Abstract
Cardiac disease is still the leading cause of non-pregnancy related maternal morbidity and mortality. Valvular disease is one of the most concerning cardiac conditions in pregnancy. Aortic stenosis (AS) is rare in young populations but deadly complications have been reported in pregnant women. This study is a retrospective review of data from the HCUP-NIS Database from 2002-2014. There were 1108 weighted discharges for both pregnancy and AS. The data contained ten or fewer unweighted discharges with AS in pregnancy that underwent a cardiac intervention: open heart surgery or percutaneous cardiac intervention. Patients who had at least one diagnosis for AS had a greater mean cost per discharge than the comparison groups. No deaths were identified in this group. We found a statistically significant increase in the billing codes for pulmonary hypertension and heart failure. Conditions commonly associated with AS such as atrial arrhythmias, ventricular arrhythmias, diastolic dysfunction, ischemic heart disease and stroke were poorly reported. Our study identified a low incidence of AS and its complications in pregnancy in the USA over our 13-year study period. Even though, the morbidity and mortality are low, it is important that clinicians be aware of this diagnosis due higher costs and risk of complications.
Keywords: Aortic valve stenosis, pregnancy, epidemiology, United States
Introduction
Valvular heart disease is not as common as other cardiac problems such as coronary artery disease and heart failure. There are fewer registries and trials of these abnormalities even though a change in the presentation and treatment of this condition was observed in recent years. In the early 20th century, rheumatic heart disease was the main cause of valvulopathy in patients of childbearing age and treatment typically involved cardiac surgery. Today, congenital heart diseases are more prevalent and the development of less invasive procedures and cardiac devices to treat valvular heart disease has become the norm [1].
Among pregnant women in the United States, corrected congenital and acquired valvular diseases are responsible for most of the non-pregnancy related cardiac complications during pregnancy [2]. Reduced aortic leaflet movement in aortic stenosis imposes an extra workload on the hearts of pregnant women which already have increased cardiac output due to physiological changes of normal pregnancy. This may cause severe impairment of cardiac output [3]. Aortic stenosis is commonly due to calcification and degeneration in the general population but the most common cause in pregnant women is bicuspid aortic valve stenosis [4,5].
The present study aims to provide more information about aortic stenosis in the USA looking for associations with other cardiac conditions and implications for maternal morbidity, mortality and epidemiology.
Methods
Objective
This retrospective study used the Healthcare Utilization Projects/Nationwide Inpatient Sample (HCUPS/NIS) database from 2002-2014 to evaluate the characteristics, risk factors, mortality, used treatments and trends over time of aortic stenosis in pregnant women (ASp) in the USA who required hospitalization.
HCUP/NIS database
The NIS is a database provided by the Health Care Utilization Project (HCUP) sponsored by the Agency for Healthcare Research and Quality (AHRQ). The National Inpatient Sample contains discharges from State Inpatient Databases and is designed to represent hospitals and discharges at a national level using a random sampling of discharges stratified by US census region, urban or rural location, teaching status, ownership, and size. The data collected includes demographic information and diagnostic and procedural codes related to the patient’s hospital stay. One of the major strengths of the database is its size, with information about millions of discharges each year. Limitations include the inability to assess causation between different diagnoses, and dependence on the coding used during the stay. Only diagnoses that were coded during the hospitalization are reflected in the data.
The NIS approximates a 20-percent stratified sample of discharges from U.S. community hospitals, excluding rehabilitation and long-term acute care hospitals. In our study we present the number of weighted discharges that would be the estimate for 100% of the US discharges for the given period.
Descriptive analysis was used to assess frequency of aortic stenosis in pregnancy (ASp) and patient characteristics. The proportion (95% confidence interval) of ASp, as indicated by inclusion criteria diagnosis codes, is reported for the study period, 2002-2014.
Inclusion criteria
Inclusion criteria consisted of the presence of at least one code for pregnancy and at least one code for aortic stenosis. As ICD9 has multiple codes for aortic stenosis, some of them combined with other valvulopathies, we only included the codes exclusive for aortic stenosis.
Statistical analysis
SAS 9.4 was used to perform all statistical analyses via the survey procedures, to account for discharge weights and sampling methodology of the NIS database. A threshold of α = 0.05 was used to determine statistical significance.
Descriptive tables were created for each variable with quantitative variables being represented as mean ± standard deviation and categorical variables represented as frequency (percent). Descriptive frequency tables were calculated for the variables mortality, costs, and treatments by whether or not the patient had aortic stenosis. No inference was done for these variables. Due to NIS confidentiality concerns, all cell counts that had a frequency less than 10 are represented with a dash instead. Those with no cases are represented by zero.
The prevalence of ASp was estimated together with its 95% confidence interval of the observed proportion. To assess the trend frequency over time, two two-sided Cochran-Armitage Trend tests were performed to assess whether the frequency of ASp increased or decreased over time.
A Chi-Square test was performed to assess whether the proportion of discharges that had at least one diagnosis code for ASp differed by hospital region. A Chi-Square test was also performed to assess whether the proportion of a particular co-diagnosis differed on the diagnosis of aortic stenosis. Co-diagnoses considered were heart failure, pulmonary hypertension, atrial arrhythmia, stroke, ventricular arrhythmias, syncope, diastolic disfunction and coronary artery disease. Due to the fact that four separate co-diagnoses were being tested, a Bonferroni multiplicity adjustment was implemented such that α = 0.05/4 = 0.0125 for each individual test.
Results
The data during the study period consists of 100,790,900 discharges accounting for 482,872,274 weighted discharges. The data contains 7,380,599 pregnancy discharges representing a weighted total discharge count of 35,249,339 pregnancy discharges. There were 1108 weighted discharges that have a code for both pregnancy and aortic stenosis (Figure 1). None of the patients in the ASp group died during the hospitalization. General characteristics are described in the Table 1.
Figure 1.
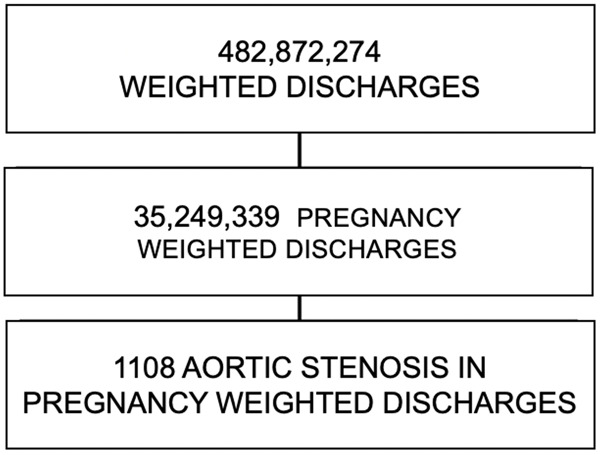
Size of ASp population.
Table 1.
General characteristics
| Characteristic | AORTIC STENOSIS GROUP | PATIENTS WITHOUT AORTIC STENOSIS |
|---|---|---|
| AGE | 27 [14-46] years old | 27 [8-57] years old |
| MEDIAN length OF STAY | 3 [0-64] days | 2 [0-365] days |
| IN HOSPITAL MORTALITY | - | |
| MEDIAN COST | 14226 (1348-228,260) USD | 9299 (25-4,490,012) USD |
| ETHNICITY | ||
| - CAUCASIAN | 65.78% | 51.10% |
| - AFRICAN AMERICAN | 7.48% | 16.75% |
| - HISPANIC | 20.32% | 22.29% |
| - ASIAN/PACIFIC | <4.3% | 4.44% |
| - NATIVE AMERICAN | 0% | 0.78% |
| - OTHER | 4.28% | 4.63% |
| MEDIAN INCOME PER ZIPCODE | ||
| - FIRST QUARTILE (LOWEST) | 23.25% | 27.4% |
| - SECOND QUARTILE (2ND LOWEST) | 26.32% | 24.98% |
| - THIRD QUARTILE (2ND HIGHER) | 26.75% | 24.18% |
| - FORTH QUARTILE (HIGHER) | 23.68% | 23.45% |
| ASP PER REGION | ||
| - NORTHEAST | 15.68% | 16.61% |
| - MIDWEST | 21.62% | 21.26% |
| - SOUTH | 30.81% | 38.43% |
| - WEST | 31.89% | 23.70% |
| PAYER | ||
| - MEDICARE | 4 (1.73%) | 0.80% |
| - MEDICAID | 99 (42.86%) | 44.82% |
| - PRIVATE INSURANCE | 114 (49.35%) | 48.00% |
| - SELF PAYER/OTHER | 14 (6.06%) | 6.16% |
| - NO CHARGE | 0 (0%) | 0.22% |
Co-occurrences (Table 2)
Table 2.
Co-occurrences
| ASp GROUP - WEIGHTED DISCHARGES (% OF TOTAL*) | CONTROL GROUP - WEIGHTED DISCHARGES (% OF TOTAL**) | ODDS ratio (95% CI) | |
|---|---|---|---|
| PULMONARY HYPERTENSION | 31 weighted discharges (2.79%) | 17110 weighted discharges (0.04%) | 59.96 (22.92-157.51) |
| ATRIAL ARRHYTHMIAS | <10 weighted discharges | 24680 weighted discharges (0.070%) | - |
| STROKE | <10 weighted discharges | 21075 weighted discharges (0.059%) | - |
| HEART FAILURE | 43 weighted discharges (3.88%) | 78018 weighted discharges (0.22%) | 18.46 (7.91-43.08) |
| DIASTOLIC DYSFUNCTION | <10 weighted discharges | 5087 weighted discharges (0.01%) | |
| ISCHEMIC HEART DISEASE | <10 weighted discharges | 10411 weighted discharges (0.03%) | |
| VENTRICULAR ARRHYTHMIAS | <10 weighted discharges | 8284 weighted discharges (0.02%) |
Number of ASp Discharges - 1108 Weighted discharges;
Number of Discharges withour AS - 35,248,230 Weighted discharges.
Thirty one weighted discharges for patients with ASp had pulmonary hypertension whereas forty three had heart failure.
The frequency of the following co-occurrences was less than or equal to 10 and therefore cannot be reported; atrial arrhythmias, stroke, diastolic dysfunction, ischemic heart disease, syncope and ventricular arrhythmias.
Figure 2 shows the proportion of discharges with ASp over time.
Figure 2.
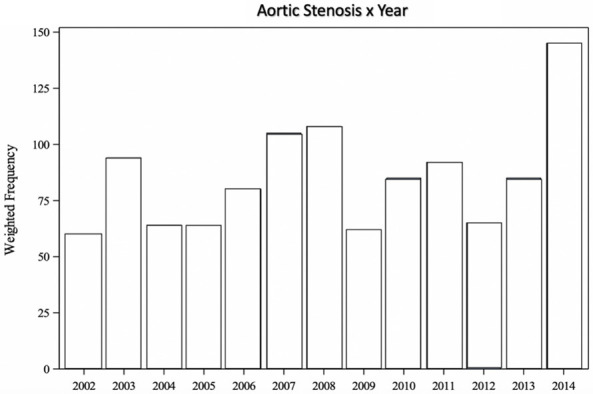
Total number of discharges with Aortic Stenosis in Pregnancy codes. A Cochran-Armitage Trend test was performed and there is sufficient evidence to conclude that the probability of having at least one diagnosis code for aortic stenosis increases over time.
Procedures
The following six ICD-9 procedures were identified in the data set: open and other replacement of aortic valve with tissue graft, open and other replacement of aortic valve, closed heart valvotomy of aortic valve, endovascular replacement of aortic valve, transapical replacement of aortic valve, and percutaneous balloon valvuloplasty. The data contained ten or fewer discharges with ASp that underwent one of the six procedures. That is, of those with ASp, fewer than 4.3% underwent one of those procedures. We also found 345 (0.0046%) weighted discharges with those procedures in pregnant patients without ASp (Table 3).
Table 3.
Procedures performed
| Procedure Performed | Weighted frequency Group with ASp |
|---|---|
| Open and other replacement of aortic valve with tissue graft ICD9-35.21 | <10 weighted discharges |
| Open and other replacement of aortic valve ICD9-35.22 | <10 weighted discharges |
| Closed heart valvotomy, aortic valve ICD9-35.01 | 0 weighted discharges |
| Endovascular replacement of aortic valve ICD9-35.05 | 0 weighted discharges |
| Transapical replacement of aortic valve ICD9-35.06 | 0 weighted discharges |
| Percutaneous balloon valvuloplasty ICD9-35.96 | <10 weighted discharges |
Discussion
NIS-HCUP is a large database with more than 480 million admissions over 13 years. Since ASp has a low prevalence in the USA, only 1,108 weighted cases were identified during the study period. Less than 0.01% of the patients with a diagnosis of pregnancy had an associated isolated aortic stenosis diagnosis. Nevertheless, we believe this number might be larger since ICD9 with ASp and other associated valvulopathies were not included in order to have a more homogenous group. The prevalence of aortic stenosis in women of childbearing age is low and most frequently associated with Bicuspid Aortic Valve. Other etiology is rheumatic fever, especially in low and middle income countries [6,7]. No large studies have been published on ASp in the USA and the incidence is not known.
Even though we cannot determine incidence and prevalence of ASp with our data, our results show there is an uptrend of hospitalizations for ASp overtime from 2002 to 2014 in the USA. As in high income countries the main etiology for ASp is bicuspid aortic valve, the reason for this uptrend is not well defined.
Aortic Stenosis reduces the flow through the aortic valve; the cardiac output is essentially limited and dependent on preload. This can become more problematic given the increased demand in pregnancy. The lower than expected cardiac output and the transmission in pressure from left ventricle to left atrium and pulmonary veins can cause the classic symptoms of heart failure, arrhythmias, chest pain and angina [2,4]. The physical exam can show the typical pulsus tardus and parvus, aortic crescendo-decrescendo systolic murmur and audible S4 [4,8].
Case reports have already shown patients with ASp due to bicuspid aortic valve presenting with cardiovascular events [9]. In our study, we found a statistically significant increase in the billing codes for pulmonary hypertension and heart failure.
Pulmonary hypertension is usually not well tolerated during pregnancy, if severe, pulmonary hypettension increases the risk of right sided heart failure, prematurity and death. In general, pregnancy should be contraindicated or terminated in case of severe pulmonary hypertension [10,11]. Even with a statistically significant increase in the number of patients with pulmonary hypertension, no patients with ASp died in our sample.
Heart failure is one of the most common presentations in patients with Asp. A similar study also showed significant risk of heart failure in patients with aortic stenosis [12]. Atrial arrhythmias, stroke, diastolic dysfunction, ischemic heart disease and ventricular arrythmias occurred; the non-weighted number is low but not zero and could not be reported.
Atrial arrhythmias, stroke, diastolic dysfunction, ischemic heart disease and ventricular arrythmias, the weighted number is low and could not be reported.
Results from a global study called Registry of Pregnancy and Cardiac Disease (ROPAC) published in 2018 registered 96 patients with moderate to severe ASp from 2008 to 2014 in an international cohort. Hospital admission happened in 35.8% of the cases. Heart failure was reported in 11.5% of the patients in this international study meanwhile we only found heart failure billing codes in fewer than 3.88% of our American population. Atrial arrhythmias, ventricular arrythmias, stroke and thrombotic complications occurred in 1%, 1%, 0% and 0% respectively in the ROPAC study, these numbers are below the range of reporting in our study for the same complications [13]. ROPAC also demonstrated increased rate of low birth weight and pre-term birth, these complications were not evaluated in our study.
Guidelines from AHA/ACC in 2014 recommend pre-conception counselling for patients with prior diagnosis of aortic stenosis, and interventions before pregnancy when indicated. Patients should be followed in a tertiary center with a dedicated heart valve team consisting of anesthesiologists, obstetricians, cardiologists and cardiovascular surgeons. There is no medication specifically recommended for patients with severe aortic stenosis [14-18].
Valve interventions only should be done in patients with severe aortic stenosis if there is hemodynamic deterioration or NYHA class III or IV. Case reports have shown successful percutaneous aortic balloon dilatation even after 20 weeks, but aortic valve replacement might be considered in selected cases [14,19,20]. In our study open valve replacement billing codes and percutaneous balloon valvuloplasty were observed in less than 10 patients with Asp.
Studies with hospital costs of ASp are scarce, the mean hospital cost in our study was $14,226 US dollars, 52% more expensive than the group without aortic stenosis over the similar length of stay [21]. The need of monitoring and complementary exams, even with no increased cardiac complications, may explain the high cost of the hospitalizations when compared to pregnant patients without ASp. Considering these costs reflect only the hospital stay and those pregnant patients likely need individualized antenatal care in reference centers, the difference might be even higher.
Even though, historic series have shown increased mortality rates in patients with ASp, recent studies had no deaths reported [12,13]. In our study, the number of deaths was zero which correlates with the literature findings.
Limitations
NIS-HCUP database does not provide individual patient information so we have to rely on ICD9 codes added during the hospitalization. The ICD9 may be nonspecific in many medical conditions. Our study was limited by:
- Multiple obstetrical ICD9 codes had to be used to determine whether or not the hospital stay was pregnancy related.
- ICD9 does not have specific codes for different severities therefore we cannot determine the severity of the aortic stenosis in our population.
- ICD9 has codes with aortic stenosis combined with other valvular pathologies such as aortic stenosis and mitral stenosis such as 396.0, 396.2, 396.8 and 396.9. As we cannot determine severity only based on the ICD9 codes and combined valvulopathies can be completely different than isolated aortic stenosis, we decided to not include those codes to have a more homogenous group. Due to this choice, we might have missed many patients with severe ASp as we identified aortic stenosis procedures done in the group without ASp.
- To protect the privacy of patients the NIS database does not allow detection of cells with fewer than ten patients. With low numbers of ASp patients we cannot make definitive conclusions about several outcomes. Zeroes can be reported and specifically, no deaths were detected by our study. However with regard to other complications of ASp up to 10 events could occur before our study would detect them.
Conclusions
This study provides additional information about demographics of patients with ASp in the USA. Even though ASp is taken as a high risk maternal condition, in our study we found a low mortality and low cardiac morbidity. If diagnosed during pregnancy aortic stenosis should be treated in a tertiary care center where the described cardiac interventions can be performed but are rarely indicated.
Disclosure of conflict of interest
None.
References
- 1.Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Barwolf C, Levang OW, Tornos P, Vanoverschelde JL, Vermeer F, Boersma E, Ravaud P, Vahanian A. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on valvular heart disease. Eur Heart J. 2003;24:1231–1243. doi: 10.1016/s0195-668x(03)00201-x. [DOI] [PubMed] [Google Scholar]
- 2.Pessel C, Bonanno C. Valve disease in pregnancy. Semin Perinatol. 2014;38:273–284. doi: 10.1053/j.semperi.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 3.Dawson J, Rodriguez Y, De Marchena E, Alfonso CE. Aortic balloon valvuloplasty in pregnancy for symptomatic severe aortic stenosis. Int J Cardiol. 2012;162:12–13. doi: 10.1016/j.ijcard.2012.04.143. [DOI] [PubMed] [Google Scholar]
- 4.Maganti K, Rigolin VH, Sarano ME, Bonow RO. Valvular heart disease: diagnosis and management. Mayo Clin Proc. 2010;85:483–500. doi: 10.4065/mcp.2009.0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silversides CK, Colman JM, Sermer M, Farine D, Siu SC. Early and intermediate-term outcomes of pregnancy with congenital aortic stenosis. Am J Cardiol. 2003;91:1386–1389. doi: 10.1016/s0002-9149(03)00340-0. [DOI] [PubMed] [Google Scholar]
- 6.Windram JD, Colman JM, Wald RM, Udell JA, Siu SC, Silversides CK. Valvular heart disease in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2014;28:507–518. doi: 10.1016/j.bpobgyn.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Crousillat DR, Wood MJ. Valvular heart disease and heart failure in women. Heart Fail Clin. 2019;15:77–85. doi: 10.1016/j.hfc.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Emmanuel Y, Thorne SA. Heart disease in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2015;29:579–597. doi: 10.1016/j.bpobgyn.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Nwabuobi C, McDowell M, Običan S. Severe bicuspid aortic stenosis in pregnancy: balancing the risk of prematurity and maternal mortality. Cardiol Young. 2018;28:756–758. doi: 10.1017/S104795111800001X. [DOI] [PubMed] [Google Scholar]
- 10.Olsson KM, Channick R. Pregnancy in pulmonary arterial hypertension. Eur Respir Rev. 2016;25:431–437. doi: 10.1183/16000617.0079-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dolgun ZN, Inan C, Sayin NC. Maternal and fetal outcomes in pregnancies with pulmonary hypertension: experience of a tertiary center. Taiwan J Obstet Gynecol. 2018;57:13–17. doi: 10.1016/j.tjog.2017.10.032. [DOI] [PubMed] [Google Scholar]
- 12.Hameed A, Karaalp IS, Tummala PP, Wani OR, Canetti M, Akhter MW, Goodwin I, Zapadinsky N, Elkayam U. The effect of valvular heart disease on maternal and fetal outcome of pregnancy. J Am Coll Cardiol. 2001;37:893–899. doi: 10.1016/s0735-1097(00)01198-0. [DOI] [PubMed] [Google Scholar]
- 13.Orwat S, Diller GP, van Hagen IM, Schmidt R, Tobler D, Greutmann M, Jonkaitiene R, Elnagar A, Johnson MR, Hall R, Roos-Hesselink JW, Baumgartner H. Risk of pregnancy in moderate and severe aortic stenosis: from the multinational ROPAC registry. J Am Coll Cardiol. 2016;68:1727–1737. doi: 10.1016/j.jacc.2016.07.750. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, O’Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM 3rd, Thomas JD, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis R, Creager MA, Curtis LH, Demets D, Guyton RA, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Selke FW, Shen WK, Stevenson WG, Yancy CW. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol. 2014;63:57–185. doi: 10.1016/j.jacc.2014.02.536. [DOI] [PubMed] [Google Scholar]
- 15.Bonnet V, Simonet T, Labombarda F, Dolley P, Milliez P, Dreyfus M, Hanouz JL. Neonatal and maternal outcomes of pregnancy with maternal cardiac disease (the NORMANDY study): years 2000-2014. Anaesth Crit Care Pain Med. 2018;37:61–65. doi: 10.1016/j.accpm.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Lau E, DeFaria Yeh D. Management of high risk cardiac conditions in pregnancy: anticoagulation, severe stenotic valvular disease and cardiomyopathy. Trends Cardiovasc Med. 2019;29:155–161. doi: 10.1016/j.tcm.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Dahl JS, Baris L, Carter-Storch R, Hall R. Aortic stenosis: what risks do the stresses of noncardiac surgery or pregnancy pose and how should they be managed? Cardiol Clin. 2020;38:139–148. doi: 10.1016/j.ccl.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Clapp MA, Bernstein SN. Preconception counseling for women with cardiac disease. Curr Trear Options Cardio Med. 2017;19:67. doi: 10.1007/s11936-017-0565-z. [DOI] [PubMed] [Google Scholar]
- 19.Yates MT, Soppa G, Smelt J, Fletcher N, van Besouw JP, Thilaganathan B, Jahangiri M. Perioperative management and outcomes of aortic surgery during pregnancy. J Thorac Cardiovasc Surg. 2015;149:607–610. doi: 10.1016/j.jtcvs.2014.10.038. [DOI] [PubMed] [Google Scholar]
- 20.Hodson R, Kirker E, Swanson J, Walsh C, Korngold EC, Ramelli S. Transcatheter aortic valve replacement during pregnancy. Circ Cardiovasc Interv. 2016;9:1–8. doi: 10.1161/CIRCINTERVENTIONS.116.004006. [DOI] [PubMed] [Google Scholar]
- 21.Torio CM, Moore BJ. Statistical brief #204 national inpatient hospital costs: the most expensive conditions by payer, 2013. Hcup. 2016;204:1–15. [PubMed] [Google Scholar]


