Abstract
Background: Non-alcoholic steatohepatitis (NASH) is well recognized as an important cardiovascular disease (CVD) risk factor. However, not many studies have described the prevalence of traditional CVD risk factors and CV diseases, and respective sex differences, in patients with NASH. Methods: In this retrospective observational cohort study using the 2016 US National Readmissions Database, we studied adults with NASH to identify the prevalence of important CVD risk factors like age, obesity, hypertension, diabetes mellitus, renal failure, dyslipidemia, smoking, and drug abuse and cardiovascular diseases like coronary artery disease, myocardial infarction and coronary revascularization, peripheral vascular disease, cerebrovascular disease, and pulmonary vascular disease. We studied sex differences using the Pearson χ2 test for categorical variables, student t-test for continuous variables, and logistic regression for age-adjusted analysis. Results: Our study sample included 41,005 patients with NASH of which 15,758 (38.4%) were male and 25,247 (61.6%) were female. Hypertension was the most prevalent CVD risk factor (68%), followed by diabetes mellitus (62%), obesity (40%), dyslipidemia (37%), smoking (30%), and renal failure in 27%. Of the cardiovascular diseases studied, coronary artery disease (20.6%) and heart failure (19.6%) were highly prevalent followed by peripheral vascular disease (5.7%), stroke (4.7%), and pulmonary vascular disease (1.4%). Men had higher rates of most risk factors and diseases compared with women, except for higher rates of obesity and diabetes mellitus in women and a similar rate of drug abuse, stroke, and pulmonary vascular disease between the sex groups. Conclusion: In patients with NASH, CVD risk factors and diseases were highly prevalent and important sex differences were present.
Keywords: NASH, cardiovascular disease, NAFLD, fatty liver, sex differences
Introduction
Non-alcoholic steatohepatitis (NASH) is a pre-cirrhotic manifestation of non-alcoholic fatty liver disease and the spectrum of fatty liver disease is well recognized as an important cardiovascular disease (CVD) risk factor [1,2]. Mechanisms by which fatty metabolic liver disease increases CVD risk include systemic inflammation, endothelial dysfunction, hepatic insulin resistance, oxidative stress, and altered lipid metabolism, which constitute important pathophysiologic changes associated with metabolic syndrome [3,4]. Several studies have evaluated the elevated risk for CVD in patients with fatty liver disease and the health care impact of the coexistence of these conditions [5-7]. In addition to metabolic risk, other CVD risk factors contribute to additional risk in patients with NASH. However, not many studies have described the prevalence of traditional CVD risk factors in patients with NASH.
In addition, certain sex differences were described in the pathogenesis of NASH [1]. Several sex differences in CVD risk factors and manifestations were described in existing literature. However, studies have not evaluated the sex differences in CVD risk factors and disease prevalence in patients with NASH. To overcome these limitations, we aimed to evaluate the prevalence of CVD risk factors and cardiovascular diseases in patients with NASH and to study the respective sex differences.
Materials and methods
Study design and data source
For this retrospective observational cohort study, data were obtained from the publicly available 2016 US National Readmissions Database (NRD). The NRD is part of the Healthcare Cost and Utilization Project (HCUP) that is sponsored by the Agency for Healthcare Research and Quality (AHRQ) and is a database of all-payer hospital inpatient stays [8].
Patient sample and study outcomes
In this study, we included all adult patients (≥ 18 years of age) from the 2016 US NRD with a diagnosis of NASH (ICD 10 code K75.81). In this sample, we identified important CVD risk factors like age, obesity, hypertension, diabetes mellitus, renal failure, dyslipidemia, smoking, and drug abuse. Study outcomes also included analyzing the prevalence of important cardiovascular diseases like coronary artery disease, myocardial infarction and coronary revascularization, peripheral vascular disease, cerebrovascular disease, and pulmonary vascular disease. These risk factors and diseases were identified using appropriate International Classification of Diseases, Ninth Edition, Clinical Modification (ICD-10-CM) diagnosis codes.
Ethical approval
This study utilized an ede-identified database and is exempt from patient consent requirement and ethical approval.
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics 25.0 (IBM Corp., Armonk, NY, USA). Weighted data were used for all statistical analyses. Categorical variables are expressed as percentages and continuous variables as mean ± standard deviation (SD). Sex differences were analyzed using the Pearson χ2 test for categorical variables and student t-test for continuous variables. Age adjusted sex differences for CVD risk factors and diseases were analyzed using the logistic regression analysis by using age and NASH status as covariates. Results of logistic regression were presented as odds ratio (CI) and 95% confidence intervals (CI). Two-sided P values with a threshold of < 0.05 were reported for all statistical analyses.
Results
Prevalence of cardiovascular risk factors and diseases
Our study sample included 41,005 adult patients with NASH of which 15,758 (38.4%) were male and 25,247 (61.6%) were female. Mean age of the study sample was 61.5±13.3 years. Hypertension (68%) was the most prevalent CVD risk factor (present in 2 out of 3 patients), followed by diabetes mellitus in 62%, obesity in 40%, dyslipidemia in 37%, smoking in 30%, and renal failure in 27%. Of the cardiovascular diseases studied, coronary artery disease (21%) and heart failure (20%) were highly prevalent with almost 1 in 5 patients having these conditions, followed by peripheral vascular disease (6%) and stroke (5%) in roughly 1 in 20 patients; Tables 1 and 2.
Table 1.
Prevalence of cardiovascular risk factors and sex differences in patients with non-alcoholic steatohepatitis
| Cardiovascular Risk Factor | Prevalence | Male | Female | P value | Age adjusted OR (95% CI) for females compared with males | P value |
|---|---|---|---|---|---|---|
| (N = 41,005) (%) | (N = 15,758) (%) | (N = 25,247) (%) | ||||
| Age | 61.5±13.3 | 60.6±13.3 | 62.0±13.2 | < 0.001 | NA | NA |
| Diabetes mellitus | 62.4 | 61.6 | 62.9 | 0.007 | 1.03 (0.99-1.07) | 0.200 |
| Obesity | 39.6 | 38.1 | 40.5 | < 0.001 | 1.16 (1.11-1.21) | < 0.001 |
| Renal failure | 26.9 | 28.3 | 26.0 | < 0.001 | 0.83 (0.80-0.87) | < 0.001 |
| Dyslipidemia | 37.4 | 40.6 | 35.4 | < 0.001 | 0.78 (0.75-0.81) | < 0.001 |
| Hypertension | 67.1 | 68.7 | 66.0 | < 0.001 | 0.84 (0.81-0.88) | < 0.001 |
| Smoking | 29.7 | 34.5 | 26.7 | < 0.001 | 0.70 (0.67-0.73) | < 0.001 |
| Drug abuse | 2.6 | 2.6 | 2.5 | 0.899 | 1.08 (0.95-1.22) | 0.263 |
Table 2.
Prevalence of cardiovascular diseases and sex differences in patients with non-alcoholic steatohepatitis
| Cardiovascular Disease | Prevalence | Male | Female | P value | Age adjusted OR (95% CI) for females compared with males | P value |
|---|---|---|---|---|---|---|
| (N = 41,005) (%) | (N = 15,758) (%) | (N = 25,247) (%) | ||||
| Coronary artery disease | 20.6 | 27.4 | 16.3 | < 0.001 | 0.46 (0.44-0.49) | < 0.001 |
| Heart failure | 19.6 | 20.4 | 19.0 | < 0.001 | 0.86 (0.82-0.90) | < 0.001 |
| Peripheral vascular disease | 5.7 | 6.8 | 5.0 | < 0.001 | 0.69 (0.63-0.65) | < 0.001 |
| Coronary artery stenting | 0.6 | 0.9 | 0.4 | < 0.001 | 0.43 (0.33-0.56) | < 0.001 |
| Coronary artery bypass grafting | 4.3 | 6.9 | 2.6 | < 0.001 | 0.32 (0.29-0.36) | < 0.001 |
| Myocardial infarction | 5.1 | 7.0 | 3.9 | < 0.001 | 0.52 (0.41-0.56) | < 0.001 |
| History of stroke | 4.7 | 4.6 | 4.7 | 0.579 | 0.99 (0.90-1.08) | 0.755 |
| Pulmonary vascular disease | 1.4 | 1.5 | 1.3 | 0.107 | 0.86 (0.74-1.04) | 0.122 |
Sex differences
Females (mean age 62.0±13.2 years) were older than males (mean age 60.6±13.3 years; P < 0.001) in this study. Females had a lower prevalence of dyslipidemia (35.4% vs 40.6%; P < 0.001), renal failure (26.0% vs 28.3%; P < 0.001), hypertension (66.0% vs 68.7%; P < 0.001), and smoking (26.7% vs 34.5%; P < 0.001) than their male counterparts but had had higher rates of diabetes mellitus (62.9% vs 61.6%; P = 0.007) and obesity (40.5% vs 38.1%; P < 0.001). After age adjustment, results remained consistent except for a similar rate of age adjusted prevalence of diabetes mellitus (adjusted odds ratio for females 1.03, 95% CI 0.99-1.07; P = 0.2) between males and females; Table 1; Figure 1.
Figure 1.
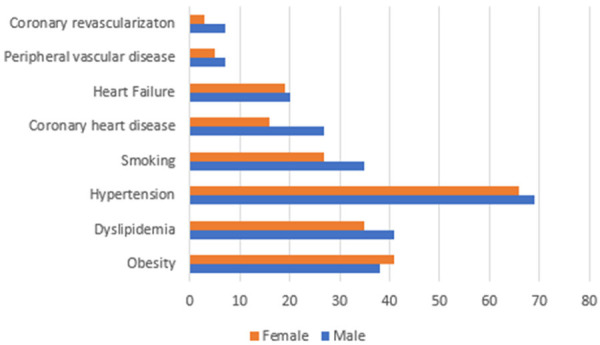
Sex differences in cardiovascular risk factors and disease in non-alcoholic steatohepatitis patients. Unique sex differences in the prevalence of important cardiovascular risk factors and cardiovascular diseases were observed in patients with non-alcoholic steatohepatitis. Males had higher rates of atherosclerotic cardiovascular disease including a higher rate of coronary artery disease (27.4% vs 16.3%; P < 0.001), coronary artery stenting (0.9% vs 0.4%; P < 0.001), coronary artery bypass surgery (6.9% vs 2.6%; P < 0.001), heart failure (20.4% vs 19.0%; P < 0.001), and peripheral vascular disease (6.8% vs 5.0%; P < 0.001). Females had a lower prevalence of dyslipidemia (35.4% vs 40.6%; P < 0.001), hypertension (66.0% vs 68.7%; P < 0.001), and smoking (26.7% vs 34.5%; P < 0.001), but had higher rates of obesity (40.5% vs 38.1%; P < 0.001).
Males had higher rates of all the CVDs evaluated than females except for a similar prevalence of stroke (4.6% vs 4.7%; P = 0.579) and pulmonary vascular disease (1.5% vs 1.3%; P = 0.107) between the sex groups. Males had a higher rate of coronary artery disease (27.4% vs 16.3%; P < 0.001), myocardial infarction (7.0% vs 3.9%; P < 0.001), coronary artery stenting (0.9% vs 0.4%; P < 0.001), coronary artery bypass surgery (6.9% vs 2.6%; P < 0.001), heart failure (20.4% vs 19.0%; P < 0.001), and peripheral vascular disease (6.8% vs 5.0%; P < 0.001) than females. Results were similar after age adjustment; Table 2. After age adjustment, compared to males, females had 54% lower odds of coronary artery disease, 48% lower odds of myocardial infarction, 57% lower odds of coronary artery stenting, 68% lower odds of coronary artery bypass surgery, 14% lower odds of heart failure, and 31% lower odds of peripheral vascular disease.
Discussion
Our study evaluated CVD prevalence and sex differences in the largest number of patients with NASH studied till date and presents important findings regarding the cardiovascular risk in these patients. Despite a relatively younger age of our study cohort (average 61 years), a substantial proportion of clinical CVD were already diagnosed in patients with NASH. These findings carry clinical relevance and future studies should evaluate the significance of these differences in the context of sex differences in NASH pathophysiology.
Nonalcoholic fatty liver disease is now recognized as an important CVD risk factor as these conditions share a common metabolic pathophysiology [3,9]. The prognostic significance of this association with regards to the development of clinical CVD events including myocardial infarction, stroke, and thromboembolism were previously reported [5,10-15]. However, data regarding the actual prevalence of clinical CVD in these high risk patients were not extensively reported. Our study fills this important gap in the literature.
Data regarding CVD risk factor prevalence is very important to plan primary and secondary preventive strategies [16,17]. It is on particular important in patients with NASH because in a large study, CVD risk factors were exclusively reported to be responsible for the increased CVD risk in patients with non-alcoholic liver disease [13]. After adjusting for these risk factors, fatty liver disease was not associated with increased CVD risk in this study [13,18]. However, as seen in our study, the burden of cardiovascular risk factors is very high in patients with NASH and early identification and management are keys to prevent CVD incidence [19,20]. It is also important for cardiologists to assess for fatty liver changes in patients with CVD to ensure co-management and to optimize disease outcomes.
Important strengths of this study are the sample size and the utilization of a well validated national database. Limitations include the administrative nature of the database which limits accurate clinical quantification of the CVDs and associated risk factors. Under-diagnosis and misclassification bias from coding inconsistencies across hospitals should be considered when interpreting the findings of this study.
Conclusion
In this study of a large sample of patients with NASH, we identified a very high prevalence of important CVD risk factors and diseases. Importantly, sex differences were present in the prevalence of these risk factors and the various diseases evaluated. Future studies should evaluate the significance of these differences in the context of sex differences in NASH pathophysiology.
Disclosure of conflict of interest
None.
References
- 1.Ballestri S, Nascimbeni F, Baldelli E, Marrazzo A, Romagnoli D, Lonardo A. NAFLD as a sexual dimorphic disease: role of gender and reproductive status in the development and progression of nonalcoholic fatty liver disease and inherent cardiovascular risk. Adv Ther. 2017;34:1291–1326. doi: 10.1007/s12325-017-0556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El Hadi H, Di Vincenzo A, Vettor R, Rossato M. Cardio-metabolic disorders in non-alcoholic fatty liver disease. Int J Mol Sci. 2019;20:2215. doi: 10.3390/ijms20092215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stahl EP, Dhindsa DS, Lee SK, Sandesara PB, Chalasani NP, Sperling LS. Nonalcoholic fatty liver disease and the heart: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:948–963. doi: 10.1016/j.jacc.2018.11.050. [DOI] [PubMed] [Google Scholar]
- 4.Francque SM, van der Graaff D, Kwanten WJ. Non-alcoholic fatty liver disease and cardiovascular risk: pathophysiological mechanisms and implications. J Hepatol. 2016;65:425–443. doi: 10.1016/j.jhep.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65:589–600. doi: 10.1016/j.jhep.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Cai J, Zhang XJ, Ji YX, Zhang P, She ZG, Li H. Nonalcoholic fatty liver disease pandemic fuels the upsurge in cardiovascular diseases. Circ Res. 2020;126:679–704. doi: 10.1161/CIRCRESAHA.119.316337. [DOI] [PubMed] [Google Scholar]
- 7.Sayiner M, Otgonsuren M, Cable R, Younossi I, Afendy M, Golabi P, Henry L, Younossi ZM. Variables associated with inpatient and outpatient resource utilization among medicare beneficiaries with nonalcoholic fatty liver disease with or without cirrhosis. J Clin Gastroenterol. 2017;51:254–260. doi: 10.1097/MCG.0000000000000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.NRD Overview. Accessed March 21, 2019. [Google Scholar]
- 9.Ismaiel A, Dumitraşcu DL. Cardiovascular risk in fatty liver disease: the liver-heart axis-literature review. Front Med. 2019;6:202. doi: 10.3389/fmed.2019.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stine JG, Niccum BA, Zimmet AN, Intagliata N, Caldwell SH, Argo CK, Northhup PG. Increased risk of venous thromboembolism in hospitalized patients with cirrhosis due to non-alcoholic steatohepatitis. Clin Transl Gastroenterol. 2018;9:140. doi: 10.1038/s41424-018-0002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdeldyem SM, Goda T, Khodeir SA, Abou Saif S, Abd-Elsalam S. Nonalcoholic fatty liver disease in patients with acute ischemic stroke is associated with more severe stroke and worse outcome. J Clin Lipidol. 2017;11:915–919. doi: 10.1016/j.jacl.2017.04.115. [DOI] [PubMed] [Google Scholar]
- 12.Kim SU, Song D, Heo JH, Yoo J, Kim BK, Park JY, Kim DY, Ahn SH, Kim KJ, Han KH, Kim YD. Liver fibrosis assessed with transient elastography is an independent risk factor for ischemic stroke. Atherosclerosis. 2017;260:156–162. doi: 10.1016/j.atherosclerosis.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Alexander M, Loomis AK, van der Lei J, Duarte-Salles T, Prieto-Alhambra D, Ansell D, Pasqua A, Lapi F, Rijnbeek P, Mosseveld M, Avillach P, Egger P, Dhalwani NN, Kendrick S, Celis-Morales C, Waterworth DM, Alazawi W, Sattar N. Non-alcoholic fatty liver disease and risk of incident acute myocardial infarction and stroke: findings from matched cohort study of 18 million European adults. BMJ. 2019;367:l5367. doi: 10.1136/bmj.l5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahfood Haddad T, Hamdeh S, Kanmanthareddy A, Alla VM. Nonalcoholic fatty liver disease and the risk of clinical cardiovascular events: a systematic review and meta-analysis. Diabetes Metab Syndr. 2017;11(Suppl 1):S209–S216. doi: 10.1016/j.dsx.2016.12.033. [DOI] [PubMed] [Google Scholar]
- 15.Wong CR, Lim JK. The association between nonalcoholic fatty liver disease and cardiovascular disease outcomes. Clin Liver Dis. 2018;12:39–44. doi: 10.1002/cld.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yandrapalli S, Nabors C, Goyal A, Aronow WS, Frishman WH. Modifiable risk factors in young adults with first myocardial infarction. J Am Coll Cardiol. 2019;73:573–584. doi: 10.1016/j.jacc.2018.10.084. [DOI] [PubMed] [Google Scholar]
- 17.Tana C, Ballestri S, Ricci F, Di Vincenzo A, Ticinesi A, Gallina S, Giamberardino MA, Cipollone F, Sutton R, Vettor R, Fedorowski A, Meschi T. Cardiovascular risk in non-alcoholic fatty liver disease: mechanisms and therapeutic implications. Int J Environ Res Public Health. 2019;16:3104. doi: 10.3390/ijerph16173104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santos RD, Valenti L, Romeo S. Does nonalcoholic fatty liver disease cause cardiovascular disease? Current knowledge and gaps. Atherosclerosis. 2019;282:110–120. doi: 10.1016/j.atherosclerosis.2019.01.029. [DOI] [PubMed] [Google Scholar]
- 19.Misra VL, Khashab M, Chalasani N. Non-alcoholic fatty liver disease and cardiovascular risk. Curr Gastroenterol Rep. 2009;11:50–55. doi: 10.1007/s11894-009-0008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patil R, Sood GK. Non-alcoholic fatty liver disease and cardiovascular risk. World J Gastrointest Pathophysiol. 2017;8:51–58. doi: 10.4291/wjgp.v8.i2.51. [DOI] [PMC free article] [PubMed] [Google Scholar]


