first occurred in Wuhan, Hubei Province, China. The disease, later named Coronavirus disease 2019 (COVID-19) by the World Health Organization (WHO), was caused by the Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), and on January 30, 2020, the WHO declared the outbreak of COVID-19 to be a public health emergency. COVID-19 is now a global pandemic impacting more than 43,438,043 patients with 1,158,596 deaths globally as of August 26th, 2020. COVID-19 is highly contagious and has caused more deaths than SARS in 2002-2003 or the Middle East Respiratory Syndrome (MERS) in 2012-2013 combined and represents an unprecedented human affliction not seen since the influenza pandemic of 1918. COVID-19 has been associated with several cardiac complications, including hypercoagulability, acute myocardial injury and myocarditis, arrhythmias, and acute coronary syndromes. Patients with pre-existing cardiovascular disease (CVD) are at the highest risk for myocardial injury and mortality among infected patients. The mechanism by which COVID-infected patients develop cardiac complications remains unclear, though it may be mediated by increased ACE-2 gene expression. Despite initial concerns, there is no evidence that angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) therapy increases risk for myocardial injury among those infected with COVID-19. In the current report, we summarize the peer-reviewed and preprint literature on cardiovascular risks and complications associated with COVID-19, as well as provide insights into its pathogenesis and management.
Keywords: COVID-19, corona, coronavirus, SARS-CoV-2, cardiovascular risks, cardiovascular complications
Introduction
The novel coronavirus disease of 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a single-stranded enveloped RNA virus first described as a novel pneumonic disease frequently resulting in acute respiratory distress syndrome (ARDS) in Wuhan, China, on December 10, 2019 [1]. COVID-19 then spread exponentially throughout China and the rest of the world in a matter of weeks. Given the rapid spread of SARS-CoV-2 and its global consequences, the World Health Organization (WHO) declared COVID-19 as a Public Health Emergency of International Concern on January 30, 2020, and subsequently declared COVID-19 as a pandemic on March 11, 2020. The transmission capacity (especially from asymptomatic carriers), risk of developing ARDS in healthy individuals, and high mortality rate has made the prevention and treatment of COVID-19 extremely challenging. Additionally, among patients with severe COVID-19 infection, there is a significant risk for cardiovascular complications including hypercoagulability, acute myocardial injury and myocarditis, arrhythmias, and acute coronary syndromes. These complications are highly associated with in-hospital mortality. In this review, we summarize the peer-reviewed and preprint literature on cardiovascular risks and complications associated with COVID-19, as well as provide insights into its pathogenesis and management.
Pathogenesis
The pathogenesis of SARS-CoV-2 and SARS-CoV are similar in some aspects. Several studies have suggested that histopathologic features in COVID-19 deaths are similar to those seen in SARS-CoV and MERS-CoV infections [2-7]. Until comparable evidence from studies of SARS-CoV-2 is available, the pathogenesis of SARS-CoV-2 remains largely unknown. However, the experience with SARS-CoV and MERS-CoV might provide the essential clues to the pathogenesis of SARS-CoV-2. There are 4 main speculative pathogenic steps based on the sequence similarities of the receptor-binding motif between SARS-CoV-2, SARS-CoV and CoV-NL63. The first step in SARS-CoV-2 infection is likely caused by binding of the viral surface spike protein to the human angiotensin-converting enzyme-2 (ACE2) receptor in Type II alveolar cells in the lung, resulting in predominant pulmonary symptoms with complete respiratory failure in some cases [8]. The ACE2 receptor is also expressed in the coronary vascular endothelium, smooth muscle cells, cardiac fibroblasts, epicardial adipocytes, and cardiac myocytes, suggesting this pathway as the mechanism for cardiac injury. Figure 1 summarizes the pathogenesis of COVID-19-related cardiovascular disease. In animal models, acute inflammatory processes or myocardial infarction may activate ACE2 gene expression [9]. The main function of ACE2 is the degradation of angiotensin II to angiotensin 1-7. ACE2 downregulation could result in the upregulation of inflammatory cytokines, a reduction in the degradation of angiotensin II, and decreased angiotensin 1-7. This could lead to decreased activation of Mas receptors on platelets, leading to acquired platelet dysfunction and intravascular thrombosis [10-12]. Moreover, increased angiotensin II could cause myocardial hypertrophy and dysfunction, interstitial fibrosis, endothelial dysfunction, enhanced inflammation, oxidative stress [13-15]. This finding could support the theory that patients with pre-existing cardiovascular disease (CVD) who develop COVID-19 are at a higher risk of myocardial injury due to increased ACE2 gene expression [13] and in high BMI individuals who have an increased amount of epicardial adipose tissue [16].
Figure 1.
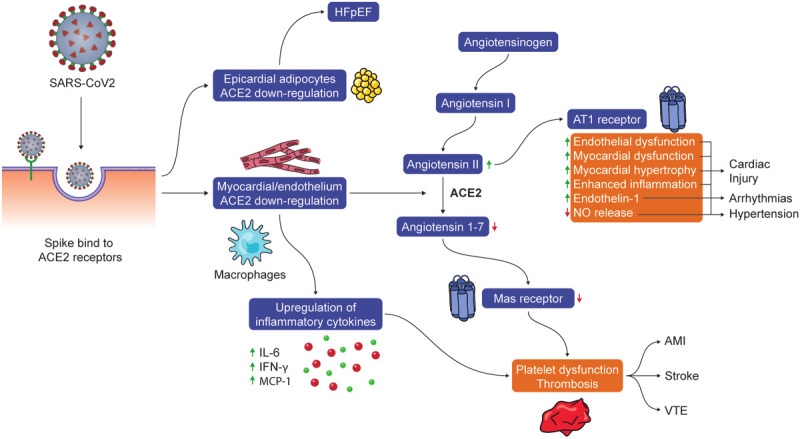
The potential pathogenesis of SARS-CoV-2 infection-related cardiovascular diseases. A spike protein of SARS-CoV-2 has high susceptibility in individuals who have increased ACE2 gene expression (e.g., pre-existing CVD), leading a downregulation of ACE2. ACE2 downregulation could result in the upregulation of inflammatory cytokines (e.g., IL-6), a reduction in the degradation of angiotensin II, and decreased angiotensin 1-7. Decreased angiotensin 1-7 could lead to subsequent decreased activation of Mas receptors on platelets which in combination of inflammatory cytokine upregulation (e.g. IL-6, IFN-, MCP-1) may result in acquired platelet dysfunction and intravascular thrombosis. Additionally, increased angiotensin II mediated AT1 receptor activation may have multiple physiologic effects including myocardial hypertrophy and dysfunction, interstitial fibrosis, endothelial dysfunction, enhanced inflammation, increased endothelin-1 expression and decreased NO release which can manifest as cardiac injury, arrhythmias and hypertension.
The second pathogenic step may involve the spike protein priming by the activation of transmembrane protease serine 2 for the SARS-CoV-2 virion to fuse between the viral and cellular membranes before entering the host cell [17]. The third step might involve the SARS-CoV-virion releasing a single-stranded RNA into the Type II alveolar cell which initiates cellular translation using the host cell ribosome. An essential product of this translation is the RNA-dependent RNA polymerase, as this is the critical machinery for RNA synthesis and viral genome replication in SARS-CoV-2.
Finally, damaged Type II alveolar cells activate macrophages which release multiple inflammatory cytokines (e.g., IL6, IFNγ), leading to T-helper-1 (Th1) and Th2 cell responses, alveolar edema, hypoxemia, and acute respiratory failure [18,19]. Theoretically, recombinant ACE2, blocking angiotensin II or ACE2 receptor, Ang 1-7 analogs, Mas receptor agonists, blocking the spike protein priming (camostat mesylate), regulating Th1 and Th2 response, blocking RNA-dependent RNA polymerase (e.g., Remdesevir), and preventing cell activation (e.g., protease inhibitors) should all be considered for targeted interventions and drug repurposing therapy. Although SARS-CoV-2 and SARS-CoV are similar, our knowledge of the pathogenesis of SARS-CoV-2 remains limited. Therefore, according to the research results of SARS-CoV, it might not be rigorous to infer the pathogenesis of SARS-CoV-2. Further research in the pathogenesis of SARS-CoV-2 is urgently needed.
Association between cardiovascular risk factors and COVID-19 infection
It is unclear whether pre-existing CVD is a risk factor for acquiring COVID-19, however some research suggests that pre-existing CV disease and risk factors for CV disease such as smoking, hypertension or diabetes can potentially upregulate ACE-2 expression [20,21]. Studies have reported that COVID-19 patients had risk factor incidence rates as follows: smoking from 10%, hypertension from 6% to 31.2%, diabetes from 10.1% to 22%, stroke from 1.4% to 22% [22-25]. Additionally, studies show that hypertension, diabetes, and smoking are associated with a higher likelihood of severe COVID-19 and adverse outcomes of COVID-19. Patients with a history of cardiovascular disease, hypertension, and diabetes are more likely to have elevated troponins than patients without these risk factors [26]. A meta-analysis of 11 studies reported a pooled odds ratio (OR) of 2.49 (95% confidence interval (CI): 1.98-3.12; I2 = 24%) for severe disease in the presence of hypertension [27]. Despite initial concerns that use of ACE inhibitors (ACEI) or angiotensin-receptor blockers (ARB) could result in severe disease due to ACE2 upregulation, based on currently available data from both experimental and clinical data, there is no evidence that ACEI and ARB therapy increases risk for myocardial injury among those infected with COVID-19 [28]. Previously, a study from Guo et al. [29] have reported outcomes associated with ACEI or ARB use and found no significant survival difference in this series. More recently, one study showed no difference in the risk of severe COVID-19 disease or mortality in COVID-19 using ACEIs or ARBs [30]. Conversely, a recent study suggested that use of ACEI or ARB may be associated a reduction in the risk of all-cause mortality [31]. Two clinical trials of losartan as additional treatment for COVID-19 are underway (e.g., NCT04311177, NCT04312009). Table 1 summarizes the cardiovascular society recommendations for COVID-19.
Table 1.
Cardiovascular society guideline for COVID-19
| Cardiovascular Society | Key Recommendations |
|---|---|
| The American College of Cardiology | ● CV-specific plans should be developed in collaboration with hospital-wide infectious disease response plans and in close collaboration with other medical specialties |
| ● Cardiovascular care team members with limited experience and/or training in personal protective equipment (PPE) donning, usage, and doffing should be trained immediately | |
| ● Clinical leadership may need to assess the risk-benefit ratio of acute MI intervention against nosocomial infection risk | |
| The American Heart Association | ● Emphasis on the need to modernize our public health efforts and engage audiences on these social media platforms |
| The European Society of Cardiology | ● It needs to be clear that “don’t come to hospital” does not apply to STEMI or other acute syndromes |
| ● Emphasis on training the staff in donning and doffing | |
| ● Staff wellbeing | |
| ● Minimize aerosolization, intubation if needed prior cath lab | |
| ● Plan for discharge medication telephone follow-up in post-PCI patients | |
| ● Continuation of NSAIDs and ACEI/ARBs for patients who are currently taking them until rigorous evidence emerges to the contrary | |
| ● Do not take chloroquine for prophylaxis | |
| The Chinese Society of Cardiology | ● All patients with severe emergent cardiovascular diseases complicated by fever should be first evaluated in the fever clinic of the local hospital, and transferred to the COVID-19-designated hospital for further treatment |
| ● It is recommended that all patients should undergo lung CT examination to evaluate for imaging features typical of COVID-19 | |
| ● An optimized medical therapy strategy should be prioritized for patients with severe emergent cardiovascular diseases who cannot be ruled out for COVID-19 | |
| ● The decision to pursue an invasive strategy for STEMI or life-threatening NSTEMI, all the following conditions should be met, 1) assuming failure of optimized, goal-directed, medical therapy; 2) taking place in a hospital designated for COVID-19; 3) cath lab with negative-pressure ventilation followed by strict peri-procedural precautions; 4) third-grade protection is adopted; 5) approval by the local health commission | |
| The ACC’s Interventional Council and SCAI | ● Patients with COVID-19 or suspected COVID-19 requiring intubation should be intubated prior to arrival to the catheterization laboratory |
| ● For known COVID-19 positive patients, restriction of cases to a dedicated laboratory may be of value | |
| ● COVID-19 and hemodynamically stable NSTEMI or type 2 MI conservative therapy should be considered | |
| The Heart Failure Society of America | ● Continuation of ACEI/ARBs for patients who are currently taking them for indications (e.g., heart failure, hypertension, or ischemic heart disease) |
| The ESC Council on Hypertension | ● There is no clinical or scientific evidence to suggest that treatment with ACEi or ARBs should be discontinued because of the COVID-19 infection |
| ● There is evidence from studies in animals suggesting that ACEI/ARBs might be rather protective against serious lung complications in patients with COVID-19 infection, but to date there is no data in humans | |
| The Canadian Cardiovascular Society | ● Continuation of ACEi, ARB, and ARNI therapy is strongly recommended in COVID-19 patients |
Cardiovascular complications associated with COVID-19
Currently, the cardiac manifestations in patients infected with COVID-19 include hypercoagulability, acute myocardial injury and myocarditis, arrhythmias, and acute coronary syndromes. Below, we further discuss each cardiovascular manifestation and summarize the currently available literature.
Hypercoagulability
Features of hypercoagulability, including disseminated intravascular coagulation (DIC) and pulmonary embolism, are highly prevalent in COVID-19. Patients with COVID-19 have been shown to have significantly higher levels of D-dimer, fibrin degradation products (FDP), and fibrinogen when compared with healthy controls [32]. DIC, which propagates multiorgan dysfunction and bleeding, has additionally been associated with thrombosis of coronary arteries, focal myocardial necrosis, and severe cardiac dysfunction [33]. Notably, a retrospective, multicentre cohort study demonstrated that D-dimer > 1 µg/mL on admission was associated with a greater odds of in-hospital death (OR: 18.4, 95% CI: 2.6 to 128.6, P = 0.003) [34]. As such, many hospitals have instituted anticoagulation protocols with some institutions investigating the use of tissue plasminogen activator (tPA).
Acute myocardial injury and myocarditis
Myocardial injury is common among patients with COVID-19 with estimates between 7.2-17% of cases, and the presence of myocardial injury correlates with disease severity. Studies have generally defined myocardial injury as the elevation of high-sensitivity cardiac troponin (hs-cTn) above the 99th percentile of its upper limit of normal or evidence of new electrocardiographic or echocardiographic abnormalities [35]. Importantly, the rate of rise and degree of elevation both carry prognostic implications. These findings suggest that cardiac troponin levels should be monitored in patients with severe disease and may portend a worsening of disease in those with mild symptoms.
Potential causes of myocardial injury include plaque rupture, coronary spasm, hypoxic injury, microthrombi, direct endothelial or vascular injury, or cytokine storm. Severe forms of myocardial injury can manifest as acute and fulminant myocarditis and portends a poor prognosis for survival. Importantly, it can develop even with mild or absent respiratory symptoms [36]. While the mechanism remains unclear, there is thought that fulminant myocarditis may be mediated by a cytokine storm caused by hyper-induction of proinflammatory cytokines, including interleukin-1, interleukin-6, T helper 1 cytokine interferon-gamma, and tumor necrosis factor-alpha [37]. These cytokines may acutely depress myocardial function through activation of the neural sphingomyelinase pathway and subacutely via nitric oxide-mediated blunting of beta-adrenergic signaling [38]. As such, similar to cardiac troponin levels, obtaining a screening echocardiogram may be an important consideration among critically ill COVID-19 patients.
Arrhythmias
Infections and systemic illnesses are associated metabolic derangements, inflammation, and activation of the sympathetic nervous system - all of which predispose to cardiac arrhythmias which include supraventricular tachycardias and atrial fibrillation, heart block, ventricular tachycardia, and ventricular fibrillation. Published studies describe a significant prevalence of cardiac arrhythmias among COVID-19 patients which increases with the degree of illness among ICU patients and those with elevated cardiac troponin levels and myocarditis [23]. Moreover, use of antibiotics and anti-inflammatory agents such as hydroxychloroquine, which have QT-prolonging properties, may further increase the predisposition for cardiac arrhythmias. Close monitoring is an important aspect of care for hospitalized COVID-19 patients. Additionally, further study regarding utility of outpatient monitors among infected patients is needed.
Acute coronary syndromes
The association of COVID-19 infection and acute coronary syndromes remains unclear. Plaque rupture and thrombus formation, resulting in both ST-elevation and non-ST-elevation myocardial infarction, has been described with severe viral infection, including SARS and influenza [39,40]. Bangalore et al. described patients with COVID-19 who presented with ST-segment elevation to have a variable presentation with high prevalence of non-obstructive disease and poor overall prognosis [41]. However, nationally, the overall number of patients presenting with STEMI has markedly decreased [42].
Differentiating between Type I and Type II myocardial infarction in this population remains difficult. The mechanism by which severe COVID-19 infection may cause Type I myocardial infarction remains poorly defined, though may be related to high pericyte expression of ACE2 levels. As such, patients may develop microvascular inflammation contributing to myocardial infarction with non-obstructive coronary arteries (MINOCA). As such, case-by-case analysis with consideration of clinical presentation, ECG, and echocardiogram can be used to determine which patients should be managed invasively versus conservatively.
Management
Patients with preexisting CVD who present with non-severe symptoms of COVID-19 should be monitored closely in an isolation room on the medical floor. Below, we further discuss management of the cardiovascular manifestations of COVID-19 and summarize the principles of treatment (Figure 2).
Figure 2.
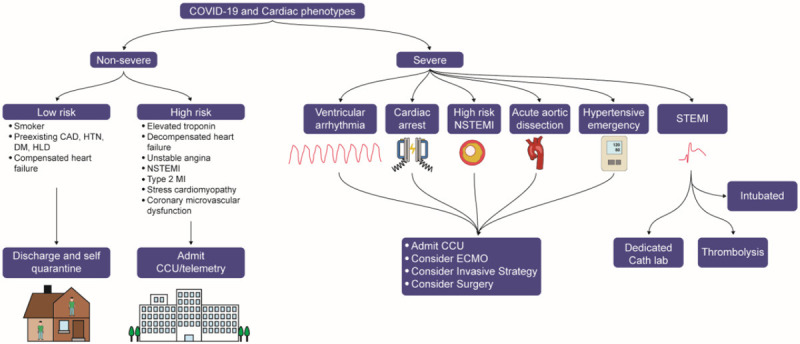
COVID-19 and Cardiac phenotypes. Patients with acute ST-elevation myocardial infarction (STEMI) requiring intubation should be intubated prior to arrival to the catheterization laboratory. If no dedicated catheterization laboratory is available, thrombolysis should be considered. Patients with severe cardiac phenotypes should be admitted to the coronary care unit (CCU) for close monitoring, and should be considered for ECMO support as well as referred to the cath lab for an invasive strategy or surgery. High risk patients with non-severe cardiac phenotypes should be hospitalized at the very least. Low risk patients with non-severe cardiac phenotypes should be discharged for self-isolation and followed up closely with telehealth platforms.
Hypercoagulability
There is a high incidence of hypercoagulable state, venous thromboembolism and both macrovascular and microvascular thrombosis in COVID-19 infected patients which has been decribed to involve multiple organs [43]. A recent study showed a 31% incidence of thrombotic complications in COVID-19 patients. A case-series of 107 COVID-19 patients showed the frequency of PE in COVID-19 period was twice as high than during the control period (patients hospitalized in the ICU during the same time interval in 2019, absolute increase risk of 14.4%, 95% CI 6.1 to 22.8%) [44]. Anticoagulation could potentially be an important component of management, particularly in severe COVID-19 patients with markedly elevated D-dimer, while non-severe COVID-19 patients would require at least DVT prophylaxis. Severe COVID-19 infection often causes development of RV failure perhaps due to progression of prothrombotic states within the pulmonary artery. A recent study showed that right ventricular longitudinal strain is a powerful predictor of higher mortality in patients with COVID-19 [45], and routine bedside echo may be needed in severe COVID patients. If RV failure occurs without evidence of PE, RV mechanical support may be needed. Venovenous extracorporeal membrane oxygenation (VV-ECMO) should be considered in severe refractory ARDS with a low cardiac risk, while venoarterial or VA-ECMO should be considered in severe refractory ARDS with RV failure, refractory ventricular arrhythmia, profound cardiac depression or cardiac arrest.
Acute myocardial injury and myocarditis
SARS-CoV2-associated immune or proinflammatory cytokines can induce myocardial inflammation, myocardial injury or myocarditis, which may cause acute heart failure and potentially lead to cardiogenic shock. In COVID-19 patients who present with acute heart failure without cardiogenic shock, current guideline-directed medical therapy for heart failure should be considered [46]. At this time, professional societies recommend continuing ACE inhibitors or ARBs [47]. A point and transthoracic echocardiogram should be considered to evaluate left ventricular function in COVID-19 patients with abnormal cardiac biomarkers. Although endomyocardial biopsy can be helpful for the definitive diagnosis of myocarditis, there remains no clear role for endomyocardial biopsy in COVID-19 patients.
In COVID-19 patients who present with cardiogenic shock, right heart catheterization should be considered to guide the use of inotropes, vasopressors or mechanical support and ECMO. The utility of temporary mechanical circulatory support, such as intra-aortic balloon pump, TandemHeart or Impella in COVID-19 patients is limited but should be considered in patients with cardiogenic shock in a case-by-case basis with consideration to age, comorbidities, and potential for recovery. VV-ECMO should be considered in COVID-19 patients who have COVID cardiomyopathy and cardiogenic shock, while VV-ECMO should be considered in those with isolated pulmonary manifestation with refractory hypoxemia despite advanced ventilator management and proning [48]. Veno-arterio-venous (VAV) or veno-veno-arterial (VVA) ECMO strategies should be considered if patients manifest with both cardiogenic shock and ARDS. Currently, a trial investigating the use and outcomes associated with ECMO in COVID-19 patients (NCT04383678) is underway.
Arrhythmias
Telemetry should be considered for those with QT prolongation obtained on initial electrocardiogram, particularly if they are placed on QT-prolonging agents for treatment. Similarly, the QTc interval should be monitored closely when using COVID related QT-prolonging agents, particularly in those who take antiarrhythmic drugs (e.g., sotalol). Patients with severe symptoms of COVID-19 should be closely monitored in an intensive care unit. Ambulatory ECG monitoring or wearable technologies should be considered as alternative options for home monitoring or telemetry in stable COVID-19 patients who have high risk of arrhythmias. Antiarrhythmic drugs (e.g., procainamide, lidocaine and mexiletine) and overdrive pacing should be considered in COVID-19 patients with tachyarrhythmias. Temporary cardiac pacing should be considered in COVID-19 patients with reversible bradyarrhythmias. Synchronized electrical cardioversion should be performed in VF and unstable VT. Amiodarone, intravenous lidocaine or esmolol should be in the event of VT storm and recurrent VF. The choice of permanent pacemaker for COVID-19 patients with bradyarrhythmias should be considered based on an individual basis. To date, no clinical evidence has suggested that COVID-19 could cause atrial fibrillation.
Acute coronary syndromes
All patients presenting with an acute coronary syndrome should be considered for possible COVID-19 infection. CT imaging of the chest may be reasonable in patients with low-to-intermediate suspicion for COVID. For patients with COVID-19 who present with unstable angina or NSTEMI, conservative therapy may be considered. Moreover, for patients who present with STEMI, fibrinolysis may be selectively considered with percutaneous coronary intervention performed as part of a rescue strategy or a pharmaco-invasive strategy. Fibrinolytic strategies may mitigate delays in reperfusion due to pending COVID-19 test results and additional time needed for the personal protection of the catheterization laboratory team. A rescue strategy can be considered once COVID-19 has been ruled out. Figure 3 summarizes the management of STEMI in COVID-19 patients. However, ST elevations in the case of COVID-19 have been described in the presence of myopericarditis. In these cases, a primary fibrinolytic strategy may not be the best strategy and may lead to harm.
Figure 3.
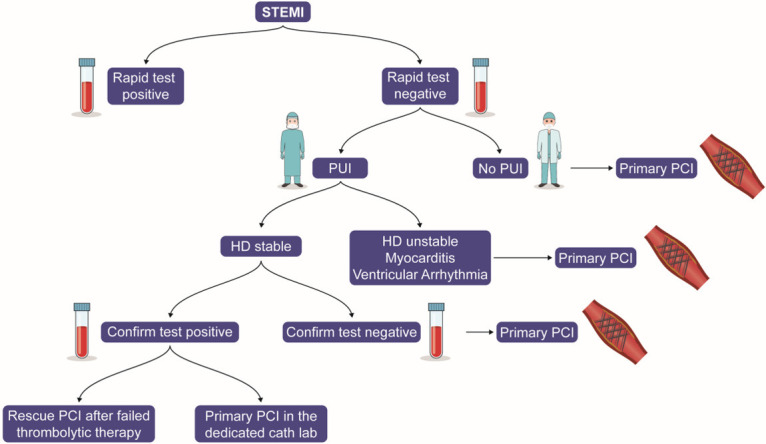
Proposed STEMI management in the COVID-19 era. Patients with acute ST-elevation myocardial infarction (STEMI) should be tested for COVID-19. If the rapid test is negative, and the patient is not a person under investigation (PUI), then primary percutaneous coronary intervention (PCI) should be considered. Primary PCI should be considered in a PUI with hemodynamic instability, myocarditis or a ventricular arrhythmia. Fibrinolytic strategy should be considered in a PUI who is hemodynamically stable. Primary PCI should be considered once COVID-19 has been ruled out. In patients with confirmed COVID-19 cath lab or rescue PCI may be needed if fibrinolytic strategy has failed.
Cardiovascular outcomes
Age, coexisting conditions, CD4 T cell count and severe pneumonia are associated with severe disease, poor prognosis and ICU admission [24]. Patients with preexisting CVD risks are associated with a higher risk of developing severe COVID-19 disease [23]. Some studies suggested that myocardial injury has a significant association with fatal outcomes of COVID-19, while the prognosis of patients with underlying CVD but without myocardial injury appears relatively favorable [23,29]. Several meta-analyses found that underlying CVD may be a risk factor severe illness and elevated troponin levels may be associated with a poor prognosis [49,50]. Preliminary data from the Mount Sinai COVID Informatics Center showed that, after adjustment for covariates, compared to patients with troponin levels in the reference range, patients with mildly elevated troponin levels (0.03-0.09 ng/dL) and moderately elevated troponin (> 0.09 ng/dL) had higher mortality rate; HR: 1.77, 95% CI 1.39-2.26 and HR: 3.23, 95% CI 2.59-4.02, respectively [26].
African American paradox
The typical biochemical profile in an African American individual with hypertension consists of a low or suppressed plasma renin activity or direct renin concentration, and suppressed angiotensin I and II activity [51]. Whether this downregulated activity of RAS extends to ACE2 is currently unknown. The reason for this is most likely multifactorial and a downregulated RAS system may simply be one pebble in the mosaic. Notably, recent data from New Orleans and Detroit have shown a devastating pulmonary outcome of COVID-19 in the African American population. However, this may well be due to the excessive prevalence of co-morbidities (e.g., hypertension, diabetes, renal disease and obesity) and lower access to care in this population.
Conclusion
The management decisions involved in COVID-19 patients with preexisting CVD should be carefully considered on a case-by-case basis. Routine bedside echo may be needed in severe COVID patients. Patients with preexisting CVD who present with either severe or non-severe symptoms of COVID-19 should be monitored closely. Mechanical support should be considered in COVID-19 patients with severe cardiac complications. Preventative strategies, such as traditional measures (e.g., regular exercise, optimal nutrition, and sleep hygiene) and social distancing should be universally advised.
Disclosure of conflict of interest
Dr. Krittanawong discloses the following relationships - Member of the American College of Cardiology Solution Set Oversight Committee, The Lancet Digital Health (Advisory Board), European Heart Journal Digital Health (Editorial board) and Journal of the American Heart Association (Editorial board). Dr. Virani discloses the following relationships: Grant from Department of Veterans Affairs, World Heart Federation, Jooma and Tahir Family. Honorarium: American College of Cardiology (Associate Editor for Innovations, acc.org). Steering Committee member: Patient and Provider Assessment of Lipid Management (PALM) registry at the Duke Clinical Research Institute (no. financial remuneration). Dr. Ballantyne discloses the following relationships: Grant/Research Support- All significant. (All paid to institution, not individual): Abbott Diagnostic, Akcea, Amgen, Esperion, Novartis, Regeneron, Roche Diagnostic, NIH, AHA, ADA. Consultant - Abbott Diagnostics, Akcea, Althera, Amarin*, Amgen, Arrowhead, Astra Zeneca, Corvidia, Denka Seiken*, Esperion, Gilead, Janssen, Matinas BioPharma Inc, New Amsterdam*, Novartis, Novo Nordisk, Pfizer, Regeneron, Roche Diagnostic, Sanofi-Synthelabo* [*Significant where noted (>$10,000); remainder modest (<$10,000)].
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martines RB, Ritter JM, Matkovic E, Gary J, Bollweg BC, Bullock H, Goldsmith CS, Silva-Flannery L, Seixas JN, Reagan-Steiner S, Uyeki T, Denison A, Bhatnagar J, Shieh WJ, Zaki SR. Pathology and pathogenesis of SARS-CoV-2 associated with fatal coronavirus disease, United States. Emerg Infect Dis. 2020;26:9. doi: 10.3201/eid2609.202095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15:700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153:725–733. doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menter T, Haslbauer JD, Nienhold R, Savic S, Deigendesch H, Frank S, Turek D, Willi N, Pargger H, Bassetti S, Leuppi JD, Cathomas G, Tolnay M, Mertz KD, Tzankov A. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77:198–209. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schröder AS, Burdelski C, de Heer G, Nierhaus A, Frings D, Pfefferle S, Becker H, Bredereke-Wiedling H, de Weerth A, Paschen HR, Sheikhzadeh-Eggers S, Stang A, Schmiedel S, Bokemeyer C, Addo MM, Aepfelbacher M, Püschel K, Kluge S. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jia HP, Look DC, Shi L, Hickey M, Pewe L, Netland J, Farzan M, Wohlford-Lenane C, Perlman S, McCray PB Jr. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J Virol. 2005;79:14614–14621. doi: 10.1128/JVI.79.23.14614-14621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burrell LM, Risvanis J, Kubota E, Dean RG, MacDonald PS, Lu S, Tikellis C, Grant SL, Lew RA, Smith AI, Cooper ME, Johnston CI. Myocardial infarction increases ACE2 expression in rat and humans. Eur Heart J. 2005;26:369–375. doi: 10.1093/eurheartj/ehi114. [DOI] [PubMed] [Google Scholar]
- 10.Fraga-Silva RA, Pinheiro SV, Goncalves AC, Alenina N, Bader M, Santos RA. The antithrombotic effect of angiotensin-(1-7) involves mas-mediated NO release from platelets. Mol Med. 2008;14:28–35. doi: 10.2119/2007-00073.Fraga-Silva. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang C, Stavrou E, Schmaier AA, Grobe N, Morris M, Chen A, Nieman MT, Adams GN, LaRusch G, Zhou Y, Bilodeau ML, Mahdi F, Warnock M, Schmaier AH. Angiotensin 1-7 and Mas decrease thrombosis in Bdkrb2-/- mice by increasing NO and prostacyclin to reduce platelet spreading and glycoprotein VI activation. Blood. 2013;121:3023–3032. doi: 10.1182/blood-2012-09-459156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraga-Silva RA, Costa-Fraga FP, De Sousa FB, Alenina N, Bader M, Sinisterra RD, Santos RA. An orally active formulation of angiotensin-(1-7) produces an antithrombotic effect. Clinics (Sao Paulo) 2011;66:837–841. doi: 10.1590/S1807-59322011000500021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuba K, Imai Y, Ohto-Nakanishi T, Penninger JM. Trilogy of ACE2: a peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol Ther. 2010;128:119–128. doi: 10.1016/j.pharmthera.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel VB, Zhong JC, Grant MB, Oudit GY. Role of the ACE2/angiotensin 1-7 axis of the renin-angiotensin system in heart failure. Circ Res. 2016;118:1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turner AJ, Hiscox JA, Hooper NM. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol Sci. 2004;25:291–294. doi: 10.1016/j.tips.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116:1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belouzard S, Chu VC, Whittaker GR. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc Natl Acad Sci U S A. 2009;106:5871–5876. doi: 10.1073/pnas.0809524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong CK, Lam CW, Wu AK, Ip WK, Lee NL, Chan IH, Lit LC, Hui DS, Chan MH, Chung SS, Sung JJ. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahallawi WH, Khabour OF, Zhang Q, Makhdoum HM, Suliman BA. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine. 2018;104:8–13. doi: 10.1016/j.cyto.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leung JM, Yang CX, Tam A, Shaipanich T, Hackett TL, Singhera GK, Dorscheid DR, Sin DD. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J. 2020;55:2000688. doi: 10.1183/13993003.00688-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uri K, Fagyas M, Kertesz A, Borbely A, Jenei C, Bene O, Csanadi Z, Paulus WJ, Edes I, Papp Z, Toth A, Lizanecz E. Circulating ACE2 activity correlates with cardiovascular disease development. J Renin Angiotensin Aldosterone Syst. 2016;17:4. doi: 10.1177/1470320316668435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang X, Yu Y, Xu J, Shu H, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krittanawong C, Virk HUH, Narasimhan B, Wang Z, Narasimhan H, Zhang HJ, Sun T, Messerli FH. Coronavirus disease 2019 (COVID-19) and cardiovascular risk: a meta-analysis. Prog Cardiovasc Dis. 2020;63:527–528. doi: 10.1016/j.pcad.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention. [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China] . Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Lala A, Johnson KW, Russak AJ, Paranjpe I, Zhao S, Manna S, Solani S, Vaid A, Chaudhry F, De Freitas JK, Fayad ZA, Pinney SP, Levin M, Charney A, Bagiella E, Narula J, Glicksberg BS, Nadkarni G, Januzzi J, Mancini DM, Fuster V. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76:533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lippi G, Wong J, Henry BM. Hypertension and its severity or mortality in coronavirus disease 2019 (COVID-19): a pooled analysis. Pol Arch Intern Med. 2020;130:304–309. doi: 10.20452/pamw.15272. [DOI] [PubMed] [Google Scholar]
- 28.Kuster GM, Pfister O, Burkard T, Zhou Q, Twerenbold R, Haaf P, Widmer AF, Osswald S. SARS-CoV2: should inhibitors of the renin-angiotensin system be withdrawn in patients with COVID-19? Eur Heart J. 2020;41:1801–1803. doi: 10.1093/eurheartj/ehaa235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1–8. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Wang X, Chen J, Zhang H, Deng A. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiol. 2020;5:1–6. doi: 10.1001/jamacardio.2020.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie J, Liu YM, Zhao YC, Huang X, Lin L, Xia M, Chen MM, Cheng X, Zhang X, Guo D, Peng Y, Ji YX, Chen J, She ZG, Wang Y, Xu Q, Tan R, Wang H, Lin J, Luo P, Fu S, Cai H, Ye P, Xiao B, Mao W, Liu L, Yan Y, Liu M, Chen M, Zhang XJ, Wang X, Touyz RM, Xia J, Zhang BH, Huang X, Yuan Y, Loomba R, Liu PP, Li H. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126:1671–1681. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han H, Yang L, Liu R, Liu F, Wu KL, Li J, Liu XH, Zhu CL. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58:1116–1120. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 33.Sugiura M, Hiraoka K, Ohkawa S, Ueda K, Matsuda T. A clinicopathological study on cardiac lesions in 64 cases of disseminated intravascular coagulation. Jpn Heart J. 1977;18:57–69. doi: 10.1536/ihj.18.57. [DOI] [PubMed] [Google Scholar]
- 34.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, Cani DS, Cerini M, Farina D, Gavazzi E, Maroldi R, Adamo M, Ammirati E, Sinagra G, Lombardi CM, Metra M. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1–6. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z, Zhang A, Wan Y, Liu X, Qiu C, Xi X, Ren Y, Wang J, Dong Y, Bao M, Li L, Zhou M, Yuan S, Sun J, Zhu Z, Chen L, Li Q, Zhang Z, Zhang X, Lu S, Doherty PC, Kedzierska K, Xu J. Early hypercytokinemia is associated with interferon-induced transmembrane protein-3 dysfunction and predictive of fatal H7N9 infection. Proc Natl Acad Sci U S A. 2014;111:769. doi: 10.1073/pnas.1321748111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mann DL. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ Res. 2015;116:1254–1268. doi: 10.1161/CIRCRESAHA.116.302317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warren-Gash C, Hayward AC, Hemingway H, Denaxas S, Thomas SL, Timmis AD, Whitaker H, Smeeth L. Influenza infection and risk of acute myocardial infarction in England and Wales: a CALIBER self-controlled case series study. J Infect Dis. 2012;206:1652–1659. doi: 10.1093/infdis/jis597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peiris JS, Chu CM, Cheng VC, Chan KS, Hung IF, Poon LL, Law KI, Tang BS, Hon TY, Chan CS, Chan KH, Ng JS, Zheng BJ, Ng WL, Lai RW, Guan Y, Yuen KY. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bangalore S, Sharma A, Slotwiner A, Yatskar L, Harari R, Shah B, Ibrahim H, Friedman GH, Thompson C, Alviar CL, Chadow HL, Fishman GI, Reynolds HR, Keller N, Hochman JS. ST-segment elevation in patients with covid-19 - a case series. N Engl J Med. 2020;382:2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia S, Albaghdadi MS, Meraj PM, Schmidt C, Garberich R, Jaffer FA, Dixon S, Rade JJ, Tannenbaum M, Chambers J, Huang PP, Henry TD. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2871–2872. doi: 10.1016/j.jacc.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang T, Chen R, Liu C, Liang W, Guan W, Tang R, Tang C, Zhang N, Zhong N, Li S. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020;7:e362–e363. doi: 10.1016/S2352-3026(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F, Jeanpierre E, Rauch A, Labreuche J, Susen S. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation. 2020;142:184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 45.Li Y, Li H, Zhu S, Xie Y, Wang B, He L, Zhang D, Zhang Y, Yuan H, Wu C, Sun W, Zhang Y, Li M, Cui L, Cai Y, Wang J, Yang Y, Lv Q, Zhang L, Xie M. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. JACC Cardiovasc Imaging. 2020 doi: 10.1016/j.jcmg.2020.04.014. S1936-878X(20)30342-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of The American College of Cardiology/American Heart Association task force on clinical practice guidelines and The Heart Failure Society of America. Circulation. 2017;136:e137–e161. doi: 10.1161/CIR.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 47.HFSA/ACC/AHA Statement Addresses Concerns Re: using RAAS Antagonists in COVID-19. https://www.acc.org/latest-in-cardiology/articles/2020/03/17/08/59/hfsa-acc-aha-statement-addresses-concerns-re-using-raas-antagonists-in-covid-19. Published March 17, 2020. Accessed July 19, 2020. [DOI] [PMC free article] [PubMed]
- 48.Fried JA, Ramasubbu K, Bhatt R, Topkara VK, Clerkin KJ, Horn E, Rabbani L, Brodie D, Jain SS, Kirtane AJ, Masoumi A, Takeda K, Kumaraiah D, Burkhoff D, Leon M, Schwartz A, Uriel N, Sayer G. The variety of cardiovascular presentations of COVID-19. Circulation. 2020;141:1930–1936. doi: 10.1161/CIRCULATIONAHA.120.047164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;63:390–391. doi: 10.1016/j.pcad.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, Ji R, Wang H, Wang Y, Zhou Y. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zilbermint M, Hannah-Shmouni F, Stratakis CA. Genetics of hypertension in African Americans and others of African descent. Int J Mol Sci. 2019;20:1081. doi: 10.3390/ijms20051081. [DOI] [PMC free article] [PubMed] [Google Scholar]


