Abstract
COVID-19, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), while having lung injury as its most prominent feature, has been increasingly shown to affect endothelial cell function and the microvasculature. In this report, a woman with COVID-19, cardiac valve disease and spherocytosis was assessed with laser Doppler perfusion monitoring. Systemic microvascular reactivity was impaired during a worsening phase of COVID-19, but improved after clinical recovery; microcirculatory dysfunction paralleled systemic inflammation and pulmonary involvement. The assessment of systemic microcirculatory function may therefore provide insights on COVID-19 pathophysiology.
Keywords: COVID-19, laser Doppler perfusion imaging, endothelial dysfunction, microvascular reactivity, cardiac valve disease
Introduction
COVID-19 is the disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), characterized by a flu-like illness, evolving to a severe systemic inflammatory syndrome in 5 to 15% of those affected [1-3]. Lung injury with hypoxemia is the most prominent feature, and may lead to the need for supplementary oxygen or even mechanical ventilation. Mortality is higher among those over 60 and those with diabetes, hypertension, cardiovascular disease, chronic kidney disease, chronic pulmonary disease, liver disease and obesity [1,2]. Pathophysiology is not fully understood but endothelial cell dysfunction plays a major role [4], and few treatment resources have proven effective [5,6]. Steroids have recently been shown to be beneficial in those with moderate to severe forms of the disease [7]. Herein we describe a case of severe microcirculatory dysfunction in a patient with valvular heart disease and COVID-19.
Case report
A 32-year-old woman with a history of rheumatic valve disease, metallic prosthetic valves in the mitral and aortic positions (three prior valve replacement surgeries), a cardiac pacemaker implant for complete heart block, and hereditary spherocytosis, was evaluated at the Cardiology outpatient clinic due to mild shortness of breath, fever (38.5°C), rhinorrhea and dry cough in the previous five days. Her ECG showed pacemaker rhythm, heart rate of 80 bpm, with left bundle branch block pattern and a ventricular spike. The corrected QT interval was of 430 msec. The echocardiogram showed left atrial enlargement, mild systolic left ventricular dysfunction (left ventricular ejection fraction of 48% by the Simpson method), and normally functioning mitral and aortic prostheses. After a chest CT (Figure 1A) was obtainedand a nasopharyngeal swab for RT-PCR of SARS-Cov2 was collected, oral azythromicin and amoxycilin + clavulanate were started, and the patient was discharged, with instructions for clinical monitoring.
Figure 1.

Chest CT images performed on three different moments of clinical evolution. A: Outpatient study, showing bilateral, rounded foci of consolidation and ground-glass images, in <50% of the lung fields. B: At admission, showing bilateral consolidations and ground-glass images, occupying >50% of the lung fields. C: On the worst day after admission, with an increase of the ground-glass opacities, which became confluent and associated with interlobular septal thickening and bilateral consolidation. Arrows point out some of the pulmonary lesions of the patient.
After three days (on day 8 after the beginning of symptoms), she returned for emergency evaluation due to bloody sputum, diarrhea and increased dyspnea. The nasopharyngeal swab was positive and COVID-19 was diagnosed. Chest CT (Figure 1B) showed a worsening pattern of viral pneumonia, and C-reactive protein (CRP) was 29 mg/dL. The patient was admitted and amoxycilin + clavulanate was changed to ceftriaxone. Azythromicin was maintained, and oseltamivir was added to the therapeutic regimen. Warfarin (used chronically) was changed to full-dose IV heparine, and oxygen therapy was delivered by face mask.
On the following four days, the patient required increasing oxygen concentrations, and had persisting fever and worsening dyspnea, with tachypnea (44 breaths/min). CRP was elevated (24.2 mg/dL), as well as ferritin (1,930 µg/L), and d-dimer (154,000 ng/mL); the chest CT also showed deterioration (Figure 1C). Blood cultures were negative. Piperacillin + tazobactam and IV methylprednisolone were initiated; oseltamivir, ceftriaxone and azythromicin were stopped (the latter due to QT interval prolongation). High-flux continuous supplemental oxygen maintained saturation at 97%, and mechanical ventilation was not necessary.
The patient improved on the following days: she became afebrile on the 16th day after the onset of symptoms, and was weaned from supplemental oxygen. CRP decreased to 4.4 mg/dL (Figure 2).
Figure 2.
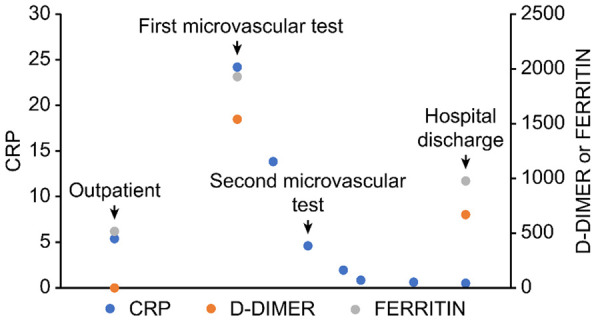
Evolution of C-reactive protein (CRP, mg/dL), d-dimer (ng/mL) and ferritin (µg/L) serum levels.
The evaluation of microvascular flow and reactivity was performed on two different occasions, the first on day 12 (when the patient presented the worst clinical features since hospital admission) and the second on day 16 (after clinical and laboratory improvement). Endothelium-dependent vasodilation of skin microcirculation was evaluated using a single-point laser doppler perfusion monitoring (LDPM) system (Periflux 5001, Perimed, Järfälla, Sweden), for noninvasive measurement of systemic microvascular perfusion changes (in arbitrary perfusion units [APU = 10 mV]), as previously described [8,9]. After measuring the resting microvascular flow on the skin of the forearm, the maximal microvascular vasodilatation was assessed using prolonged (20 minutes) local heating of the laser probe to 44°C (local thermal hyperemia, LTH) (Figure 3).
Figure 3.
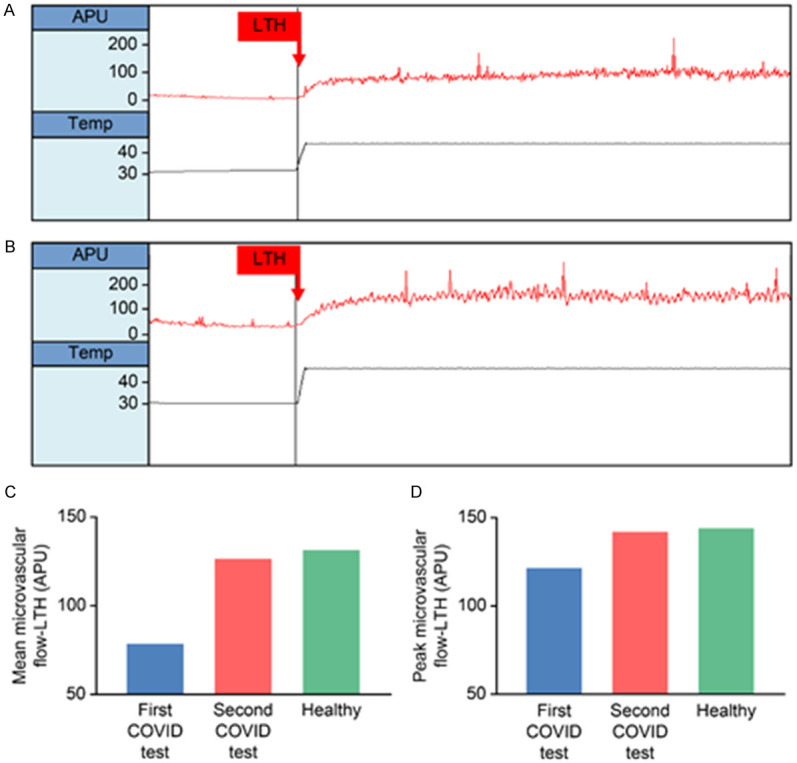
Upper panels: Recordings of skin microvascular flow using laser doppler perfusion monitoring, with local thermal hyperemia (LTH) stimulus. (A) Patient with COVID-19, first test (worst day during clinical evolution). (B) Same patient, second test, after clinical and laboratory improvement. There is higher baseline flow, as well as post-LTH flow on the second test. Lower panels: Bar graphs showing the effect of LTH on mean (C) and peak (D) cutaneous microvascular flow of the patient, in the first test (blue bars) and on the second test (red bars), compared to a sex- and age-matched healthy control (green bars). APU, arbitrary perfusion units; LTH, start of local thermal hyperemia; TEMP, skin temperature.
This case report is part of a prospective study, which investigates systemic microvascular flow and reactivity in patients in the acute phase of COVID-19. The study was approved by the Institutional Review Board (IRB) of the National Institute of Cardiology and was registered and made public at ClinicalTrials.gov (NCT4406545). The subject included in this case report read and signed the informed consent form approved by the IRB before inclusion.
Discussion
An association between the presence of cardiovascular disease or myocardial injury and adverse prognosis has been demonstrated in patients with COVID-19, with increases of up to 10 times in mortality [10,11]. As an initial process, SARS-CoV-2 virus, anchored in the transmembrane angiotensin-converting enzyme 2 (ECA2) receptor, penetrates host cells, including endothelial cells [12,13]. The ensuing endothelial dysfunction may contribute-together with the chronic endothelial dysfunction present incomorbidities such as hypertension, diabetes, coronary artery disease and obesity [14,15]- to more severe presentations, faster evolution and worse outcomes.
The cutaneous microcirculation is an accessible and representative vascular bed for the evaluation of systemic microcirculatory flow and reactivity [16]. LDPM, which was employed in this study, is a noninvasive method for the evaluation of systemic microvascular endothelial function in diverse clinical or surgical conditions [9,17,18]. The skin vasodilatory response resulting from LTH assesses microvascular function and reactivity, and consists of a biphasic response. The initial peak mediated by a calcitonin gene-related peptide, dependent on the axonal reflex of the sensory nerves, is followed by long-term plateau phase, mediated primarily by substances produced by the vascular endothelium, such as nitric oxide and hyperpolarizing factors derived from the endothelium [8].
In this patient, systemic endothelium-dependent microvascular reactivity was severely impaired on day 12, but recovered to levels similar to those of a sex- and age-matched healthy control on day 16 (Figure 2). Both resting microvascular flow, as well as mean and peak flows during LTH, were higher in the second test, when the patient was improved (Table 1). These changes occurred in parallel with improvements of CRP. This biomarker is increased in patients with COVID-19, most likely due to systemic inflammation [19,20]. Systemic inflammation may account for endothelial function abnormalities [21], as endothelial cells and microvascular function are known to be affected by circulating inflammatory cytokines and reactive oxygen species, for example, which are increased in sepsis- the release of inflammatory mediators and reactive molecules to destroy pathogens may ultimately cause endothelial damage [22,23].
Table 1.
Summary of the case report
| Case description | A 32-year-old woman with cardiac valve disease and spherocytosis, diagnosed with COVID-19. Systemic microvascular reactivity assessed with cutaneous laser doppler perfusion monitoring, with thermal hyperemia |
| First microcirculation test | Performed in the worst day of clinical evolution-severely impaired microvascular vasodilation, compared to a healthy control |
| Second microcirculation test | Performed after clinical and laboratory improvement-marked increase in baseline microvascular flow and endothelial-dependent vasodilation |
Bearing in mind that the presence and intensity of systemic microvascular changes during the acute phase of COVID-19 may be related to disease progression and prognosis, the importance of the evaluation of microvascular reactivity in these patients should be underscored. To the best of our knowledge, this is the first report on the detrimental effect of COVID-19 on endothelium-dependent systemic microvascular reactivity in human beings. The current findings may point towards a unique opportunity for further evaluation of the microcirculation in this patient population, with a fast, simple and noninvasive method.
Conclusions
Systemic microvascular dysfunction may occur in patients with COVID-19, paralleling the inflammatory status of the disease. Laser doppler perfusion monitoring with local thermal hyperemia on the skin may be valuable to noninvasively evaluate the systemic microcirculation in COVID-19, offering useful information, which may have therapeutic and prognostic implications.
Acknowledgements
The work was funded by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; grant # 305234/2017-0) and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ; grant # E26/202.822/2018). The authors thank Marcio Gonzalez for technical assistance.
Disclosure of conflict of interest
None.
References
- 1.Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, Holden KA, Read JM, Dondelinger F, Carson G, Merson L, Lee J, Plotkin D, Sigfrid L, Halpin S, Jackson C, Gamble C, Horby PW, Nguyen-Van-Tam JS, Ho A, Russell CD, Dunning J, Openshaw PJ, Baillie JK, Semple MG ISARIC4C investigators. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. 2020;369:1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cevik M, Bamford CGG, Ho A. COVID-19 pandemic-a focused review for clinicians. Clin Microbiol Infect. 2020;26:842–847. doi: 10.1016/j.cmi.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teuwen LA, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20:389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC ACTT-1 Study Group Members. Remdesivir for the treatment of covid-19 - preliminary report. N Engl J Med. 2020;383:994. doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 6.Rose MR, Hiltz KA, Stephens RS, Hager DN. Novel viruses, old data, and basic principles: how to save lives and avoid harm amid the unknown. Lancet Respir Med. 2020;8:661–663. doi: 10.1016/S2213-2600(20)30236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horby P, Lim WS, Emberson J, Mafham M, Bell J, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cracowski JL, Roustit M. Current methods to assess human cutaneous blood flow: an updated focus on laser-based-techniques. Microcirculation. 2016;23:337–344. doi: 10.1111/micc.12257. [DOI] [PubMed] [Google Scholar]
- 9.Salgado MA, Salgado-Filho MF, Reis-Brito JO, Lessa MA, Tibirica E. Effectiveness of laser Doppler perfusion monitoring in the assessment of microvascular function in patients undergoing on-pump coronary artery bypass grafting. J Cardiothorac Vasc Anesth. 2014;28:1211–1216. doi: 10.1053/j.jvca.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei JF, Huang FY, Xiong TY, Liu Q, Chen H, Wang H, Huang H, Luo YC, Zhou X, Liu ZY, Peng Y, Xu YN, Wang B, Yang YY, Liang ZA, Lei XZ, Ge Y, Yang M, Zhang L, Zeng MQ, Yu H, Liu K, Jia YH, Prendergast BD, Li WM, Chen M. Acute myocardial injury is common in patients with covid-19 and impairs their prognosis. Heart. 2020;106:1154–1159. doi: 10.1136/heartjnl-2020-317007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kreutz R, Algharably EAE, Azizi M, Dobrowolski P, Guzik T, Januszewicz A, Persu A, Prejbisz A, Riemer TG, Wang JG, Burnier M. Hypertension, the renin-angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for COVID-19. Cardiovasc Res. 2020;116:1688–1699. doi: 10.1093/cvr/cvaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pons S, Fodil S, Azoulay E, Zafrani L. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit Care. 2020;24:353. doi: 10.1186/s13054-020-03062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Lorenzo A, Escobar S, Tibirica E. Systemic endothelial dysfunction: a common pathway for COVID-19, cardiovascular and metabolic diseases. Nutr Metab Cardiovasc Dis. 2020;30:1401–1402. doi: 10.1016/j.numecd.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tibirica E, De Lorenzo A. Increased severity of COVID-19 in people with obesity: are we overlooking plausible biological mechanisms? Obesity (Silver Spring) 2020;28:1374. doi: 10.1002/oby.22887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holowatz LA, Thompson-Torgerson CS, Kenney WL. The human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol (1985) 2008;105:370–372. doi: 10.1152/japplphysiol.00858.2007. [DOI] [PubMed] [Google Scholar]
- 17.de Moraes R, Van Bavel D, Gomes MB, Tibirica E. Effects of non-supervised low intensity aerobic excise training on the microvascular endothelial function of patients with type 1 diabetes: a non-pharmacological interventional study. BMC Cardiovasc Disord. 2016;16:23. doi: 10.1186/s12872-016-0191-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ugenti V, Romano AC, Tibirica E. Microvascular endothelial dysfunction during cardiopulmonary bypass in surgery for correction of cyanotic and acyanotic congenital heart disease. Microvasc Res. 2018;120:55–58. doi: 10.1016/j.mvr.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 22.Ait-Oufella H, Maury E, Lehoux S, Guidet B, Offenstadt G. The endothelium: physiological functions and role in microcirculatory failure during severe sepsis. Intensive Care Med. 2010;36:1286–1298. doi: 10.1007/s00134-010-1893-6. [DOI] [PubMed] [Google Scholar]
- 23.Ince C, Mayeux PR, Nguyen T, Gomez H, Kellum JA, Ospina-Tascon GA, Hernandez G, Murray P, De Backer D. The endothelium in sepsis. Shock. 2016;45:259–270. doi: 10.1097/SHK.0000000000000473. [DOI] [PMC free article] [PubMed] [Google Scholar]


