Abstract
Background: Following severe burn injury, patients undergo profound metabolic changes, including insulin resistance and hyperglycemia. Hyperglycemia has been linked to impaired wound healing, increased risk of skin graft loss, increased muscle catabolism, increased infections, and mortality. Sitagliptin is a dipeptidyl peptidase-4 (DPP-4) inhibitor that improves glycemic control by slowing the inactivation of incretin hormones, increasing insulin synthesis and release from pancreatic beta cells and lowering glucagon secretion from pancreatic alpha cells. The objective of this study was to describe our institution’s experience with using sitagliptin to help mitigate insulin resistance after burn injury. Methods: This was a retrospective chart review that included 22 adult burn patients. Burn patients were prescribed sitagliptin regardless of their previous medical history of type 2 diabetes mellitus. Patients were included in this analysis if they were adults admitted for burn injury during a 13-month period and received at least 3 consecutive doses of sitagliptin. Patients were excluded if they did not have insulin use data 3 days pre- and 3 days post-sitagliptin initiation. The first day of sitagliptin initiation was considered day 0; data from day 0 were not included in either the pre- or post-sitagliptin analysis. Results: In the 3 days prior to sitagliptin initiation, patients received a median of 114.3 units per day (IQR 49.1, 228) in an attempt to maintain a blood glucose goal of less than 180 mg/dL. In the 3 days after sitagliptin was started, exogenous insulin requirements significantly decreased to a median to 36.3 units per day (IQR 11.7, 95) (P=0.009). Seven patients were on insulin infusions at the time of sitagliptin initiation. After sitagliptin was started, it took a median of 3 days (IQR 2, 3.25) to be liberated from the insulin infusion. In terms of safety, there were two episodes of hypoglycemia (BG<70 mg/dL) after sitagliptin initiation, compared to three episodes prior to sitagliptin initiation (P=0.7). Conclusion: The addition of sitagliptin to burn patients’ medication regimens significantly reduced insulin requirements over a 3-day period and allowed liberation from insulin drips.
Keywords: Insulin resistance, hyperglycemia, sitagliptin
Introduction
Metabolic changes following severe burn injury include inflammation, hypermetabolism, and hyperdynamic circulation. This physiologic stress, in turn, can lead to hyperglycemia and insulin resistance [1,2]. Sustained increases in catecholamines, glucocorticoids, and glucagon occur. Catecholamines mediate glycogenolysis, thereby increasing blood glucose (BG) levels. Additionally, pro-inflammatory cytokines present after burn injury, including tumor necrosis factor (TNF), interleukin (IL)-6, and monocyte chemotactic protein-1 directly contribute to hyperglycemia by influencing skeletal muscle insulin resistance [2]. Critical illness adds another layer of complexity, causing shifts in metabolism that augment hepatic glucose production not suppressed by systemic hyperglycemia. Concurrently, glucose breakdown and disposal via the insulin-signaling pathway and glucose transporter-4 are inhibited, resulting in peripheral insulin resistance [2]. Sitagliptin works to improve glycemic control by slowing the inactivation of GLP-1 and GIP, increasing insulin synthesis and release from pancreatic beta cells and lowering glucagon secretion from pancreatic alpha cells [3]. This increase in endogenous insulin production can potentially lower exogenous insulin requirements.
Persistent or sustained hyperglycemia has been associated with poor outcomes after burn injury, including increased risk of infection (pneumonia and bloodstream infections), augmented muscle catabolism, impaired wound healing, increased skin graft loss, and mortality [1,2,4,5]. Additionally, achievement of intensive glycemic control has been associated with decreased incidence of pneumonia, including ventilator-associated pneumonia, and urinary tract infections [6]. Murphy and colleagues found that patients who did not achieve early glycemic control had a 6.75-fold increased risk of mortality even after adjusting for age, burn size, and presence of inhalation injury [4].
While the ideal BG target after burn injury has yet to be determined, current evidence indicates that BG should be maintained less than 180 mg/dL, with a goal of less than 150 mg/dL for trauma patients [7]. Some experts in managing burn injury and post-burn metabolic changes have suggested that the BG target should be lower in this patient population, with a goal less than 140 mg/dL or even less than 130 mg/dL [8,9]. While intensive insulin therapy (targeting BG less than 110 mg/dL) has demonstrated increased survival and decreased infections in burn patients, these benefits must be carefully weighed against the risks of hypoglycemia [10,11]. A recent study found that a standardized insulin protocol was able to achieve moderate glycemic control (targeting BG 110-140 mg/dL) in burn patients, while decreasing glucose variability and hypoglycemia [12].
Many burn patients require an insulin infusion to maintain BG levels within goal. For the past several years, in an effort to decrease insulin requirements and liberate patients from insulin infusions to facilitate transfer out of the burn intensive care unit (BICU), our center has utilized sitagliptin. Sitagliptin is a dipeptidyl peptidase-4 (DPP-4) inhibitor that is currently indicated as an adjunctive treatment to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus (DM) [3]. Sitagliptin improves glycemic control by slowing the inactivation of incretin hormones (glucagon-like peptide-1 [GLP-1] and glucose-dependent insulinotropic polypeptide [GIP]), thereby increasing insulin synthesis and release from pancreatic beta cells and lowering glucagon secretion from pancreatic alpha cells [3]. Based on its mechanism of action, sitagliptin can theoretically mitigate insulin resistance, decrease insulin requirements, and improve glycemic control both in patients with pre-existing type 2 DM and in patients without this diagnosis. Sitaglipin has been shown to have a minimal risk of hypoglycemia, making it an attractive adjunctive agent [3,13].
The purpose of this study was to describe our clinical experience and evaluate our previous use of sitagliptin in patients admitted to our BICU, focusing on change in insulin requirements and ability to be liberated from insulin infusions.
Methods
This was a retrospective chart review was approved by the US Army Institute of Surgical Research’s (USAISR) Research Regulatory Compliance Division as a performance improvement project (project number H-18-041nr). As this project was approved as a retrospective performance improvement project, informed consent was not required. All privacy and ethics standards were met. This project included all adult patients admitted to the burn intensive care unit (BICU) at the USAISR between June 1, 2017 and June 30, 2018. The primary endpoints included pre- and post-sitagliptin insulin requirements and time to liberation from insulin infusion. Safety endpoints included incidence of hypoglycemia (BG less than 70 mg/dL).
Patients were initiated on sitagliptin at the discretion of the BICU medical team if they were on an insulin infusion or needed more intensive BG monitoring and control, but were otherwise medically stable to be downgraded to ward-level care. Our institution utilizes a computerized decision support system (EndoTool®) for insulin infusion titration; a paper titration protocol is used in the event EndoTool® is unavailable. This system targets a BG less than 150 mg/dL. If for any reason Endotool® could not be used, a non-electronic insulin infusion titration protocol was used (Appendix A). Patients who were not on an insulin infusion were receiving sliding scale insulin every 6 hours (Appendix B).
Inclusion and exclusion criteria
Patients without burns (i.e. skin diseases, toxic epidermal necrolysis, necrotizing fasciitis) were excluded from this analysis. Patients were included if they received at least 3 consecutive doses of sitagliptin. Patients were excluded if they did not have insulin use data for the 3 days pre- and post-sitagliptin initiation. Patients who received sitagliptin were included regardless of their previous medical history of type 2 DM. Patients with a prior history of type 1 DM were not given sitagliptin, and therefore were not included in this chart review.
Data collection
The day of sitagliptin initiation was considered ‘day 0’; data from this data were not included in our analysis. Data were collected from the electronic medical record and included demographic information, admission diagnosis, percent total body surface area burned (%TBSA), DM status, admission hemoglobin A1C, creatinine clearance (CrCl, as per the Cockroft-Gault equation) at the time of sitagliptin initiation, dose of sitagliptin ordered, insulin requirements in the 3 days pre- and post-sitagliptin initiation, and time to insulin infusion discontinuation after sitagliptin was started. All subjects on enteral nutrition received our institution’s standard formula of Promote® (Abbott Nutrition), which provides 25% of kcal from protein, 23% of kcal from fat, and 52% of kcal from carbohydrate. Diabetic enteral nutrition formulas were not used in our burn ICU. Carbohydrate intake data were recorded for each day, if available. When carbohydrate intake was not available, the analysis for carbohydrate intake pre- and post-sitagliptin was not used in the analysis for that patient.
Appropriate dosing was defined per the sitagliptin package insert. Patients with a CrCl, based on the Cockroft-Gault equation of 45 mL/min or higher, were recommended to receive sitagliptin 100 mg daily; patients with a CrCl of 30-44 mL/min were recommended to receive 50 mg daily; and patients with a CrCl of 29 mL/min or less were recommended to receive 25 mg daily [3].
Statistical analysis
Data were reported as mean ± standard deviation (SD) or median and interquartile range (IQR). Insulin requirements in the 72 hours pre-sitagliptin initiation and 72 hours post-sitagliptin initiation were compared and analyzed using a related-samples Wilcoxon signed rank test. Incidence of hypo- or hyperglycemia pre- and post-sitagliptin initiation was compared using Fisher’s exact tests. Statistical significance was defined as an α-level of 0.05. Statistical analysis was performed using SPSS version 22 (IBM, Armonk, NY).
Results
Demographics
Fifty-two subjects were identified for possible inclusion, and 22 subjects were included in this analysis. Figure 1 shows the number of patients included and excluded, along with reasons for exclusion. Demographic and baseline characteristics of these patients are included in Table 1. Ten (45.5%) patients had a formal diagnosis of type 2 DM and/or a hemoglobin A1C value indicative of DM type 2 (≥6.5%) at hospital admission. Patients had an average burn size of 31.2% ± 23.2% TBSA, and 11 (50%) patients had inhalation injury. Sitagliptin was initiated a median of 22 days post-burn (IQR 11.5, 32 days). Adjusted for burn size, sitagliptin was initiated 0.8 days per %TBSA (IQR 0.5, 1.3 days per %TBSA) post-burn. The median duration of therapy was 10 days (IQR 7, 17 days).
Figure 1.
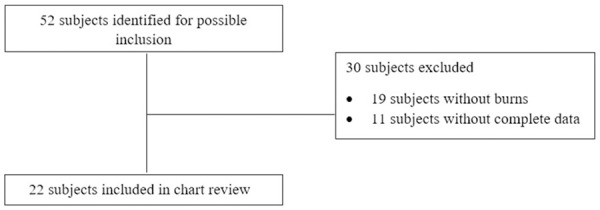
Inclusion and exclusion criteria.
Table 1.
Demographic characteristics
| Characteristic | N=22 |
|---|---|
| Age, years, mean ± SD | 49.8 ± 19.9 |
| Male gender, n (%) | 17 (77.3) |
| BMI, kg/m2, mean ± SD | 32.2 ± 6.6 |
| TBSA, %, mean ± SD | 31.2 ± 23.2 |
| Inhalation injury present, n (%) | 11 (50) |
| Hemoglobin A1C on admission, mean ± SD | 6.5 ± 2 |
| Diagnosis of type 2 DM prior to admission, n (%) | 6 (27.3) |
| A1C indicative of DM on admission, but without prior diagnosis of DM2 (≥6.5%), n (%) | 4 (18.2) |
| Diabetics (A1C indicative or prior diagnosis), n (%) | 10 (45.5) |
| CrCl (Cockroft-Gault) when sitagliptin started, mean ± SD | 155.9 ± 75.8 |
| Renal replacement therapy when sitagliptin initiated, n (%) | 2 (9.1) |
Insulin requirements
In the 3 days prior to sitagliptin initiation, patients received a median of 114.3 units per day (IQR 49.1, 228.0) in an attempt to maintain a BG goal of less than 180 mg/dL. In the 3 days after sitagliptin was started, exogenous insulin requirements significantly decreased to a median of 36.3 units per day (IQR 11.7, 95.0) (P=0.031) (Table 2). Figure 2 shows insulin requirements over time. Patients had a median 33.9% decrease in insulin requirements after starting sitagliptin (IQR-89.9, +9.9). The average enteral nutrition rate was 122 ± 43 mL/hr. The carbohydrate intake did not significantly differ before and after initiation of sitagliptin (1288.2 g vs. 1326.5 g; p=0.613) in the 17 patients who had carbohydrate intake data available (Table 2). Seven patients were on insulin infusions at the time of sitagliptin initiation. After sitagliptin was started, it took a median of 3 days (IQR 2.0, 3.3) to be liberated from the insulin infusion. The remaining patients had the insulin infusion discontinued on the day of or prior to sitagliptin initiation.
Table 2.
Insulin and carbohydrate intake (median, IQR)
| Pre-sitagliptin | Post-sitagliptin | P-value | |
|---|---|---|---|
| Insulin requirement, units | 114.3 (49.1, 228.1) | 36.3 (11.7, 95) | 0.009 |
| Carbohydrates, g | 1288.2 (910.8, 1643.3) | 1326.5 (841.4, 1835.1) | 0.613 |
Figure 2.
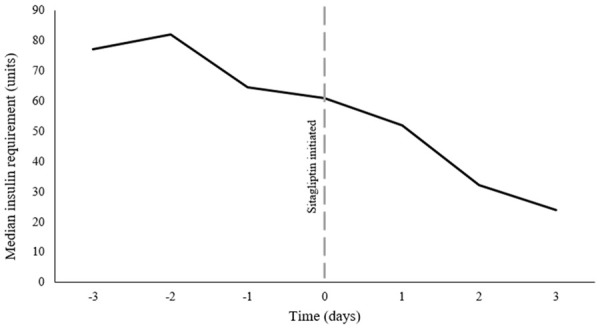
Insulin use over time.
Blood glucose control
The median minimum BG was 99 (IQR 99, 121) mg/dL on day 1, 105 (IQR 89, 123) mg/dL on day 2, and 110 (IQR 90, 118) mg/dL on day 3. The median maximum BG was 202 (IQR 172, 240) mg/dL on day 1, 195 (IQR 163, 221) mg/dL on day 2, and 206 (IQR 149, 259) mg/dL on day 3. Figure 3 shows the distribution of BG levels over the three days pre- and post-sitagliptin initiation. Twenty (90.9%) patients had at least one episode of hyperglycemia (BG>180 mg/dL) prior to sitagliptin initiation, as compared to 17 (77.3%) patients after sitagliptin initiation (P=0.043).
Figure 3.
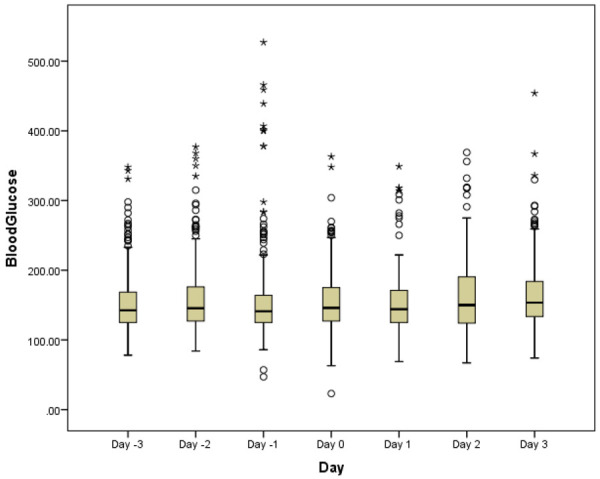
Blood glucose by day.
Appropriate sitagliptin dosing
Fifteen patients (68.2%) were started an appropriate dose based on their renal function; 6 patients (27.3%) were started on too low of a dose, while 1 patient (4.5%) was started on too high of a dose. Four patients (18.2%) developed an acute kidney injury (AKI), defined as an increase in serum creatinine of ≥0.3 mg/dL per the AKIN criteria, during the first 3 days of sitagliptin therapy. Only one patient (4.5%) had an increase in serum creatinine >50% from the day of sitagliptin initiation to day 3 of sitagliptin therapy. The median change in serum creatinine from the day of initiation to day 3 of sitagliptin was an increase of 0.11 mg/dL (IQR -0.05, +0.09 mg/dL).
Hypoglycemia
Prior to sitagliptin initiation, 3 patients (13.6%) had at least one episode of hypoglycemia. Two patients (9.1%) experienced hypoglycemia (BG<70 mg/dL) within the first 3 days after sitagliptin initiation; each patient only had 1 instance of hypoglycemia. One of these had pre-existing uncontrolled type 2 DM (admission hemoglobin A1C was 11.4%). This patient had an average pre-sitagliptin insulin requirement of 124.7 units per day and a post-sitagliptin insulin requirement of 5.3 units per day. The other patient had an average requirement of 225 units per day and 2.7 units per day, respectively. Incidence of hypoglycemia was not significantly different pre- and post-sitagliptin initiation (13.6% vs. 9.1; P=0.7).
Discussion
We conducted a retrospective chart review to evaluate the use of sitagliptin as an adjunct anti-hyperglycemic agent post-burn injury. We found that the addition of sitagliptin was associated with decreased exogenous insulin requirements by an average of 33.9% in burn patients. Patients were able to be liberated from the insulin infusion and started on sliding-scale subcutaneous insulin an average of 3 days after sitagliptin initiation.
Hyperglycemia is a common metabolic consequence of burn injury. Exogenous insulin is the mainstay of therapy for treating hyperglycemia. To date, the only oral antidiabetic agent investigated to combat hyperglycemia after burn injury is metformin [14-16]. However, its potential for lactic acidosis precludes its use in most critically ill burn patients. We sought to explore the use of sitagliptin as an adjunct to insulin therapy for the management of hyperglycemia after burn injury.
In critically ill burn patients, it is important to achieve glycemic control, as this has been associated with decreased mortality and morbidity. Murphy and colleagues conducted a retrospective study, which showed that patients who failed to achieve early glycemic control (by post-burn day 3) had an increased risk of mortality [4]. In order to achieve early glycemic control, some burn experts recommend targeting a lower blood glucose level, as previously mentioned [1]. Lack of BG control has been associated with increased morbidity, Hyperglycemia has been associated with increased risk of morbidity, including pneumonia, bloodstream infections, and decreased graft take [17,18].
While a BG level greater than 180 mg/dL should be avoided in critically ill burn/trauma patients, the ideal goal level remains unknown [5]. Studies examining the safety and efficacy of intensive insulin therapy (targeting a BG level of 110 mg/dL or less) in burn patients have shown that intensive insulin therapy is associated with increased survival and decreased infections [10,11]. In 2010, 73% of burn centers verified by the American Burn Association had insulin protocols that targeted BG levels less than 120 mg/dL [19]. An intensive insulin protocol implemented in a burn-trauma unit showed achievement of BG less than 120 mg/dL with only a 5% incidence of hypoglycemia per day [20]. However, intensive insulin therapy has been associated with increased risk of hypoglycemia in critically ill patient populations, with the risk of morbidity and mortality increasing with prolonged or frequent episodes of hypoglycemia [1,2,4,5,21].
The results from our study show that the addition of sitagliptin was associated with decreased episodes of hyperglycemia. In fact, 90.9% of patients had at least one episode of hyperglycemia (BG>180 mg/dL) prior to sitagliptin initiation, as compared to 77.3% of patients after sitagliptin initiation (P=0.043). Other studies in patients undergoing general and cardiac surgical procedures have not found the same results. It is estimated that 30% of non-diabetic patients undergoing non-cardiac procedures will develop stress hyperglycemia within 72 hours post-operatively. Fayfman and colleagues conducted a randomized pilot study to determine if sitagliptin could lower the risk of post-operative hyperglycemia in general surgery patients. Eighty patients were enrolled in this study; however, no significant differences in the rate of post-operative stress hyperglycemia, glycemic control, or complications were seen [22]. Brackbill and colleagues conducted a double-blind, placebo-controlled study to determine if there was a role for sitagliptin therapy for the acute control of BG management in the post-operative cardiac-surgery setting. They found that there was no difference in mean BG control between the sitagliptin and placebo groups, with both groups averaging about 150 mg/dL throughout the study period (P=0.388). Both groups required a mean of 13 units of insulin per day (P=0.942). Additionally, there was no difference in the incidence of post-operative infection, antibiotic use, and post-operative length of stay (LOS). In this study, there were 7 episodes of hypoglycemia (BG less than 60 mg/dL); 5 occurred in the sitagliptin group while 2 occurred in the placebo group [23]. Lastly, Cardona and colleagues conducted a double-blinded, placebo-controlled study to determine if sitagliptin could prevent stress hyperglycemia in patients undergoing coronary artery bypass surgery. While sitagliptin statistically significantly decreased BG levels pre-surgery, this was not clinically significant (101 mg/dL vs. 107 mg/dL; P=0.01). However, sitagliptin did not affect the proportion of patients who developed post-operative stress hyperglycemia (21% vs. 22%, P>0.99) [24]. In these studies, sitagliptin was initiated either on the day of or the day prior to surgery and may not have reached steady state during the study period. Additionally, both of these studies had a primary endpoint of BG levels, whereas daily insulin requirement was the primary endpoint in the present study.
To our knowledge, this is the first published report on sitagliptin in burn patients. However, there are several limitations to our study. This was a small, single-center, retrospective chart review, and carries inherent limitations due to the study design. Additionally, detailed carbohydrate data were not available for every patient on every day pre- or post-sitagliptin initiation; carbohydrate intake could have affected BG levels and insulin requirements. Lastly, other clinical data were not collected, such as the prevalence of infection/sepsis, time from burn injury, and percent total body surface area open. All of these factors have been associated with (or are anecdotally associated with) insulin resistance, exogenous insulin requirements, and BG levels.
Conclusion
The addition of sitagliptin to burn patients’ medication regimens was associated with reduced exogenous insulin requirements over a 3-day period and allowed liberation from insulin drips. Larger studies are needed to validate these findings.
Appendix A.
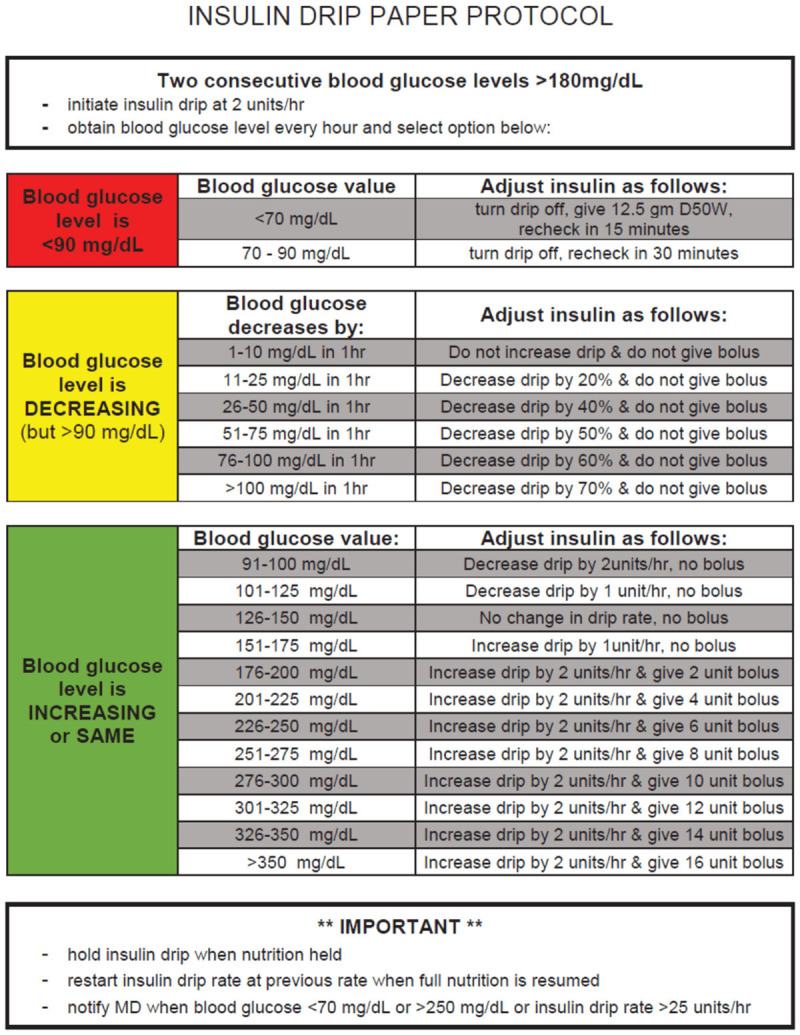
Insulin infusion titration protocol.
Appendix B.
Sliding scale insulin protocol
| Blood Glucose | Units of Insulin Aspart | |
|---|---|---|
| Very low dose | 70-130 mg/dL | 0 units |
| 131-180 mg/dL | 1 unit | |
| 181-240 mg/dL | 2 units | |
| 241-300 mg/dL | 3 units | |
| 301-350 mg/dL | 4 units | |
| 351-400 mg/dL | 5 units | |
| >400 mg/dL | 6 units and then call provider | |
| Low dose | 70-130 mg/dL | 0 units |
| 131-180 mg/dL | 2 units | |
| 181-240 mg/dL | 4 units | |
| 241-300 mg/dL | 6 units | |
| 301-350 mg/dL | 8 units | |
| 351-400 mg/dL | 10 units | |
| >400 mg/dL | 12 units and then call provider | |
| Moderate dose | 70-130 mg/dL | 0 units |
| 131-180 mg/dL | 4 units | |
| 181-240 mg/dL | 6 units | |
| 241-300 mg/dL | 8 units | |
| 301-350 mg/dL | 10 units | |
| 351-400 mg/dL | 12 units | |
| >400 mg/dL | 14 units and then call provider | |
| High dose | 70-130 mg/dL | 0 units |
| 131-180 mg/dL | 8 units | |
| 181-240 mg/dL | 12 units | |
| 241-300 mg/dL | 16 units | |
| 301-350 mg/dL | 20 units | |
| 351-400 mg/dL | 24 units | |
| >400 mg/dL | 28 units and then call provider | |
| Very high dose | 70-130 mg/dL | 0 units |
| 131-180 mg/dL | 10 units | |
| 181-240 mg/dL | 14 units | |
| 241-300 mg/dL | 18 units | |
| 301-350 mg/dL | 22 units | |
| 351-400 mg/dL | 26 units | |
| >400 mg/dL | 30 units and then call provider |
Disclosure of conflict of interest
The opinions or assertions contained herein are the private views of the authors, and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense.
References
- 1.Jeschke MG. Clinical review: glucose control in severely burned patients - current best practice. Crit Care. 2013;17:232. doi: 10.1186/cc12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gauglitz GG, Herndon DN, Jeschke MG. Insulin resistance postburn: underlying mechanisms and current therapeutic strategies. J Burn Care Res. 2008;29:683–694. doi: 10.1097/BCR.0b013e31818481ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Januvia [package insert] Whitehouse Station, NJ: Merck & Co. Inc; 2018. [Google Scholar]
- 4.Murphy CV, Coffey R, Cook CH, Gerlach AT, Miller SF. Early glycemic control in critically ill patients with burn injury. J Burn Care Res. 2011;32:583–590. doi: 10.1097/BCR.0b013e31822dc3da. [DOI] [PubMed] [Google Scholar]
- 5.Jacobi J, Bircher N, Krinsley J, Agus M, Braithwaite SS, Deutschman C, Freire AX, Geehan D, Kohl B, Nasraway SA, Rigby M, Sands K, Schallom L, Taylor B, Umpierrez G, Mazuski J, Schunemann H. Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit Care Med. 2012;40:3251–3276. doi: 10.1097/CCM.0b013e3182653269. [DOI] [PubMed] [Google Scholar]
- 6.Hemmila MR, Taddonio MA, Arbabi S, Maggio PM, Wahl WL. Intensive insulin therapy is associated with reduced infectious complications in burn patients. Surgery. 2013;144:629–637. doi: 10.1016/j.surg.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stanojcic M, Abdullahi A, Rehou S, Parousis A, Jeschke MG. Pathophysiological response to burn injury in adults. Ann Surg. 2018;267:576–584. doi: 10.1097/SLA.0000000000002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finney SJ, Zekveld C, Elia A, Evans TW. Glucose control and mortality in critically ill patients. JAMA. 2003;290:2041–2047. doi: 10.1001/jama.290.15.2041. [DOI] [PubMed] [Google Scholar]
- 9.Jeschke MG, Kraft R, Emdad F, Kulp GA, Williams FN, Herndon DN. Glucose control in severely thermally injured pediatric patients: what glucose range should be the target? Ann Surg. 2010;252:521–528. doi: 10.1097/SLA.0b013e3181f2774c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pham TN, Warren AJ, Phan HH, Molitor F, Greenhalgh DG, Palmieri TL. Impact of tight glycemic control in severely burned children. J Trauma. 2005;59:1148–1154. doi: 10.1097/01.ta.0000188933.16637.68. [DOI] [PubMed] [Google Scholar]
- 11.Gauglitz GG, Toliver-Kinsky TE, Williams FN, Song J, Cui W, Herndon DN, Jeschke MG. Insulin increases resistance to burn wound infection associated sepsis. Crit Care Med. 2010;38:202–208. doi: 10.1097/CCM.0b013e3181b43236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stoecklin P, Delodder F, Pantet O, Berger MM. Moderate glycemic control safe in critically ill adult burn patients: a 15 year cohort study. Burns. 2016;42:63–70. doi: 10.1016/j.burns.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 13.Miller S, St Onge EL. Sitagliptin: a dipdptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. Ann Pharmacother. 2006;40:1336–1343. doi: 10.1345/aph.1G665. [DOI] [PubMed] [Google Scholar]
- 14.Gore DC, Wolf SE, Sanford A, Herndon DN, Wolfe RR. Influence of metformin on glucose intolerance and muscle catabolism following severe burn injury. Ann Surg. 2005;241:334–342. doi: 10.1097/01.sla.0000152013.23032.d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gore DC, Wolf SE, Herndon DN, Wolfe RR. Metformin blunts stress-induced hyperglycemia after thermal injury. J Trauma. 2003;54:555–561. doi: 10.1097/01.TA.0000026990.32856.58. [DOI] [PubMed] [Google Scholar]
- 16.Hamblin MR. Novel pharmacotherapy for burn wounds: what are the advancements. Expert Opin Pharmacotehr. 2019;20:305–321. doi: 10.1080/14656566.2018.1551880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kraft R, Herndon DN, Micak RP, Finnerty CC, Cox RA, Williams FN, Jeschke MG. Bacterial respiratory tract infections are promoted by systemic hyperglycemia after severe burn injury in pediatric patients. Burns. 2014;3:428–435. doi: 10.1016/j.burns.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gore DC, Chinkes D, Heggers J, Herndon DN, Wolf SE, Desai M. Association of hyperglycemia with increased mortality after severe burn injury. J Trauma. 2001;51:540–544. doi: 10.1097/00005373-200109000-00021. [DOI] [PubMed] [Google Scholar]
- 19.Mecott GA, Al-Mousawi AM, Gauglitz GG, Herndon DN. The role of hyperglycemia in burned patients: evidence-based studies. Shock. 2010;33:5–13. doi: 10.1097/SHK.0b013e3181af0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cochran A, Davis L, Morris SE, Saffle JR. Safety and efficacy of an intensive insulin protocol in a burn-trauma intensive care unit. Burns. 2008;29:187–191. doi: 10.1097/BCR.0b013e318160d066. [DOI] [PubMed] [Google Scholar]
- 21.NICE-SUGAR Study Investigators. Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hébert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 22.Fayfman M, Davis G, Duggan EW, Urrutia M, Chackhiani D, Schindler J, Pasquel FJ, Galindo RJ, Vellanki P, Reyes-Umpierrez D, Wang H, Umpierrez GE. Sitagliptin for the prevention of stress hyperglycemia in patients without diabetes undergoing general surgery: a pilot randomized study. J Diabetes Complications. 2018;32:1091–1096. doi: 10.1016/j.jdiacomp.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brackbill ML, Rahman A, Sandy JS, Stam MD, Harralson AF. Adjunctive sitagliptin therapy in postoperative cardiac surgery patients: a pilot study. Int J Endocrinol. 2012;2012:810926. doi: 10.1155/2012/810926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cardona S, Tsegka K, Pasquel FJ, Fayfman M, Peng L, Jacobs S, Vellanki P, Halkos M, Guyton RA, Thourani VH, Galindo RJ, Umpierrez G. Sitagliptin for the prevention of stress hyperglycemia in patients without diabetes undergoing coronary artery bypass graft (CABG) surgery. BMJ Open Diabetics Res Care. 2019;7:e000703. doi: 10.1136/bmjdrc-2019-000703. [DOI] [PMC free article] [PubMed] [Google Scholar]


