Abstract
Lower limb salvage in severely injured burned patients with bone or tendon exposure may be a reconstructive challenge. In this cases, local or regional flaps and other more conservative therapies such as dermal substitutes and negative-pressure wound therapy are usually not available or are not good enough to solve the problem. In such situations, microsurgical reconstruction with distant flaps seems to be the best option, even though the particularities of the severe burn patient may decrease free flaps’ success rate. We report the case of a patient with severe electrical injuries affecting 70% of the total body surface area who had full-thickness burns to the lower extremity with wide bone exposure and extensively drug-resistant Pseudomonas aeruginosa infection. We achieved limb salvation using rectus femoris muscle free flap plus lateral and medial gastrocnemius muscle flaps and soleus muscle flap, after two failed microsurgical coverture attempts and a long not useful periplus with conservative therapies such us negative-pressure wound therapy and dermal substitutes. After 3 years of follow-up, the patient can walk without aid, and he has recovered his social and employment situation prior to the accident.
Keywords: Burn, electrical injury, lower limb reconstruction, microsurgery, free flap, flaps
Introduction
Severely damaged lower limb reconstruction with bone and tendon exposure is still a reconstructive challenge for plastic surgeons. There are many different reconstructive techniques to deal with this problem: full-thickness skin grafts, dermal substitutes, negative-pressure wound therapy (NPWT), tissue expansion, local flaps, regional flaps, and free microvascular flaps. Nevertheless, limb amputation is still a relatively frequent outcome. The limb reconstruction attempt is harder in the severe burn patient due to the affectation of the general condition and the limitation of the reconstructive alternatives as a result of the extent of the burn itself, increasing even more the difficulty for the limb salvage success. In the case of high voltage electrical injuries, the need for amputation of one or more limbs can occur in almost 20% of cases [1]. Besides, in this type of burns, the success rate of free microsurgical flaps is under 85%, which is lower than in the general population, according to the existing literature [2].
In this article, we report our experience with a patient who had an electrical burn injury affecting 70% of the total body surface area. The patient presented an extensive bone exposure in the right tibia (25 × 4 cm) and wound infection by an extensively drug-resistant Pseudomonas aeruginosa. The aim of this manuscript is to discuss the alternatives for limb salvage in each reconstructive stage and to explain the foundation for our decisions. As well as to expose the lessons we learnt from this complex case and to propose a decision algorithm based on existing scientific evidence.
In this work, no data is revealed that would allow the identity of the patient to be known. Besides, we obtained verbal informed consent of the patient for taking the pictures and for their publication for scientific purposes.
Case report
A 25 year old male arrived at the emergency department of our Burn Centre with an electrical injury and kindling of his clothes in 2016. He showed deep second-degree burns and third-degree burns in 70% of the total body surface area (TBSA), preserving only the head, upper extremities in a discontinuous fashion, left flank, and both soles. In the course of his hospitalization, the patient required surgery up to 7 times for tangential and fascia level burn excision and coverage with homograft skin, which was then sequentially replaced by autograft skin when the available donor areas healed.
After serial debridement, on the 21st day after the accident, the patient had an exposure of the right tibia about 25 × 4 cm wide with exposure of the anterior compartment tendons as well (Figure 1). The rest of the limb had already undergone fascial excision and had been covered with MEEK micrografting technique.
Figure 1.
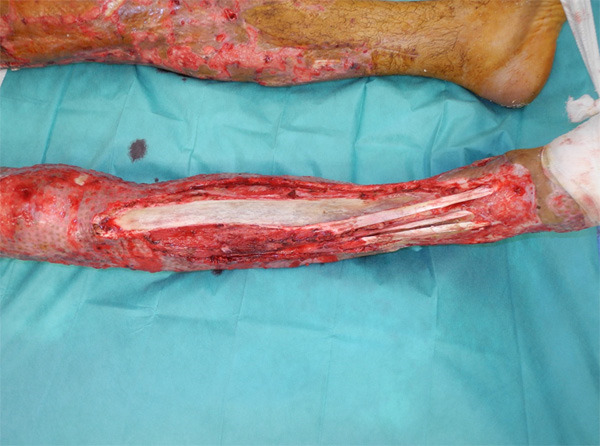
Large initial defect exposing the tibia and anterior compartment tendons.
Given this soft-tissue defect, on the 30th day after the accident, we proceeded to remove necrotic, sclerotic and avascular bone with high speed burr until pinpoint bleeding bone (paprika sign) was obtained and to cover it with a chimeric serratus and latissimus dorsi free muscle flap. It was end-to-end anastomosed to the anterior tibial vessels and covered with meshed autograft skin. 24 hours after surgery the muscle displayed signs of congestion. It was revised in the operation room (OR), and it revealed thrombosis of the vein. We performed thrombectomy and venous reanastomosis. Despite the reintervention, the flap had an unfavorable evolution, becoming completely necrotic 12 days after the operation.
Considering that the contralateral subescapular system was not available because of the burn injury, we opted for partial coverage of the proximal third of the wound with both gastrocnemius muscle flaps and medial hemisoleus muscle flap (Figure 2); we grafted all three muscles with split-thickness meshed skin. There were no complications in this surgery. However, an extensively drug-resistant Pseudomonas aeruginosa (XDR-PA), only susceptible to colistin, grew in the soft-tissue culture obtained in the OR. The former occurred in the context of an outbreak that our Burn Center suffered that year [3].
Figure 2.
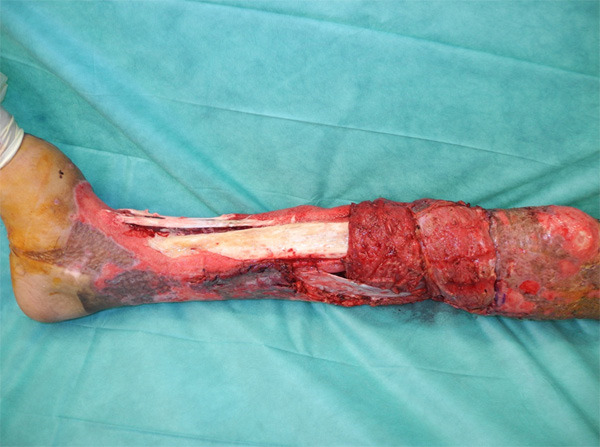
Wound size reduction using regional flaps: medial gastrocnemius muscle flap and hemisoleus muscle flap.
The resultant soft tissue defect was reduced to 15 × 4 cm, therefore we projected a new attempt for microsurgical coverage on the 55th day after the accident. Seeing the size and shape of the defect, a contralateral rectus femoris muscle free flap was selected, and we anastomosed it end-to-end to the posterior tibial vessels. Previously to the flap coverage, we performed chiseling of the cortical bone.
After 72 hours without complications, it appeared a relevant pulsating bleeding which seemed to emerge from the vascular pedicle area. The bleeding was controlled with local pressure and the patient was then transferred to the OR. An arterial thrombosis was found, with purulent secretion underneath the flap. Although we performed a thrombectomy, we did not obtain any arterial flow. Given that the flap presented no signs of vitality, we decided to remove it and to install an NPWT with -120 mmHg of continuous pressure.
Despite the failure of the previous 2 attempts of microsurgical coverage and the XDR-PA infection of the area, we resolved, in a multidisciplinary team meeting, that attempting to preserve the limb had more potential benefits than an amputation. We made the decision on the grounds that it would imply a short and shoddy below-the-knee stump (the limb had been debrided to the fascia level and autografted with split-thickness skin) which would not be suitable for conventional prosthesis rehabilitation; thus, it would have required above-the-knee amputation. We decided to wait until the granulation tissue, stimulated by the NPWT, covered the whole wound before planning another coverage. Since the evolution was sluggish, on the 85th day after the accident, we performed scattered perforations in the cortical bone down to the bone marrow to promote granulation from these cortical holes. One month later (day 115th), the whole bone was practically covered with granulation tissue, and we proceeded to its coverage with Integra® (Integra Lifesciences Corp., USA); but we had to remove it a few days later because of infection.
We decided to perseverate with the NWPT, aiming for complete granulation once again. However, the evolution was torpid, alternating periods of improvement and periods of reinfection when much of the granulation tissue was lost. After three months there was still a 14 × 2 cm defect over the tibia. Computerized tomography (CT) ruled out osteomyelitis and showed only significant osteopenia. We assembled a new multidisciplinary team meeting and the resolution was still in favor of limb salvage and against amputation. We started intravenous antibiotic treatment with Colistin to treat the XDR-PA infection and scheduled a joint surgery with the orthopedic surgeon on the 235th day. Despite CT did not identify osteomyelitis, XDR-PA grew in soft tissue cultures, so in the OR we proceeded to perform anterior tibial decortication, bone marrow reaming, obliteration of the dead space with Stimulan® (Biocomposites Ltd, England) and coverage of the defect with an ipsilateral rectus femoris muscle free flap (Figure 3) with a left cephalic vein graft to reach the tibiofibular trunk, where we performed an end-to-side anastomosis. Both flap and vascular graft were covered with a skin graft. This flap had an optimal evolution (Figure 4) and one month after surgery the patient could begin to ambulate with partial weight load and orthosis. After 3 years of follow-up, the patient can walk without aid, and he has recovered his social and employment situation prior to the accident.
Figure 3.
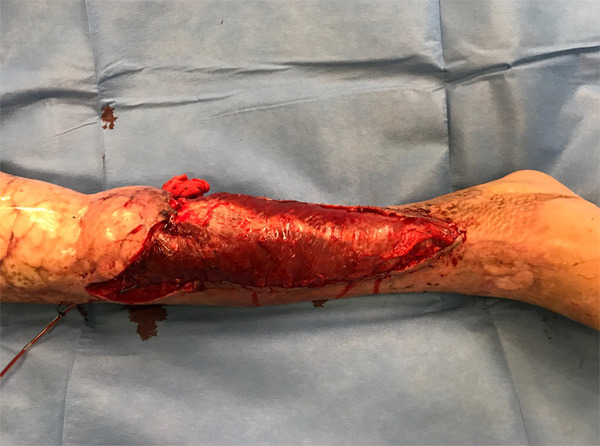
Muscle rectus femoris free flap for covering the wound resulting after the partial coverage with regional flaps.
Figure 4.
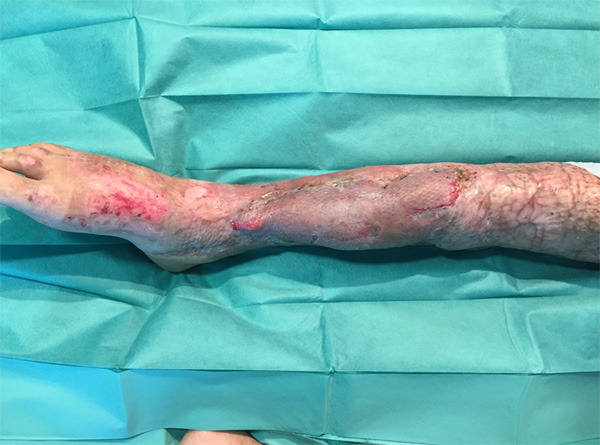
Final result. Two months after the last surgery.
Discussion
One of the first aspects to consider in a patient with severely injured lower extremity is to assess whether he will benefit more from limb salvage or, conversely, from below-the-knee amputation (Table 1). Scales developed for this purpose have shown limited utility [4]. Besides, we have not found specific literature addressing this issue in burn patients.
Table 1.
Summary of the bibliography used on the most controversial topics
| TOPIC | AUTHORS | STUDI TYPE | YEAR | RESULTS |
|---|---|---|---|---|
| Amputation vs. lower limb reconstruction | Russell Esposito et al [5] | Observational retrospective (humans) | 2017 | A general similarity in key lower extremity biomechanics between amputation and reconstruction. However, reconstruction group exhibited less ankle power and range of motion while the amputee group exhibited lower knee flexor and extensor moments and power generation. |
| Akula et al [6] | Meta-analysis (humans) | 2011 | There was no statistically significant difference in terms of physical outcomes. However, reconstruction group had a better outcome with SF-36 (p = 0.008) and psychosocial SIP (P = 0.05). | |
| Mackenzie et al [7] | Observational retrospective (humans) | 2007 | Including initial hospitalization, rehospitalizations, post-acute care and prosthesis-related costs during the first two years, the difference of mean health-care costs was significant ($81.316 reconstruction vs. $91.106 amputation; P < 0.05). The projected lifetime health-care cost for the patients who had undergone amputation was three times higher than that for those treated with reconstruction ($509.275 vs. 163.282). | |
| Mackenzie et al [8] | Observational retrospective (humans) | 2005 | No significant differences between rates of return to work and self-reported disability in amputation vs. reconstruction. | |
| Microsurgical flaps | Ofer et al [2] | Observational retrospective (humans) | 2007 | Muscle free flaps were the most common option (58%) for limb salvage in electrical burn injuries. Flap survival rate was 85%. All the failed flaps were performed within 5-21 days after trauma (early reconstruction). |
| Villaverde-Doménech et al [11] | Observational retrospective (humans) | 2015 | Fasciocutaneous free flaps were the most common option (66.6%) in burn patients (this study included secondary and sequelae reconstructions and it does not differentiate between them). Flap survival rate was 80.05%. All the failed flaps in primary reconstruction were performed in early reconstruction. | |
| De Lorenzi et al [12] | Observational retrospective (humans) | 2001 | Fasciocutaneous free flaps were the most common option (67.9%), but the majority of cases were secondary reconstructions or sequelae (65%). Flap survival rate was 94%. | |
| Baumeister et al [13] | Observational retrospective (humans) | 2005 | Muscle free flaps were the most common option (42.6%) and most flaps were performed in the first six weeks after the trauma (57%). Flap survival rate was 86.6%. 80% of the failed flaps were performed in early reconstruction. | |
| Pan et al [26] | Observational retrospective (humans) | 2007 | 38 free fasciocutaneous flaps in acute burn-hand injuries. Flap survival rate was 100% and most of the flaps (60.5%) were performed in early reconstruction. | |
| Kuo [32] | Experimental (rabbits) | 1990 | Blood vessels 3 cm beyond the margin of the electrical injury are safe if they have normal elasticity of vessel wall, intact endothelium with no separation from the media and good arterial bleeding. | |
| Muscle flaps vs. fasciocutaneous flaps | Kovar et al [14] | Meta-analysis (humans) | 2020 | No statistically significant differences in osteomyelitis recurrence (P = 0.165), partial flap loss (P = 0.701) and hematomas (P = 0.235). |
| Cho et al [15] | Observational retrospective (humans) | 2018 | No significant differences in cumulative limb salvage rates in acute trauma (P = 0.56) or chronic trauma (P = 0.51). Neither in rates of flap thrombosis, flap loss, tibial nonunion and secondary flap refinement. | |
| Cherubino et al [16] | Systematic review | 2017 | Both techniques are equal in terms of efficiency. The choice should be relegated to the experienced surgeon independently from the result. | |
| Calderon et al [17] | Experimental (dogs) | 1986 | After inoculation of bacterial concentration under the flap, musculocutaneous flaps showed a rapid decrease in bacterial load. Fasciocutaneous flaps showed a slight decrease in bacterial load (P < 0.01). | |
| Gosain et al [18] | Experimental (dogs) | 1990 | Musculocutaneous flaps demonstrated significantly lower bacterial concentrations on postoperative days 1, 3 and 6 (P < 0.001). | |
| Harry et al [19] | Experimental (mice) | 2008 | Muscle flap group showed faster bridging of the fracture site by callus, with almost 50% higher bone mineral content (P < 0.001). Biomechanical investigations demonstrated a threefold stronger union in the muscle group. | |
| Harry et al [20] | Experimental (mice) | 2009 | Fasciocutaneous tissue covering of an open tibial fracture showed a higher vascular density at all times (P < 0.0001). | |
| Glass et al [21] | Experimental (mice) | 2011 | Cells isolated from muscle adjacent to the fracture clustered and expressed alkaline phosphatase (a marker of early osteogenic differentiation) and they produced bone nodules when they were cultured to day 28. None of this happened with cells isolated from fasciocutaneous tissue adjacent to the fracture. | |
| Evans et al [22] | Experimental (rabbits) | 2009 | Adenovirus vector (Ad.BMP-2) activated muscle graft and fat graft showed promise as tissue for use in endogenous facilitated bone repair, but muscle grafts showed more bone mineral formation (P < 0.05). | |
| Hamrick et al [23] | Review | 2011 | The muscle may be a source of secreted osteogenic factors (myokines) that can influence bone mass, such as interleukin 6 and leukemia inhibitory factor. | |
| Hamrick et al [24] | Experimental (mice) | 2010 | Three injections of a recombinant myostatin inhibitor increased muscle mass (P < 0.01) and bony callus tissue formation (P < 0.05). | |
| McPherron et al [25] | Experimental (mice) | 1997 | Increased skeletal muscle mass in myostatin null mice compared to wild-type (existed hyperplasia and hypertrophy). |
SF-36: Short Form-36; SIP: Sickness Impact Profile.
Improvement in surgical technique has elevated our capability of limb salvage. However, amputation is technically easier, it requires less hospitalization time and the evolution of prosthetic devices has improved its functional outcomes. Literature shows no significant differences between these two therapeutic alternatives in terms of physical perspectives and future biomechanical functioning of the limb [5,6]. Despite this, statistically significant differences have been found in the psychological results, obtained with the Short Form-36 and Sickness Impact Profile scales, in favor of reconstruction of the lower limb [6].
Once we have established that functional outcomes will be similar, we have to consider the costs for the healthcare system of these two therapeutic options. A priori, if amputation requires a simpler surgical technique and less hospitalization days, one could assume that this option should be cheaper for the health care system. A North American study published in 2007 shows that the lifetime estimated cost of an amputee patient is more than three times higher than that of a patient undergoing lower extremity reconstruction [7]. This difference lies mainly in the purchase and maintenance expenses for the different prostheses that the patient is going to need during his life. In this study, the acute treatment cost, rehospitalization, follow-up visits, rehabilitation, prostheses and orthoses were considered. The same authors previously reported that there are no significant differences regarding the indirect cost associated with the loss of the job or with the residual disability [8].
Despite this evidence, there are currently no widely accepted protocols for treatment of gravely damaged extremities, even less in severe burn patients. Hence, personalized assessment of each case is essential, and so is considering the functional outcome that will be achieved throughout limb reconstruction [9].
In severe burn patients we have to consider other nuances. For example, that an amputation stump made up with debrided and grafted tissues may be less suitable for conventional prosthesis rehabilitation.
Once we have justified the reconstruction of gravely damaged lower extremities, we will discuss the reconstructive techniques and its peculiarities. Standard treatment for deep burns consists of tangential excision and coverage of the debrided areas with skin grafts [11]. Microsurgical flaps play a minor role in the treatment of burn patients, they are only performed in 1.5-2% of the total of patients hospitalized in a Burn Center [11,12]. Despite this marginal role, at present, free flaps have become an essential tool in selected acute cases for coverage of noble structures (bone, tendon, nerves, etc.) which might be exposed after aggressive burn excisions; in this way amputation could be avoided. They are also useful in secondary cases for scar contractures releasing [12,13]. In the past, before the introduction of microsurgery and free flaps, severely damaged lower limbs with bone and tendon exposure in severe burn patients were doomed to amputation or, when feasible, to cross-leg flaps.
Choosing between muscle and fasciocutaneous free flap in lower extremity traumatic reconstruction is controversial (Table 1). It is clear, that clinical, prospective, randomized studies are needed to answer to this question, unfortunately, this kind of evidence is not available yet. Considering clinical results, both techniques seem comparable [14-16]. There is agreement that muscle flaps are useful for obliterating dead spaces and that they fit well the contour of the lower limb. However, fasciocutaneous and perforator flaps provide a more “like-to-like” tissue and they are easier and safer to re-elevate when secondary procedures are required. Muscle flaps have their main disadvantage in terms of donor-site morbidity. On the other hand, fasciocutaneous flaps may be too bulky and could require surgical refinement [16]. Notwithstanding, experimental studies suggest that muscle flaps may be better, since they showed further decrease in bacterial load in infected wounds [17,18], further bone synthesis and more resistant bone union in open tibial fractures [19,20]. Moreover, muscle-derived stromal cells showed greater potential for osteogenesis, therefore it is natural to think that they may be better at promoting bone repair, than skin-derived stromal cells and fat-derived stromal cells [21,22]. Finally, it has been said that healthy muscles release trophic factors that stimulate bone formation, promoting the differentiation of stem cells in osteoblast [23-25]. The most commonly used flaps are latissimus dorsi muscle flap and chimeric flaps from the subscapular system for large defects, and the gracilis muscle flap for small defects [2,12,13]. For large defects, it is recommended the use of chimeric flaps over the use of pre-expanded free flaps or the combination of free and local flaps [2,12,13,26]. For these reasons we initially opted for a chimeric flap of latissimus dorsi and serratus muscles. Given its failure and the impossibility to use the contralateral subscapular system due to burn injury, we decided to combine regional flaps (which allowed us to reduce the size of the defect) with a rectus femoris muscle free flap, which is a large flap, but smaller than the chimeric one. In accordance with the literature and our personal experience, we propose a decision algorithm for the coverage of extensive defects in the lower limb in a major burn patient (Figure 5). We think muscle flaps could be better for covering infected wounds when there are bones, joints or tendons exposed than fasciocutaneous. We do self-criticism, and we advise to insist on microsurgical reconstruction when it is possible, even if there have been one or more reconstructive failures.
Figure 5.
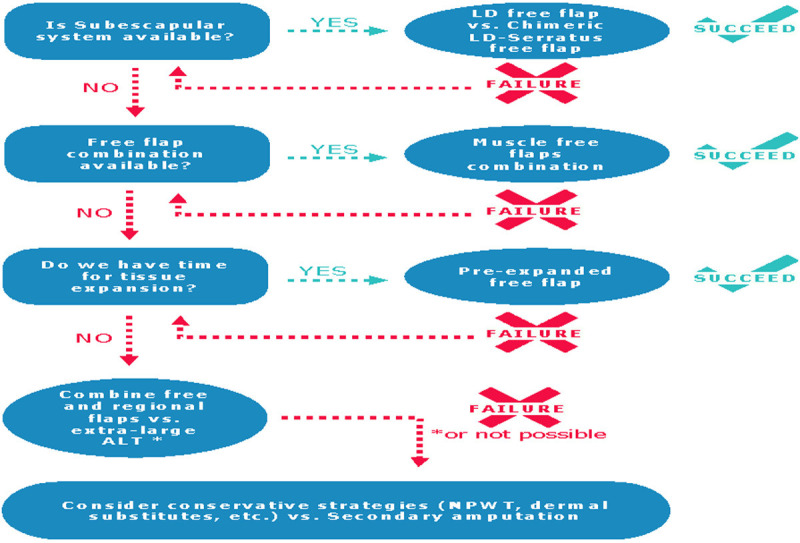
Decision algorithm for the reconstruction of wide defects with bone exposure in the lower limb in a burned patient. LD: Latissimus Dorsi; ALT: Antero Lateral Tight; NPWT: Negative Pressure Wound Therapy.
In case that the patient is not a candidate for a microsurgical free flap, there are some more conservative strategies that may also achieve salvage of the gravely damaged limb. Direct coverage of poorly vascularized structures (bone and tendon) with Integra® is possible as long as the foil sits on well vascularized tissue in adjacent areas. From these areas, cells will grow to completely colonize the structural framework [27]. A statistically significant decrease in the number of amputations has been reported in a group of patients, similar to ours, treated with Integra® vs. another group treated with local flaps and skin grafts [28]. The main risk of Integra® coverage is that infections are relatively frequent, although it is true that the infection can be easily spotted with visual inspection and it can be rapidly solved by cutting out the silicone sheet and applying a topical antiseptic cure [27]. Another technique that can be used in these cases is a good preparation of the wound bed through complete burn excision, tibial cortical bone perforations are optional, and application of NPWT to promote the coverage of the bone by granulation tissue optimal to be grafted [29,30]. Despite this, skin grafts over bone or tendon with a granulation tissue layer do not provide stable coverture over time, thus some authors advise to apply a dermal substitute over this granulation tissue [31]. However, the results achieved with these techniques are usually not satisfactory in the long term.
There is controversy in literature with regard to when microsurgical coverage should be performed (Table 1), since there seems to be a higher rate of complications (10-20%), especially if it is performed early after the injury [2,11-13]. One of the reasons for this controversy is that there is no unique classification of temporality. Some authors define primary reconstruction as the one that takes place in the first 6 weeks, and as secondary reconstruction the one that takes place after 6 weeks. Primary reconstruction can be subdivided into three groups: immediate, when it takes place in the first 5 days; early, when it takes place between day 5th and day 21th; and intermediate if it takes place between day 21th and the 6th week [11,13]. Following this classification, some series show lower survival rate of free flaps in early primary reconstruction [2,11,13], although others argue that flap survival is independent from when it is performed [26]. This greater complication rate after the 5th day could be explained by the particularities of the burn patient (immune alteration and higher infection risk, higher levels of proinflammatory cytokines which alter the equilibrium between intravascular and extravascular spaces, release of prothrombotic factors, etc.). In case of electric burns, such the case of this patient, there are also specific supplementary features to consider (vascular damage in endothelium and muscular wall, vascular occlusions, arteritis, aneurism formation, thrombosis and narrowing of the main vessels of the limbs and a notable decrease in the density of small-caliber vessels) [2,13]. These complications will appear even more often when the injury is caused by high voltage [13]. Despite this, a Chinese experimental study on rabbits defends that free flaps could be performed safely if the anastomosis is performed at least 3 cm away from the wound margins and if the recipient vessels look normal and they bleed with good flow when cut [32].
There are not many reported cases of immediate reconstruction in burn patients, since hemodynamic instability in the acute phase of this patients with extensive BSA affected usually contraindicates this type of reconstruction. Furthermore, in such an early stage, the need for a microsurgical flap may not be evident yet. Even though there may be a higher complication rate in primary reconstruction, we, as well as the authors who report this handicap, believe that in the attempt to save a limb, benefits outweigh the risks.
As it has been discussed earlier, if the patient is psychologically more satisfied with reconstruction than with amputation, the functional outcome is at least similar, and the long-term cost for society is lower; then we strongly believe that a 10-20% of failure rate is justifiable.
Conclusion
Salvaging a gravely injured lower limb in a severe burn patient is a great surgical challenge. We defend, supported by scientific literature, that the salvaging of these limbs is better than its amputation, for both patient and healthcare system; and that microsurgical reconstruction with muscle flaps could be the best option in such cases. Despite failures, if the patient still wants to preserve the limb, we consider that it must be attempted to salvage the extremity as long as it is feasible using this technique. Further investigation to establish therapeutic protocols is needed.
Acknowledgements
We would like to express our gratitude to the septic orthopedic surgery team: Dr. Lluis Carrera, Dr. Pablo Corona and Dr. Carles Amat.
Disclosure of conflict of interest
None.
References
- 1.Aguilera-Saez J, Binimelis MM, Collado JM, Dos Santos BP, Garcia V, Ruiz-Castilla M, Serracanta J, Barret JP. Electrical burns in times of economic crisis: a new epidemiologic profile. Burns. 2016;42:1861–1866. doi: 10.1016/j.burns.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 2.Ofer N, Baumeister S, Megerle K, Germann G, Sauerbier M. Current concepts of microvascular reconstruction for limb salvage in electrical burn injuries. J Plast Reconstr Surg. 2007;60:724–730. doi: 10.1016/j.bjps.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Aguilera-Saez J, Andreu-Sola V, Larrosa Escartin N, Rodríguez Garrido V, Armadans Gil L, Sanchez Garcia JM, Campins M, Baena Caparros J, Barret JP. Extensively drug-resistant Pseudomonas aeruginosa outbreak in a burn unit: management and solutions. Ann Burns Fire Disasters. 2019;32:47–55. [PMC free article] [PubMed] [Google Scholar]
- 4.Bosse MJ, Mackencie EJ, Kellam JF, Burgess AR, Webb LX, Swiontkowski MF, Sanders RW, Jones AL, McAndrew MP, Patterson BM, McCarthy ML, Cyril JK. A prospective evaluation of the clinical utility of the lower-extremity injury severety scores. J Bone Join Surg. 2001;83:3–14. doi: 10.2106/00004623-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Russell Esposito E, Stinner DJ, Fergason JR, Wilken JM. Gait biomechanics following lower extremity trauma: amputation vs. reconstruction. Gait Posture. 2017;54:167–173. doi: 10.1016/j.gaitpost.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 6.Akula M, Gella S, Shaw CJ, McShane P, Mohsen AM. A meta-analysis of amputation versus limb salvage in mangled lower limb injuries--the patient perspective. Injur. 2011;42:1194–1197. doi: 10.1016/j.injury.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Mackenzie EJ, Jones AS, Bosse MJ, Castillo RC, Pollak AN, Webb LX, Swiontkoski MF, Kellam JF, Smith DG, Sanders RW, Jones AL, Starr AJ, McAndrew MP, Patterson BM, Burgess AR. Health-care costs associated with amputation or reconstruction of a limb-threatening injury. J Bone Joint Surg Am. 2007;89:1685–1692. doi: 10.2106/JBJS.F.01350. [DOI] [PubMed] [Google Scholar]
- 8.Mackenzie EJ, Bosse MJ, Pollak AN, Webb LX, Swiontkowski MF, Kellam JF, Smith DG, Sanders RW, Jones AL, Starr AJ, McAndrew MP, Patterson BM, Burgess AR, Castillo RC. Long-term persistence of disability following severe lower-limb trauma. Results of a seven-year follow-up. J Bone Joint Surg Am. 2005;87:1801–1809. doi: 10.2106/JBJS.E.00032. [DOI] [PubMed] [Google Scholar]
- 9.Camporro-Fernandez D, Ontaneda-Rubio A, Castellanos-Moran M. Tratamiento de fracturas abiertas de tibia grado IIIB-IIIC de Gustilo con colgajos libres microvascularizados. Cir Plast Iberolatinoam. 2015;41:283–293. [Google Scholar]
- 10.Papini R. ABC of burns: management of burn injuries of various depths. BMJ. 2004;329:158–160. doi: 10.1136/bmj.329.7458.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villaverde-Domenech ME, Simon-Sanz E, Delgado-Ruiz T, Perez-Ramos L, Safont-Albert J. El reto de las transferencias de colgajos libres en pacientes quemados: ¿cual es el momento para la cirugia? Cir Plast Iberolatinoam. 2015;41:117–126. [Google Scholar]
- 12.De Lorenzi F, Van der Hulst R, Boeckx W. Free flaps in burn reconstruction. Burns. 2001;27:603–612. doi: 10.1016/s0305-4179(01)00006-7. [DOI] [PubMed] [Google Scholar]
- 13.Baumeister S, Köller M, Dragu A, Germann G, Sauerbier M. Principles of microvascular reconstruction in burn and electrical burn injuries. Burns. 2005;31:92–98. doi: 10.1016/j.burns.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Kovar A, Colakoglu S, Iorio ML. Choosing between muscle and fasciocutaneous free flap reconstruction in the treatment of lower extremity osteomyelitis: available evidence for a function-specific Approach. J Reconstr Microsurg. 2020;36:197–203. doi: 10.1055/s-0039-1698469. [DOI] [PubMed] [Google Scholar]
- 15.Cho EH, Shammas RL, Carney MJ, Weissler JM, Bauder AR, Glener AD, Kovach SJ, Hollenbeck SJ, Levin LS. Muscle versus fasciocutaneous free flaps in lower extremity traumatic reconstruction: a multicenter outcomes analysis. Plast Reconstr Surg. 2018;141:191–199. doi: 10.1097/PRS.0000000000003927. [DOI] [PubMed] [Google Scholar]
- 16.Cherubino M, Corno M, D’Arpa S, Di Summa P, Pellegatta I, Valdatta L, Ronga M. Muscle versus fasciocutaneous flap in lower limb reconstruction: is there a best option? J Reconstr Microsurg. 2017;33:S27–S33. doi: 10.1055/s-0037-1606559. [DOI] [PubMed] [Google Scholar]
- 17.Calderon W, Chang N, Mathes SJ. Comparison to the effect of bacterial inoculation in musculocutaneous and fasciocutaneous flaps. Plast Reconstr Surg. 1986;77:785–794. doi: 10.1097/00006534-198605000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Gosain A, Chang N, Mathes S, Hunt TK, Vasconez L. A study of the relationship between blood flow and bacterial inoculation in musculocutaneous and fasiocutaneous flap. Plast Reconstr Surg. 1990;86:1152–1163. [PubMed] [Google Scholar]
- 19.Harrry LE, Sandison A, Paleolog EM, Hansen U, Pearse MF, Nanchahal J. Comparison of the healing of open tibial fractures covered with either muscle or fasciocutaneous tissue in a murine model. J Orthop Res. 2008;26:1238–1244. doi: 10.1002/jor.20649. [DOI] [PubMed] [Google Scholar]
- 20.Harry LE, Sandison A, Pearse MF, Paleolog EM, Nanchahal J. Comparison of the vascularity of fasciocutaneous tissue and muscle for coverage of open tibial fractures. Plast Recontr Surg. 2009;124:1211–1219. doi: 10.1097/PRS.0b013e3181b5a308. [DOI] [PubMed] [Google Scholar]
- 21.Glass GE, Chan JK, Freidin A, Feldmann M, Horwood NJ, Nanchahal J. TNF-alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc Natl Acad Sci U S A. 2011;108:1585–1590. doi: 10.1073/pnas.1018501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans CH, Liu FJ, Glatt V, Hoyland JA, Kirker-Head C, Walsh A, Betz O, Wells JW, Betz V, Porter RM, Saad FA, Gerstenfeld LC, Einforn TA, Harris MB, Vrahas MS. Use of genetically modified muscle and fat grafts to repair defects in bone and cartilage. Eur Cell Mater. 2009;18:96–111. doi: 10.22203/ecm.v018a09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamrick MW. A role for myokines in muscle-bone interactions. Exerc Sport Sci Rev. 2011;39:43–47. doi: 10.1097/JES.0b013e318201f601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamrick MW, Arounleut P, Kellum E, Cain M, Immel D, Liang LF. Recombinant myostatin (GDF-8) propeptide enhances the repair and regeneration of both muscle and bone in a model of deep penetrant musculoskeletal injury. J Trauma. 2010;69:579–583. doi: 10.1097/TA.0b013e3181c451f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 26.Pan CH, Chuang SS, Yang JY. Thirty eight free fasciocutaneous flap transfers in acute burned-hand injuries. Burns. 2007;33:230–235. doi: 10.1016/j.burns.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 27.Jeng JC, Fidler PE, Sokolich JC, Jaskille AD, Khan S, White PM, Street JH 3rd, Light TD, Jordan MH. Seven years’ experience with Integra as a reconstructive tool. J Burn Care Res. 2007;28:120–126. doi: 10.1097/BCR.0b013E31802CB83F. [DOI] [PubMed] [Google Scholar]
- 28.Verbelen J, Hoeksema H, Pirayesh A, Van Landuyt K, Monstrey S. Exposed tibial bone after burns: flap reconstruction versus dermal substitute. Burns. 2016;42:e31–e37. doi: 10.1016/j.burns.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 29.De Juan Perez FJ. Terapia VAC® en traumatismo grave de pierna izquierda. Cirugia Plastica Ibero-Latinoamericana. 2010;36:247–254. [Google Scholar]
- 30.Bernal-Martinez AJ, Lopez-Cabrera P, Sampietro de Luis JM, Puertas-Peña J, Agullo-Domingo A. La terapia de vacio como alternativa terapeutica en quemaduras con exposicion osea. Cir Plast Iberolatinoam [Internet] 2016;42:355–360. [Google Scholar]
- 31.Lee LF, Porch JV, Spenler W, Garner WL. Integra in lower extremity reconstruction after burn injury. Plast Reconstr Surg. 2008;121:1256–1262. doi: 10.1097/01.prs.0000304237.54236.66. [DOI] [PubMed] [Google Scholar]
- 32.Kuo ET. Experimental study of free flap transplantation after debridement in early stage o electric burn. Zhonghua Zheng Xing Shao Shang Wai Ke Za Zhi. 1990;6:285–2. [PubMed] [Google Scholar]


