Key Points
Question
Is visit-to-visit variability in kidney function and serum electrolyte indexes associated with risk of adverse clinical outcomes among patients with chronic, stable heart failure with preserved ejection fraction?
Findings
This cohort study of patients with chronic heart failure with preserved ejection fraction suggests that higher visit-to-visit variability in creatinine, blood urea nitrogen, sodium, and potassium levels is significantly associated with a higher risk of adverse clinical outcomes independent of other potential confounders and changes in these parameters.
Meaning
In heart failure with preserved ejection fraction, visit-to-visit variability in laboratory indexes of kidney function and certain serum electrolytes may identify a higher-risk disease state with worse long-term clinical outcomes.
Abstract
Importance
Although kidney dysfunction and abnormalities in serum electrolyte levels are associated with poor clinical outcomes in patients with heart failure with preserved ejection fraction (HFpEF), the association of visit-to-visit variability in such laboratory measures with long-term outcomes is unclear.
Objective
To evaluate the associations of visit-to-visit variability in indexes of kidney function (creatinine and blood urea nitrogen [BUN] levels) and serum electrolyte (sodium, chloride, and potassium) with the risk of adverse clinical outcomes among patients with chronic, stable HFpEF.
Design, Setting, and Participants
This cohort analysis used data from the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial. All participants with 3 or more serial laboratory measurements who were event free within the first 4 months of enrollment were included. Data were analyzed from March 1, 2019, to January 31, 2020.
Main Outcomes and Measures
Adjusted associations between indexes of variability in serum laboratory measurements during the first 4 months of follow-up and risk of the primary composite outcome (a composite of aborted cardiac arrest, hospitalization for heart failure, or cardiovascular death) and all-cause mortality were assessed using Cox proportional hazards regression models.
Results
Of the 3445 patients enrolled in the TOPCAT trial (mean [SD] age, 68-69 [10] years; 49.7%-51.5% female), 2479 (BUN) to 3195 (potassium) were analyzed, depending on availability of serial measurements. Participants with higher laboratory variability in kidney function parameters were older, had more comorbidities, and had more severe symptoms of HFpEF. Higher visit-to-visit variability in BUN (hazard ratio [HR] per 1-SD higher average successive variability [ASV], 1.21; 95% CI, 1.10-1.33) and creatinine (HR per 1-SD higher ASV, 1.13; 95% CI, 1.04-1.22) were independently associated with a higher risk of the primary composite outcome as well as mortality independent of other baseline confounders, changes in kidney function, changes in medication dosages, and variability in other cardiometabolic parameters (systolic blood pressure and body mass index). The higher risk associated with greater variability in kidney function was consistent across subgroups of patients stratified by the presence of chronic kidney disease (CKD) at baseline (CKD: HR per 1-SD higher ASV, 1.39; 95% CI, 1.16-1.67 and no CKD: HR per 1-SD higher ASV, 1.13; 95% CI, 1.01-1.27), among placebo and spironolactone treatment arms separately (spironolactone arm: 1.30; 95% CI, 1.03-1.65 and placebo arm: HR per 1-SD higher ASV, 1.27; 95% CI, 1.04-1.56). Among serum electrolytes, variability in sodium and potassium measures were also significantly associated with a higher risk of primary composite events (sodium: HR per 1-SD higher ASV, 1.14; 95% CI, 1.01-1.30 and potassium: HR per 1-SD higher ASV, 1.21; 95% CI, 1.02-1.44).
Conclusions and Relevance
In HFpEF, visit-to-visit variability in laboratory indexes of kidney function and serum electrolytes is common and independently associated with worse long-term clinical outcomes.
This secondary cohort study evaluates the associations of visit-to-visit variability in indexes of kidney function and serum electrolytes with the risk of adverse clinical outcomes among patients with chronic, stable heart failure with preserved ejection fraction.
Introduction
Chronic derangements in kidney function or serum electrolytes are common in patients with heart failure (HF) with preserved ejection fraction (HFpEF), indicate an advanced disease state, and are associated with increased risks of adverse outcomes. Specifically, kidney dysfunction and electrolyte disturbances in HFpEF are associated with reduced exercise capacity, increased hospitalizations for HF, and mortality.1,2,3,4,5,6 Furthermore, short-term deteriorations in kidney function are associated with worse outcomes in individuals with chronic HFpEF.7,8 To date, reported associations between kidney dysfunction or electrolyte abnormalities and long-term adverse events in HFpEF have relied on single laboratory measurements or short-term changes in such markers.
Visit-to-visit variability in serum creatinine levels is associated with increased risk of mortality among patients with chronic kidney disease (CKD)9,10 and myocardial infarction.11 The prognostic significance of visit-to-visit variability in laboratory measurements of kidney function or serum electrolyte levels in patients with HFpEF is currently unclear. In the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial, multiple serum laboratory markers, including kidney function and serum electrolyte indexes, were measured serially as part of the trial protocol.12 In this study, we aimed to evaluate the associations of visit-to-visit variability in indexes of kidney function and serum electrolytes with risk of adverse clinical outcomes among participants in the TOPCAT trial. We hypothesized that higher visit-to-visit variability in laboratory markers would be associated with higher risk of adverse clinical outcomes.
Methods
Study Population and Laboratory Assessment
In this secondary observational cohort analysis, we used deidentified data from the TOPCAT trial obtained from the National Heart, Lung, and Blood Institute’s Biologic Specimen and Data Repository Information Coordinating Center. The details about the study design and protocol have been previously published and are described in the eMethods in the Supplement.12 Briefly, the TOPCAT trial was a double-blind randomized clinical trial that evaluated the efficacy of spironolactone (vs placebo) in 3445 participants with chronic HFpEF. The mean (SD) study follow-up time was 3.3 (1.7) years. All participants enrolled in the TOPCAT trial provided written informed consent. The clinical trial protocol was approved by the institutional review board at all participating institutions. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
The current study analysis was performed from March 1, 2019, to January 31, 2020. Participants underwent detailed evaluation at baseline, including medical history questionnaires, physical examination, and blood sampling for measuring laboratory parameters of kidney function and electrolytes. These laboratory parameters were measured at baseline, 4 weeks, 8 weeks, and 4 months after randomization at local institutions. The present analysis included all participants with 3 or more serial laboratory measurements and were free of the primary outcome event within the first 4 months. In the current study, percentage change in laboratory values were calculated using data obtained from the baseline visit until up to 4 months of follow-up.
Definition of Visit-to-Visit Variability in Kidney Function and Serum Electrolyte Indexes
Primary exposure variables of interest for this analysis were visit-to-visit variability in kidney function parameters of blood urea nitrogen (BUN) and creatinine. Secondary exposure variables of interest were variability in other electrolytes, such as sodium, potassium, and chloride. Variability of laboratory values was defined as the fluctuation between visits for each participant and was calculated using repeated measures of BUN, creatinine, potassium, sodium, or chloride between baseline and 4 months. Each of these electrolytes was measured locally across trial sites. The mean laboratory value by visit is displayed in eFigure 1 in the Supplement. The following 3 established measures of laboratory variability were used: average successive variability (ASV: mean absolute difference between successive laboratory values), coefficient of variation (CV: SD divided by the mean laboratory value), and SD.13,14 We prespecified ASV as the primary exposure variable of interest. Participants with less than 3 available laboratory measurements were excluded from analyses.
Clinical Outcomes
The primary outcome for the current analysis was a composite of aborted cardiac arrest, hospitalization for management of HF, or cardiovascular death, which is consistent with the primary outcome of the TOPCAT trial. The secondary outcome of interest was all-cause mortality. All the outcome events included in the primary composite outcome were clinically adjudicated by the TOPCAT Trial Clinical End Point Adjudication Committee using an established protocol as previously described.12 Follow-up was recorded in all patients, and 4 months from randomization was defined as time origin (landmark) for this analysis. Time to event was measured as the number of months from randomization to the date of first event occurrence. Patients with a primary outcome event within 4 months from enrollment were excluded from the present analysis.
Statistical Analysis
Patients were stratified into data-derived quintiles (from lowest to highest) of ASV, and baseline characteristics at the time of randomization were reported across study groups as number (percentage) for categorical variables and mean (SD) for continuous variables. As previously reported, natriuretic peptides (NPs) measured at baseline (brain natriuretic peptide and N-terminal prohormone brain NP) were combined into a single log-transformed z score.15 Differences in participant characteristics across groups were evaluated by χ2 tests for categorical and 1-way analysis of variance for continuous variables. Separate stepwise-forward selection generalized linear models with minimization of the Akaike information criterion were used to determine the optimal factors associated with variability (ASV) in BUN and creatinine.
Unadjusted rates of the primary outcome were compared across ASV quintiles of the kidney function parameters (BUN and creatinine) and further stratified by the presence of CKD (defined as an estimated glomerular filtration rate <60 mL/min per 1.73 m2 at baseline) and categories of percentage change in BUN and creatinine from baseline to 4-month follow-up (≥10% loss, <10 change, or ≥10% gain). Multivariable Cox proportional hazards regression models were constructed to evaluate the associations of continuous measures of variability in each laboratory parameter with primary and secondary outcomes. Separate models were constructed using different measures of variability (ASV, CV, and SD) in each laboratory parameter with sequential adjustment for potential confounders based on biologic plausibility as follows: model 1: age, sex, race/ethnicity, educational level, treatment arm, country of enrollment, and enrollment stratum; model 2: model 1 plus body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), systolic blood pressure (SBP), diabetes status, alcohol use, smoking history, history of atrial fibrillation, angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use, history of cardiovascular disease, New York Heart Association (NYHA) class, and baseline respective laboratory value (BUN, creatinine, potassium, sodium, or chloride); and model 3: model 2 plus percentage change in respective laboratory values, variability (defined by ASV) in SBP and BMI (baseline to month 4), and spironolactone and loop diuretic medication doses as time-varying covariates. Loop diuretics were converted to furosemide equivalents as previously reported.16,17
Several additional analyses were performed to evaluate the robustness of the association between variability in laboratory measures and risk of the primary outcome. First, in the subset of participants with available data on NPs, the NP z score was included as a covariate in the most adjusted model. Second, sensitivity analyses were performed using data limited to participants enrolled in North and South America.18,19 Third, sensitivity analysis was also performed using a longer follow-up period to estimate variability in kidney function parameters (baseline to 1 year and 4 months to 1 year). Fourth, adjusted associations between variability in laboratory values and risk of the primary outcome were assessed across subgroups stratified by the treatment arm (spironolactone and placebo groups), baseline CKD status (yes vs no), and tertiles of Meta-Analysis Global Group in Chronic Heart Failure (MAGGIC) risk score, a well-established quantitative risk score to estimate HF severity.20 To evaluate whether visit-to-visit variability in kidney function and percentage change in kidney function improved discrimination performance for estimating all-cause mortality over the well-established measures of disease severity, such as the MAGGIC risk score, C indexes were compared for models, including the MAGGIC risk score, MAGGIC risk score plus ASV in BUN and creatinine, and MAGGIC risk score plus percentage change in BUN and creatinine.
Analyses were performed using R statistical software, version 3.6.0 (R Foundation for Statistical Computing) with P < .05 indicating statistical significance.
Results
Of the 3445 patients enrolled in the TOPCAT trial (mean [SD] age, 68-69 [10] years; 49.7%-51.5% female), 2479 (BUN) to 3195 (potassium) were analyzed, depending on availability of serial measurements. The median number of BUN measurements between enrollment and month 4 was 4. The mean (SD) variability of BUN, as measured by ASV, was 5.3 (5.0) mg/dL (to convert to millimoles per liter, multiply by 0.357). Baseline characteristics of participants included vs excluded from the current analysis are given in eTable 1 in the Supplement.
The baseline characteristics of participants stratified by quintiles of visit-to-visit BUN variability are given in Table 1. The 472 participants with higher BUN variability (quintile 5) were more commonly older (mean [SD] age, 70.6 [9.7] years), Black (57 [12.1%]), and enrolled in the spironolactone treatment arm (297 [62.9%]) and had a higher BMI (34.0 [7.9]). These participants also had a higher NYHA class (198 [41.9%]) and higher loop diuretic dosages (106.5 [159.3] mg at baseline and 120.7 [180.2] mg at month 4 of oral furosemide equivalent dose); a higher prevalence of cardiovascular disease (203 [43.0%]), diabetes (133 [28.2%] with insulin-dependent diabetes and 98 [20.8%] with non–insulin-dependent diabetes), and anemia (hemoglobin, 12.7 [2.3] g/dL [to convert to grams per liter, multiply by 10); and higher levels of BUN (28.1 [13.4] mg/dL [to convert to millimoles per liter, multiply by 0.357]), creatinine (1.3 [0.4] mg/dL [to convert to micromoles per liter, multiply by 88.4]), and glucose (126.5 [63.9] mg/dL [to convert to millimoles per liter, multiply by 0.0555]). No statistically significant differences were found in NP levels across variability quintiles (BNP: 374.3 [420.0] pg/mL in quintile 1 vs 417.1 [385.8] pg/mL in quintile 5; P = .20; NT-proBNP: 1184.8 [1859.6] pg/mL in quintile 1 vs 1638.6 [2058.8] pg/mL in quintile 5; P = .48 [to convert to nanograms per liter, multiply by 1]).
Table 1. Baseline Characteristics Across Quintiles of BUN Variability Assessed by ASV.
| Characteristic | Quintile | P value | ||||
|---|---|---|---|---|---|---|
| 1 (n = 526) | 2 (n = 483) | 3 (n = 503) | 4 (n = 495) | 5 (n = 472) | ||
| ASV, mg/dL | 1.0 (0.3) | 2.3 (0.4) | 3.6 (0.5) | 5.7 (0.8) | 13.8 (9.1) | |
| Demographica | ||||||
| Age, y | 65.8 (8.9) | 67.1 (9.4) | 69.5 (9.3) | 70.7 (10.0) | 70.6 (9.7) | .002 |
| Female sex, No. (%) | 285 (54.2) | 234 (48.4) | 242 (48.1) | 250 (50.5) | 222 (47.0) | .16 |
| Race/ethnicity, No. (%) | <.001 | |||||
| White | 489 (93.0) | 443 (91.7) | 442 (87.9) | 429 (86.7) | 403 (85.4) | |
| Black | 31 (5.9) | 28 (5.8) | 49 (9.7) | 52 (10.5) | 57 (12.1) | |
| Other | 6 (1.1) | 12 (2.5) | 12 (2.4) | 14 (2.8) | 12 (2.5) | |
| North and South America, No. (%) | 151 (28.7) | 200 (41.4) | 296 (58.8) | 333 (67.3) | 375 (79.4) | <.001 |
| Spironolactone treatment arm, No. (%) | 238 (45.2) | 216 (44.7) | 256 (50.9) | 241 (48.7) | 297 (62.9) | .008 |
| Clinical characteristics | ||||||
| SBP, mm Hg | 132.3 (13.1) | 128.4 (12.6) | 128.8 (14.1) | 127.3 (14.1) | 127.7 (14.9) | <.001 |
| BMI | 31.0 (6.3) | 31.6 (7.0) | 32.0 (7.2) | 32.2 (6.9) | 34.0 (7.9) | <.001 |
| NYHA class III-IV | 110 (21.0) | 142 (29.4) | 157 (31.2) | 188 (38.0) | 198 (41.9) | <.001 |
| Alcoholic drinks per week | .04 | |||||
| 0 | 426 (81.0) | 346 (71.8) | 371 (73.8) | 381 (77.0) | 345 (73.1) | |
| 1-5 | 85 (16.2) | 107 (22.2) | 100 (19.9) | 79 (16.0) | 91 (19.3) | |
| 5-10 | 10 (1.9) | 18 (3.7) | 21 (4.2) | 24 (4.8) | 25 (5.3) | |
| >10 | 5 (1.0) | 11 (2.3) | 10 (2.0) | 11 (2.2) | 11 (2.3) | |
| Current smoking | 60 (11.4) | 59 (12.2) | 54 (10.7) | 33 (6.7) | 38 (8.1) | .04 |
| Variability in SBP | 8.3 (6.3) | 10.1 (6.4) | 11.7 (7.6) | 11.9 (8.1) | 13.7 (8.1) | <.001 |
| Variability in BMI | 0.5 (0.5) | 0.6 (0.6) | 0.6 (0.6) | 0.6 (0.5) | 0.7 (0.8) | <.001 |
| Medical history, No. (%) | ||||||
| History of CVD | 144 (27.4) | 175 (36.2) | 206 (41.0) | 176 (35.6) | 203 (43.0) | .005 |
| Atrial fibrillation | 143 (27.2) | 157 (32.6) | 202 (40.2) | 243 (49.1) | 189 (40.0) | <.001 |
| Diabetes status | <.001 | |||||
| None | 404 (76.8) | 354 (73.4) | 329 (65.4) | 323 (65.3) | 241 (51.1) | |
| Insulin dependent | 31 (5.9) | 31 (6.4) | 64 (12.7) | 72 (14.5) | 133 (28.2) | |
| Non–insulin dependent | 91 (17.3) | 97 (20.1) | 110 (21.9) | 100 (20.2) | 98 (20.8) | |
| ACEI or ARB medication | 473 (89.9) | 402 (83.2) | 417 (82.9) | 409 (82.6) | 394 (83.5) | .003 |
| Loop diuretic medication | 443 (84.2) | 388 (80.3) | 419 (83.3) | 438 (88.5) | 431 (91.3) | <.001 |
| Loop diuretic dose, mgb | ||||||
| At baseline | 47.5 (35.4) | 57.9 (42.7) | 64.7 (58.1) | 74.6 (85.9) | 106.5 (159.3) | <.001 |
| At month 4 | 62.5 (77.6) | 59.9 (46.6) | 78.7 (79.1) | 87.5 (86.1) | 120.7 (180.2) | <.001 |
| Laboratory values | ||||||
| Sodium, mEq/L | 142.0 (4.2) | 141.4 (4.2) | 140.8 (4.0) | 140.6 (3.7) | 140.1 (3.8) | <.01 |
| Potassium, mEq/L | 4.3 (0.5) | 4.3 (0.4) | 4.2 (0.4) | 4.2 (0.4) | 4.2 (0.4) | .09 |
| Chloride, mEq/L | 100.6 (14.8) | 100.6 (15.9) | 99.8 (18.3) | 100.1 (17.7) | 99.7 (16.7) | .85 |
| BUN, mg/dL | 14.2 (7.4) | 18.5 (8.8) | 20.8 (8.8) | 22.3 (9.3) | 28.1 (13.4) | <.001 |
| Creatinine, mg/dL | 1.0 (0.2) | 1.0 (0.3) | 1.1 (0.3) | 1.1 (0.3) | 1.3 (0.4) | .001 |
| Glucose, mg/dL | 107.2 (33.6) | 107.8 (39.6) | 116.7 (56.5) | 118.0 (53.2) | 126.5 (63.9) | <.001 |
| Hemoglobin, g/dL | 13.3 (2.1) | 13.2 (1.7) | 13.2 (2.2) | 13.0 (2.5) | 12.7 (2.3) | <.001 |
| BNP, pg/mL | 374.3 (420.0) | 330.0 (380.2) | 430.7 (608.2) | 335.7 (290.5) | 417.1 (385.8) | .20 |
| NT-proBNP, pg/mL | 1184.8 (1859.6) | 1497.9 (1949.8) | 1376.5 (1586.4) | 1656.8 (1939.7) | 1638.6 (2058.8) | .48 |
| z Scorec | −0.1 (0.9) | −0.1 (0.9) | 0.0 (1.2) | −0.0 (0.8) | 0.1 (0.9) | .35 |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ASV, average successive variability; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BNP, brain natriuretic peptide; BUN, blood urea nitrogen; CVD, cardiovascular disease; NT-proBNP, N-terminal prohormone brain natriuretic peptide; NYHA, New York Heart Association; SBP, systolic blood pressure.
SI conversion factors: To convert BNP and NT-proBNP to nanograms per liter, multiply by 1; BUN to millimoles per liter, multiply by 0.357; chloride, sodium, and potassium to millimoles per liter, multiply by 1; creatinine to micromoles per liter, multiply by 88.4; hemoglobin to grams per liter, multiply by 10; and glucose to millimoles per liter, multiply by 0.0555.
Data are presented as mean (SD) unless otherwise indicated.
Loop diuretic dose is represented as oral furosemide equivalent.
Natriuretic peptide (BNP or NT-proBNP) levels were available in 1039 participants.
Factors Associated With Kidney Function Variability
In adjusted analysis, patient characteristics significantly associated with higher visit-to-visit variability in both BUN and creatinine were baseline levels of the laboratory parameter (BUN: coefficient, 0.20; 95% CI, 0.18-0.22 and creatinine: coefficient, 0.13; 95% CI, 0.12-0.15), spironolactone use (BUN: coefficient, 0.99; 95% CI, 0.59-1.39 and creatinine: coefficient, 0.021; 95% CI, 0.01-0.03), loop diuretic dose at baseline (BUN: coefficient, 0.01; 95% CI, 0.008-0.16 and creatinine: coefficient, 0.00024; 95% CI, 0.0002-0.0003), prior hospitalization for HF (BUN: coefficient, 0.78; 95% CI, 0.30-1.25 and creatinine: coefficient, 0.02; 95% CI, 0.008-0.03), and country of enrollment (North or South America vs Russia or Georgia) (BUN: coefficient, 1.20; 95% CI, 0.68-1.72 and creatinine: coefficient, 0.03; 95% CI, 0.02-0.04) (eTable 2 in the Supplement). Spironolactone dose at month 4 was not associated with variability in BUN or creatinine. Change in loop diuretic dose was associated with greater variability in BUN but not creatinine (BUN: coefficient, 0.006; 95% CI, 0.0001-0.01).
Visit-to-Visit Variability in BUN and Risk of Adverse Outcomes
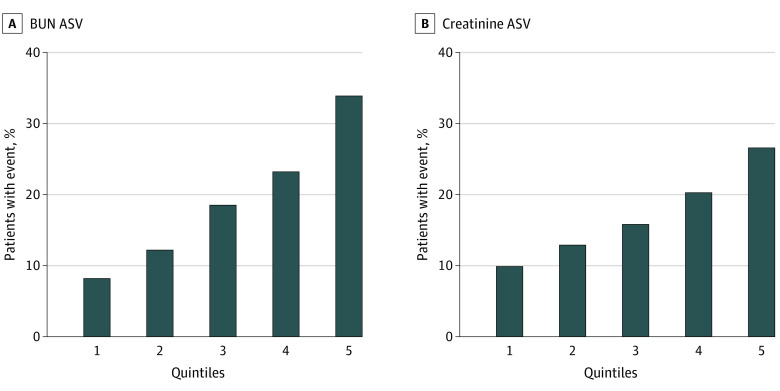
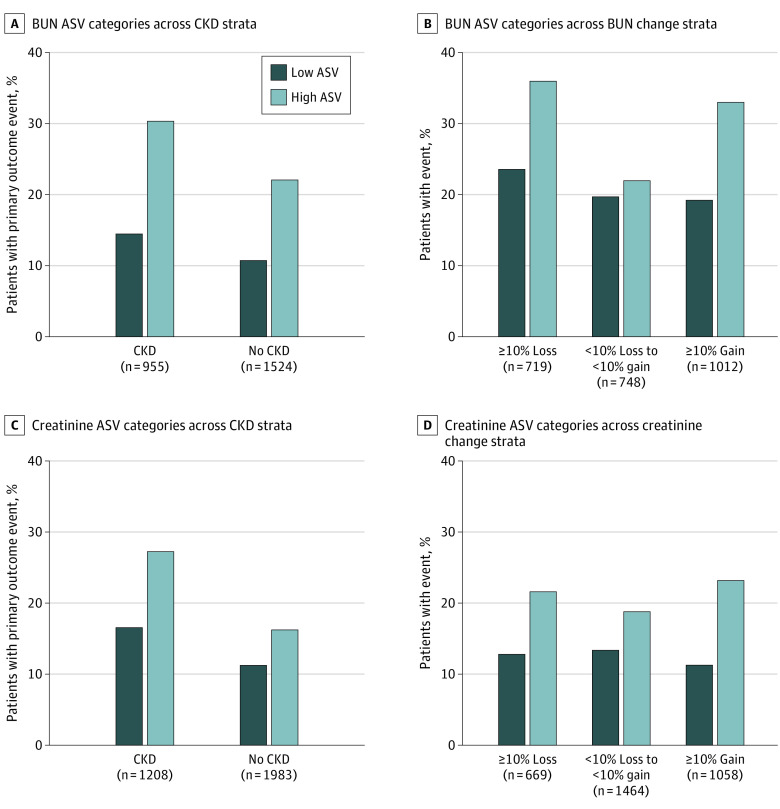
During a mean (SD) follow-up of 3.1 (1.7) years from the end of BUN variability assessment at month 4, 475 primary outcome events (19.2%) occurred. The risk of primary outcome increased in a graded fashion across quintiles of ASV (8.4% in quintile 1 and 34.1% in quintile 5) (Figure 1A). The higher risk of primary outcome among individuals with higher BUN variability was consistent across subgroups of patients with vs without CKD and across categories of percentage change in BUN (Figure 2A and B). In adjusted analyses that accounted for baseline confounders, greater variability in BUN was associated with significantly higher risk of the primary composite outcome (model 1: hazard ratio [HR] per 1-SD higher ASV, 1.22; 95% CI, 1.15-1.30) (Table 2). This association remained significant after further adjustment for percentage change in BUN, variability in other cardiometabolic parameters (SBP and BMI), and time-varying medication dosage adjustments (model 3: HR per 1-SD higher ASV, 1.21; 95% CI, 1.10-1.33) (Table 2). In contrast, percentage change in BUN was not associated with risk of the primary outcome in the most adjusted model (HR, 0.98; 95% CI, 0.96-1.01). In addition, no significant interaction was noted between ASV and the treatment group for the risk of primary outcome (P = .10 for interaction). Similar patterns of association were also observed for other measures of BUN variability (CV and SD) and risk of the primary outcome (model 3: HR per 1-SD higher CV, 1.24; 95% CI, 1.11-1.39 and HR per 1-SD higher SD, 1.23; 95% CI, 1.09-1.38) (eTables 3 and 4 in the Supplement). In secondary analyses, higher measures of BUN variability were also significantly associated with higher risk of all-cause mortality in the partially and fully adjusted models (model 3: HR per 1-SD higher ASV, 1.15; 95% CI, 1.03-1.30) (Table 2 and eTables 3 and 4 in the Supplement).
Figure 1. Quintiles of Visit-to-Visit Average Successive Variability (ASV) in Blood Urea Nitrogen (BUN) and Creatinine and Rates of Primary Composite Outcome.
Primary composite outcome is a composite of aborted cardiac arrest, hospitalization for management of heart failure, or cardiovascular death.
Figure 2. Blood Urea Nitrogen (BUN) and Creatinine Average Successive Variability (ASV) and Risk of Primary Composite Outcome Across Chronic Kidney Disease (CKD) Status and Percentage Change in BUN and Creatinine Values.
Primary composite outcome is a composite of aborted cardiac arrest, hospitalization for management of heart failure, or cardiovascular death. High and low variability indicates variability above and below the median, respectively. Chronic kidney disease was defined as an estimated glomerular filtration rate less than 60 mL/min per 1.73 m2.
Table 2. Associations of ASV Among 5 Laboratory Measures With the Primary Composite Outcomea and All-Cause Mortality .
| Variable | No. (%) of events | Model 1b | Model 2c | Model 3d | |||
|---|---|---|---|---|---|---|---|
| HR per 1-SD higher ASV (95% CI) | P value | HR per 1-SD higher ASV (95% CI) | P value | HR per 1-SD higher ASV (95% CI) | P value | ||
| Primary composite outcome | |||||||
| BUN | 475 (19.2) | 1.22 (1.15-1.30) | <.001 | 1.15 (1.07-1.24) | <.001 | 1.21 (1.10-1.33) | <.001 |
| Creatinine | 548 (17.2) | 1.25 (1.18-1.32) | <.001 | 1.17 (1.09-1.25) | <.001 | 1.13 (1.04-1.22) | .003 |
| Sodium | 548 (17.2) | 1.18 (1.08-1.29) | <.001 | 1.17 (1.07-1.29) | <.001 | 1.14 (1.01-1.30) | .04 |
| Potassium | 549 (17.2) | 1.17 (1.08-1.26) | <.001 | 1.15 (1.06-1.25) | <.001 | 1.21 (1.02-1.44) | .03 |
| Chloride | 542 (17.5) | 1.09 (1.00-1.19) | .05 | 1.11 (1.01-1.22) | .04 | 1.19 (0.96-1.40) | .08 |
| All-cause mortality | |||||||
| BUN | 374 (15.1) | 1.22 (1.13-1.31) | <.001 | 1.14 (1.04-1.26) | .005 | 1.15 (1.03-1.30) | .02 |
| Creatinine | 450 (14.1) | 1.15 (1.09-1.22) | <.001 | 1.12 (1.04-1.20) | .002 | 1.16 (1.04-1.32) | .01 |
| Sodium | 450 (14.1) | 1.15 (1.05-1.27) | .004 | 1.13 (1.03-1.25) | .01 | 1.14 (1.02-1.27) | .02 |
| Potassium | 451 (14.1) | 1.13 (1.04-1.23) | .004 | 1.11 (1.02-1.21) | .02 | 1.10 (1.01-1.20) | .04 |
| Chloride | 440 (14.2) | 1.13 (1.04-1.23) | .006 | 1.14 (1.03-1.25) | .008 | 1.19 (1.02-1.40) | .03 |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ASV, average successive variability; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BUN, blood urea nitrogen; CVD, cardiovascular disease; HR, hazard ratio; NYHA, New York Heart Association; SBP, systolic blood pressure.
Primary composite outcome is a composite of aborted cardiac arrest, hospitalization for management of heart failure, or cardiovascular death.
Model 1 was adjusted for age, sex, race/ethnicity, educational level, treatment arm, country of enrollment, and enrollment stratum (elevated natriuretic peptide levels or hospitalization for heart failure).
Model 2 was adjusted for the covariates in model 1 plus BMI, SBP, diabetes status (none, insulin dependent, or non–insulin dependent), alcohol use, smoking history, history of atrial fibrillation, ACEI or ARB use (yes or no), history of CVD, NYHA class, and baseline respective laboratory value (BUN, creatinine, potassium, sodium, or chloride).
Model 3 was adjusted for the covariates of model 2 plus percentage change in respective laboratory value, variability in SBP and BMI, and spironolactone and loop diuretic medication dosages as time-varying covariates.
Visit-to-Visit Variability in Creatinine and Risk of Adverse Outcomes
Among 3191 participants without a primary outcome event within 4 months of follow-up and 3 or more available values of serum creatinine, the mean (SD) creatinine variability, as measured by ASV, was 0.15 (0.12) mg/dL. A total of 548 primary outcome events (17.2%) occurred during the mean (SD) follow-up of 3.1 (1.7) years. The baseline characteristics of participants stratified by quintiles of visit-to-visit creatinine variability are given in eTable 5 in the Supplement. In unadjusted analysis, a significant graded association was observed between higher variability in creatinine and risk of the primary outcome in the overall cohort (quintile 1: 10.1% vs. quintile 5: 26.8%) as well as across subgroups stratified by presence of CKD at baseline and percentage change in creatinine (Figure 1B and Figure 2C and D). In adjusted analysis, greater variability in creatinine was significantly associated with a higher risk of the primary outcome in partially and fully adjusted models (model 3: HR per 1-SD higher ASV, 1.13; 95% CI, 1.04-1.22) (Table 2). Consistent patterns of association were observed with alternative measures of creatinine variability, including CV and SD (model 3: HR per 1-SD higher CV, 1.16; 95% CI, 1.04-1.29 and HR per 1-SD higher SD, 1.15; 95% CI, 1.03-1.29) (eTables 3 and 4 in the Supplement). No significant interaction between ASV and treatment arm was found for the risk of the primary composite outcome (P = .79 for interaction). In secondary analyses, greater creatinine variability was also significantly associated with higher risk of all-cause mortality (model 3: HR per 1-SD higher ASV, 1.16; 95% CI; 1.04-1.32) (Table 2 and eTables 3 and 4 in the Supplement).
Sensitivity Analysis
In sensitivity analysis, greater variability in kidney function parameters was significantly associated with a higher risk of the primary outcome after additional adjustment for NP z score (model 3: BUN: HR per 1-SD higher ASV, 1.26; 95%, 1.10-1.44 and creatinine: HR per 1-SD higher ASV, 1.29; 95% CI, 1.13-1.47) (eTable 6 in the Supplement), with restriction of the study cohort to participants recruited in North or South America (eTable 7 in the Supplement) and with estimation of variability during longer-term follow-up (baseline to 12 months as well as 4 months to 12 months of follow-up) (eFigure 2 in the Supplement). A consistent pattern of association between greater variability in kidney function parameters and higher risk of the primary outcome was also observed in adjusted analysis across key subgroups of interest, including placebo vs spironolactone arms (model 3: BUN: HR per 1-SD higher ASV, 1.27; 95% CI, 1.04-1.56 and creatinine: HR per 1-SD higher ASV, 1.39; 95% CI, 1.14-1.69 in the placebo arm and BUN: HR per 1-SD higher ASV, 1.30; 95% CI, 1.03-1.65 and creatinine: HR per 1-SD higher ASV, 1.19; 95% CI, 1.02-1.42 in the spironolactone arm) (eTable 8 in the Supplement), among individuals with vs without CKD at baseline (BUN: HR per 1-SD higher ASV, 1.39; 95% CI, 1.16-1.67 and creatinine: HR per 1-SD higher ASV, 1.21; 95% CI, 1.07-1.37 in the group with CKD and BUN: HR per 1-SD higher ASV, 1.13; 95% CI, 1.01-1.27 and creatinine: HR per 1-SD higher ASV, 1.15; 95% CI, 0.99-1.34 in the group without CKD) (Table 3), and across the tertiles of the MAGGIC risk score (tertile 1: BUN: HR per 1-SD higher ASV, 1.14; 95% CI, 1.01-1.29 and creatinine: HR per 1-SD higher ASV, 1.35; 95% CI, 1.16-1.57 and tertile 3: BUN: HR per 1-SD higher ASV,1.19; 95% CI, 1.02-1.38 and creatinine: HR per 1-SD higher ASV, 1.21; 95% CI, 1.04-1.41) (eTable 9 and eFigure 3 in the Supplement). Finally, addition of variability in the kidney function parameters (BUN or creatinine ASV) to the MAGGIC risk score significantly improved the discrimination performance for estimating all-cause mortality (BUN: change in C index from 0.64 to 0.67; P < .001 and creatinine: change in C index from 0.65 to 0.67; P < .001) (eTable 10 in the Supplement). In contrast, no significant change occurred in the discrimination performance of the MAGGIC risk score with addition of percentage change in kidney function parameters (BUN: change in C index from 0.64 to 0.64; P = .35 and creatinine: change in C index from 0.65 to 0.65; P = .22).
Table 3. Associations of ASV in Kidney Function Laboratory Measures With the Primary Composite Outcome Among Individuals With and Without CKD at Baseline.
| Variable | Model 1a | Model 2b | Model 3c | |||
|---|---|---|---|---|---|---|
| HR per 1-SD higher ASV (95% CI) | P value | HR per 1-SD higher ASV (95% CI) | P value | HR per 1-SD higher ASV (95% CI) | P value | |
| CKDd | ||||||
| BUN | 1.23 (1.12-1.34) | <.001 | 1.15 (1.03-1.28) | .01 | 1.39 (1.16-1.67) | <.001 |
| Creatinine | 1.21 (1.10-1.32) | <.001 | 1.20 (1.08-1.33) | .001 | 1.21 (1.07-1.37) | .002 |
| No CKD | ||||||
| BUN | 1.18 (1.09-1.27) | <.001 | 1.13 (1.03-1.23) | .008 | 1.13 (1.01-1.27) | .04 |
| Creatinine | 1.39 (123-1.57) | <.001 | 1.36 (1.18-1.56) | <.001 | 1.15 (0.99-1.34) | .06 |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ASV, average successive variability; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BUN, blood urea nitrogen; CKD, chronic kidney disease; CVD, cardiovascular disease; HR, hazard ratio; NYHA, New York Heart Association; SBP, systolic blood pressure.
Model 1 was adjusted for age, sex, race/ethnicity, educational level, treatment arm, country of enrollment, and enrollment stratum (elevated natriuretic peptide levels or hospitalization for heart failure).
Model 2 was adjusted for the covariates in model 1 plus BMI, SBP, diabetes status (none, insulin dependent, or non–insulin dependent), alcohol use, smoking history, history of atrial fibrillation, ACEI or ARB use (yes or no), history of CVD, NYHA class, and baseline respective laboratory value (BUN, creatinine, potassium, sodium, or chloride).
Model 3 was adjusted for covariates of model 2 plus percentage change in respective laboratory value, variability in SBP and BMI, and spironolactone and loop diuretic medication dose as a time-varying covariate.
CKD was defined as an estimated glomerular filtration rate less than 60 mL/min per 1.73 m2.
Visit-to-Visit Variability of Serum Electrolytes and Risk of Adverse Outcomes
The mean (SD) laboratory values, as measured by ASV, were 2.8 (1.7) mEq/L for sodium, 0.4 (0.2) mEq/L for potassium, and 3.2 (2.5) mEq/L for chloride (to convert to millimoles per liter, multiply by 1). Baseline characteristics for each of the cohorts across quintile are given in eTables 11 through 13 in the Supplement. In adjusted analysis, greater variability in sodium and potassium levels, as assessed using different measures of variability (ASV, CV, and SD), was significantly associated with higher risk of the primary outcome in the partially as well as fully adjusted models (model 3: sodium: HR per 1-SD higher ASV, 1.14; 95% CI, 1.01-1.30 and potassium: HR per 1-SD higher ASV, 1.21; 95% CI, 1.02-1.44) (Table 2 and eTables 3 and 4 in the Supplement). Variability in chloride levels, as assessed by ASV, was not significantly associated with the risk of primary outcome in the most adjusted model (model 3: HR per 1-SD higher ASV, 1.19; 95% CI, 0.96-1.40). However, other measures of chloride variability (CV and SD) were significantly associated with risk of primary outcome (model 3: HR per 1-SD higher CV, 1.25; 95% CI, 1.09-1.44 and HR per 1-SD higher SD, 1.27; 95% CI, 1.05-1.53) (eTables 3 and 4 in the Supplement). For the secondary outcome, greater variability in sodium, potassium, and chloride levels were all significantly associated with higher risk of all-cause mortality with consistent patterns of association using different measures of variability (sodium: HR per 1-SD higher ASV, 1.14; 95% CI, 1.02-1.27 and potassium: HR per 1-SD higher ASV, 1.10; 95% CI, 1.01-1.20 and chloride: HR per 1-SD higher ASV, 1.19; 95% CI, 1.02-1.40) (Table 2 and eTables 3 and 4 in the Supplement). Sodium and potassium, but not chloride, variability was also associated with an increased risk of the primary composite outcome after further adjusting for NP levels (model 3: sodium: HR per 1-SD higher ASV, 1.32; 95% CI, 1.08-1.62 and potassium: HR per 1-SD higher ASV, 1.22; 95% CI, 1.03-1.45 and chloride: HR per 1-SD higher ASV, 1.24; 95% CI, 0.99-1.55) (eTable 6 in the Supplement). In sensitivity analysis, similar patterns of association were observed between visit-to-visit variability in sodium, potassium, and chloride levels and the risk of the primary composite outcome when limited to the North and South America cohort (model 3: sodium: HR per 1-SD higher ASV, 1.27; 95% CI, 1.03-1.57 and potassium: HR per 1-SD higher ASV, 1.21; 95% CI, 1.01-1.44 and chloride: HR per 1-SD higher ASV, 1.26; 95% CI, 1.01-1.59) (eTable 7 in the Supplement) and across both treatment arms (sodium: HR per 1-SD higher ASV, 1.52; 95% CI, 1.03-2.26 and potassium: HR per 1-SD higher ASV, 1.39; 95% CI, 1.08-1.79 and chloride: HR per 1-SD higher ASV, 1.38; 95% CI, 0.94-2.02 in the placebo arm and sodium: HR per 1-SD higher ASV, 1.22; 95% CI, 1.05-1.49 and potassium: HR per 1-SD higher ASV, 1.16; 95% CI, 1.00-1.41 and chloride: HR per 1-SD higher ASV, 1.20; 95% CI, 0.87-1.66 in the spironolactone arm) (eTable 8 in the Supplement).
Discussion
We observed several key findings in this study. First, variability in measures of kidney function (BUN and creatinine) identified a subset of patients with chronic stable HFpEF with more advanced disease characterized by older age, higher burden of medical comorbidities, and increased symptom burden. Second, higher variability in kidney function parameters was significantly associated with higher risk of the adverse clinical outcomes independent of other potential confounders. Third, the associations of greater variability in kidney function parameters with the higher risk of the primary composite outcome were consistent across several subgroups stratified by treatment arm (placebo vs spironolactone) disease severity (MAGGIC risk score), baseline CKD status, and changes in kidney function. Fourth, higher variability in serum electrolyte indexes, particularly sodium and potassium, was significantly associated with higher risk of primary composite outcome and all-cause mortality. To our knowledge, our study is the first to evaluate visit-to-visit variability in such laboratory parameters and risk of adverse events in HFpEF.
Isolated measurements of worse kidney function are associated with more severe dysfunction in cardiac mechanics,1 lower exercise capacity,2 and greater risk of adverse clinical outcomes in HFpEF.3,4 Moreover, short-term worsening in kidney function as defined by changes in estimated glomerular filtration rate or serum creatinine during 1 to 2 months is independently linked to poor clinical outcomes in HFpEF.7,8,21 Our study adds to the existing literature and indicates that visit-to-visit fluctuations in kidney function and certain electrolytes is associated with a higher risk of adverse outcomes in patients with chronic HFpEF. We observed that these associations were independent of baseline measures of disease severity, baseline and changes in kidney function, changes in doses of medications, and variability in SBP and BMI. These observations suggest that the variability in kidney function and certain other electrolytes may capture a unique risk phenotype that is not accounted for by other clinical risk factors, preexisting kidney dysfunction, disease severity, and associated medication changes. Furthermore, we observed that variability but not percentage change in the kidney function parameters improved the discrimination performance of established disease severity measures, such as the MAGGIC risk score. Taken together, these findings suggest significant prognostic importance of substantial fluctuations in both kidney function and electrolytes among patients with HFpEF in identifying future risk of adverse events.
Serial measurements of kidney function parameters and electrolytes is common in HFpEF to monitor for effects of therapies, such as response to diuretics, and overall disease progression. Likewise, fluctuations in such levels are frequently noted with the initiation and/or titration of medication use. Such variability in laboratory parameters creates challenges in identifying a patient’s true baseline level of kidney function or serum electrolytes. Indeed, rapid correction of laboratory values to normal levels after certain interventions (eg, initiation of the use of certain drugs or changes in volume status) may lead to a false sense of reassurance by practitioners. Our primary findings reveal that high variability in several biomarkers, even in the setting of normal baseline levels or varying percentage changes in values over time, are associated with substantially increased risk of clinical events.
The mechanisms underlying the observed associations of variability in kidney function parameters and serum electrolytes with the risk of adverse clinical outcomes require further investigation. Patients with HFpEF who have perturbations in kidney and electrolyte homeostasis after relatively routine events, including titration of diuretics, initiation of the use vasoactive medications, or changes in volume status, may be considered to have early stages of cardiorenal syndrome. Visit-to-visit fluctuations might help to better identify a patient population that has less kidney reserve to withstand these subtle perturbations and seemingly routine changes in evidence-based interventions for HF management (eg, medication changes or volume changes).22 Further investigation is required to understand the prevalence of subclinical right ventricular dysfunction, central venous pressures, and venous congestion among those with high variability in creatinine, BUN, or electrolytes, given the contributions of systemic venous congestion to the imbalance of the cardiorenal axis.23 In addition, the associations between macrovascular arterial stiffness (and resultant pulsatile pressure load), microvascular dysfunction, and kidney function or electrolyte variability require further investigation.24 Prior studies25,26,27 have found a strong association between decreases in kidney blood flow and GFR and increased sodium avidity and resultant venous congestion, all of which are frequently seen before presentation with acute decompensated HF. It also remains plausible that variability in kidney function and sodium may be an important hemodynamic risk marker that identifies an enriched cohort for clinical deterioration. Variability in such laboratory indexes may be a sign of hemodynamic or intravascular volume fluctuations, which may lead to upregulation of neurohormonal dysregulation and proinflammatory states, leading to a more advanced HFpEF phenotype.28,29 Specifically, sodium variability may also be associated with an increase in salt and water retention, leading to poor clinical outcomes, as it does in HF with reduced EF.7,30
Our study findings have important clinical implications. Variability in routinely monitored laboratory values can be performed to improve risk stratification of patients with HF. With the use of contemporary electronic health records, serial laboratory data are readily available during clinical visits, and such measures are already frequently obtained in the HFpEF population. In addition, at-home fingerstick blood testing of biomarkers is feasible among high-risk individuals with HF and can be easily incorporated into the electronic medical record.31 Although actual laboratory values will remain important for medication selection and titration, objective measures of variability can augment risk assessment. Such integration may allow for practitioners to readily identify patients with HFpEF with higher variability in kidney function and/or electrolytes who are at particularly high risk for clinical events. Once identified, patients with HFpEF with high variability in certain laboratory indexes may benefit from closer monitoring, such as more frequent clinic visits to assess volume status, implantation of wireless pulmonary artery pressure sensors to understand subtle changes to central hemodynamics, or cardiac imaging to delineate interval alterations to right ventricular structure or function.
Limitations
Our study has some noteworthy limitations. First, because the primary results of the TOPCAT trial were neutral, our secondary analyses can be considered only exploratory, and the results need confirmation in other cohorts of patients with stable HFpEF. Second, consistent with the observational nature of our study, specific inclusion and exclusion criteria for the trial, and the requirement of availability of additional measures of kidney function and electrolytes, our study findings may be prone to selection bias and unmeasured confounding and may not be generalizable to other more racially diverse HFpEF cohorts. Third, we cannot completely rule out the possibility of reverse causation in the observed associations between variability in kidney parameters and risk of adverse outcomes. However, the consistent pattern of association across multiple adjusted models, using different estimates of variability (ASV, CV, and SD) for varying follow-up durations (short-term and long-term variability), and across clinically relevant subgroups of interest (stratified by baseline CKD status, MAGGIC risk score, and treatment arm) suggests robustness in our study findings. Fourth, core laboratory measurements were not performed for all the biomarkers assessed in this study. However, laboratory measurements of the basic metabolic panel are unlikely to vary considerably across institutions, and patients are likely to have obtained all additional measurements at a single local-site laboratory.
Conclusions
In this study, among patients with chronic HFpEF, visit-to-visit variability in kidney function and serum electrolyte indexes was significantly associated with a higher risk of adverse outcomes. High variability in these parameters may identify subsets of patients with HFpEF who could benefit from more aggressive monitoring for clinical deterioration.
eMethods. Study Population and Laboratory Assessment
eTable 1. Baseline Characteristics Among Participants Included and Excluded From the Primary Analysis Across Laboratory Values
eTable 2. Significant Predictors of BUN and Creatinine Variability Assessed by ASV
eTable 3. Associations of Coefficient of Variation (CV) Among 5 Laboratory Measures and Outcomes
eTable 4. Associations of Standard Deviation (SD) Among 5 Laboratory Measures and Outcomes
eTable 5. Baseline Characteristics Across Quintiles of Creatinine Variability Assessed by ASV
eTable 6. Sensitivity Analysis: Associations of ASV and Primary Composite Outcome After Further Adjustment for NP Levels
eTable 7. Sensitivity Analyses: Associations of Variability and Primary Composite Outcome in the Americas Cohort
eTable 8. Sensitivity Analyses: Associations of ASV and Primary Composite Outcome Across Treatment Arms
eTable 9. Sensitivity Analyses: Associations of ASV and Primary Composite Outcome Across Tertiles of MAGGIC Risk Score
eTable 10. Comparison of Model Discrimination With Variability or Percent Change in Laboratory Value and MAGGIC Risk Score
eTable 11. Baseline Characteristics Across Quintiles of Sodium Variability Assessed by ASV
eTable 12. Baseline Characteristics Across Quintiles of Potassium Variability Assessed by ASV
eTable 13. Baseline Characteristics Across Quintiles of Chloride Variability Assessed by ASV
eFigure 1. The Mean Laboratory Value at Each Visit
eFigure 2. Sensitivity Analyses: Associations of ASV and Varying Variability Time Ranges and Risk of the Primary Composite Outcome.
eFigure 3. Sensitivity Analyses: Variability and Risk of Primary Composite Across Tertiles of the MAGGIC Risk Score
References
- 1.Unger ED, Dubin RF, Deo R, et al. Association of chronic kidney disease with abnormal cardiac mechanics and adverse outcomes in patients with heart failure and preserved ejection fraction. Eur J Heart Fail. 2016;18(1):103-112. doi: 10.1002/ejhf.445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel RB, Mehta R, Redfield MM, et al. Renal dysfunction in heart failure with preserved ejection fraction: insights from the RELAX trial. J Card Fail. 2020;26(3):233-242. doi: 10.1016/j.cardfail.2020.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Damman K, Valente MA, Voors AA, O’Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J. 2014;35(7):455-469. doi: 10.1093/eurheartj/eht386 [DOI] [PubMed] [Google Scholar]
- 4.Hillege HL, Nitsch D, Pfeffer MA, et al. ; Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Investigators . Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006;113(5):671-678. doi: 10.1161/CIRCULATIONAHA.105.580506 [DOI] [PubMed] [Google Scholar]
- 5.Grodin JL, Testani JM, Pandey A, et al. Perturbations in serum chloride homeostasis in heart failure with preserved ejection fraction: insights from TOPCAT. Eur J Heart Fail. 2018;20(10):1436-1443. doi: 10.1002/ejhf.1229 [DOI] [PubMed] [Google Scholar]
- 6.Patel YR, Kurgansky KE, Imran TF, et al. Prognostic significance of baseline serum sodium in heart failure with preserved ejection fraction. J Am Heart Assoc. 2018;7(12):e007529. doi: 10.1161/JAHA.117.007529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damman K, Perez AC, Anand IS, et al. Worsening renal function and outcome in heart failure patients with preserved ejection fraction and the impact of angiotensin receptor blocker treatment. J Am Coll Cardiol. 2014;64(11):1106-1113. doi: 10.1016/j.jacc.2014.01.087 [DOI] [PubMed] [Google Scholar]
- 8.Beldhuis IE, Streng KW, Ter Maaten JM, et al. Renin-angiotensin system inhibition, worsening renal function, and outcome in heart failure patients with reduced and preserved ejection fraction: a meta-analysis of published study data. Circ Heart Fail. 2017;10(2):e003588. doi: 10.1161/CIRCHEARTFAILURE.116.003588 [DOI] [PubMed] [Google Scholar]
- 9.Nakazato Y, Kurane R, Hirose S, Watanabe A, Shimoyama H. Variability of laboratory parameters is associated with frailty markers and predicts non-cardiac mortality in hemodialysis patients. Clin Exp Nephrol. 2015;19(6):1165-1178. doi: 10.1007/s10157-015-1108-0 [DOI] [PubMed] [Google Scholar]
- 10.Suzuki A, Obi Y, Hayashi T, et al. Visit-to-visit variability in estimated glomerular filtration rate predicts hospitalization and death due to cardiovascular events. Clin Exp Nephrol. 2019;23(5):661-668. doi: 10.1007/s10157-019-01695-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jose P, Skali H, Anavekar N, et al. Increase in creatinine and cardiovascular risk in patients with systolic dysfunction after myocardial infarction. J Am Soc Nephrol. 2006;17(10):2886-2891. doi: 10.1681/ASN.2006010063 [DOI] [PubMed] [Google Scholar]
- 12.Pitt B, Pfeffer MA, Assmann SF, et al. ; TOPCAT Investigators . Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383-1392. doi: 10.1056/NEJMoa1313731 [DOI] [PubMed] [Google Scholar]
- 13.Gorst C, Kwok CS, Aslam S, et al. Long-term glycemic variability and risk of adverse outcomes: a systematic review and meta-analysis. Diabetes Care. 2015;38(12):2354-2369. doi: 10.2337/dc15-1188 [DOI] [PubMed] [Google Scholar]
- 14.Echouffo-Tcheugui JB, Zhao S, Brock G, Matsouaka RA, Kline D, Joseph JJ. Visit-to-visit glycemic variability and risks of cardiovascular events and all-cause mortality: the ALLHAT study. Diabetes Care. 2019;42(3):486-493. doi: 10.2337/dc18-1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anand IS, Claggett B, Liu J, et al. Interaction between spironolactone and natriuretic peptides in patients with heart failure and preserved ejection fraction: from the TOPCAT trial. JACC Heart Fail. 2017;5(4):241-252. doi: 10.1016/j.jchf.2016.11.015 [DOI] [PubMed] [Google Scholar]
- 16.Brater DC, Day B, Burdette A, Anderson S. Bumetanide and furosemide in heart failure. Kidney Int. 1984;26(2):183-189. doi: 10.1038/ki.1984.153 [DOI] [PubMed] [Google Scholar]
- 17.Vargo DL, Kramer WG, Black PK, Smith WB, Serpas T, Brater DC. Bioavailability, pharmacokinetics, and pharmacodynamics of torsemide and furosemide in patients with congestive heart failure. Clin Pharmacol Ther. 1995;57(6):601-609. doi: 10.1016/0009-9236(95)90222-8 [DOI] [PubMed] [Google Scholar]
- 18.Pfeffer MA, Claggett B, Assmann SF, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation. 2015;131(1):34-42. doi: 10.1161/CIRCULATIONAHA.114.013255 [DOI] [PubMed] [Google Scholar]
- 19.de Denus S, O’Meara E, Desai AS, et al. Spironolactone metabolites in TOPCAT: new insights into regional variation. N Engl J Med. 2017;376(17):1690-1692. doi: 10.1056/NEJMc1612601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pocock SJ, Ariti CA, McMurray JJ, et al. ; Meta-Analysis Global Group in Chronic Heart Failure . Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J. 2013;34(19):1404-1413. doi: 10.1093/eurheartj/ehs337 [DOI] [PubMed] [Google Scholar]
- 21.Damman K, Solomon SD, Pfeffer MA, et al. Worsening renal function and outcome in heart failure patients with reduced and preserved ejection fraction and the impact of angiotensin receptor blocker treatment: data from the CHARM-study programme. Eur J Heart Fail. 2016;18(12):1508-1517. doi: 10.1002/ejhf.609 [DOI] [PubMed] [Google Scholar]
- 22.Mullens W, Damman K, Testani JM, et al. Evaluation of kidney function throughout the heart failure trajectory: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2020;22(4):584-603. doi: 10.1002/ejhf.1697 [DOI] [PubMed] [Google Scholar]
- 23.Zannad F, Rossignol P. Cardiorenal syndrome revisited. Circulation. 2018;138(9):929-944. doi: 10.1161/CIRCULATIONAHA.117.028814 [DOI] [PubMed] [Google Scholar]
- 24.Cooper LL, Palmisano JN, Benjamin EJ, et al. Microvascular function contributes to the relation between aortic stiffness and cardiovascular events: the Framingham Heart Study. Circ Cardiovasc Imaging. 2016;9(12):e004979. doi: 10.1161/CIRCIMAGING.116.004979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Testani JM, McCauley BD, Chen J, Coca SG, Cappola TP, Kimmel SE. Clinical characteristics and outcomes of patients with improvement in renal function during the treatment of decompensated heart failure. J Card Fail. 2011;17(12):993-1000. doi: 10.1016/j.cardfail.2011.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doty JM, Saggi BH, Sugerman HJ, et al. Effect of increased renal venous pressure on renal function. J Trauma. 1999;47(6):1000-1003. doi: 10.1097/00005373-199912000-00002 [DOI] [PubMed] [Google Scholar]
- 27.Firth JD, Raine AE, Ledingham JG. Raised venous pressure: a direct cause of renal sodium retention in oedema? Lancet. 1988;1(8593):1033-1035. doi: 10.1016/S0140-6736(88)91851-X [DOI] [PubMed] [Google Scholar]
- 28.Damman K, Testani JM. The kidney in heart failure: an update. Eur Heart J. 2015;36(23):1437-1444. doi: 10.1093/eurheartj/ehv010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ter Maaten JM, Damman K, Verhaar MC, et al. Connecting heart failure with preserved ejection fraction and renal dysfunction: the role of endothelial dysfunction and inflammation. Eur J Heart Fail. 2016;18(6):588-598. doi: 10.1002/ejhf.497 [DOI] [PubMed] [Google Scholar]
- 30.Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53(7):582-588. doi: 10.1016/j.jacc.2008.08.080 [DOI] [PubMed] [Google Scholar]
- 31.Maisel A, Barnard D, Jaski B, et al. Primary results of the HABIT trial (Heart Failure Assessment With BNP in the Home). J Am Coll Cardiol. 2013;61(16):1726-1735. doi: 10.1016/j.jacc.2013.01.052 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Study Population and Laboratory Assessment
eTable 1. Baseline Characteristics Among Participants Included and Excluded From the Primary Analysis Across Laboratory Values
eTable 2. Significant Predictors of BUN and Creatinine Variability Assessed by ASV
eTable 3. Associations of Coefficient of Variation (CV) Among 5 Laboratory Measures and Outcomes
eTable 4. Associations of Standard Deviation (SD) Among 5 Laboratory Measures and Outcomes
eTable 5. Baseline Characteristics Across Quintiles of Creatinine Variability Assessed by ASV
eTable 6. Sensitivity Analysis: Associations of ASV and Primary Composite Outcome After Further Adjustment for NP Levels
eTable 7. Sensitivity Analyses: Associations of Variability and Primary Composite Outcome in the Americas Cohort
eTable 8. Sensitivity Analyses: Associations of ASV and Primary Composite Outcome Across Treatment Arms
eTable 9. Sensitivity Analyses: Associations of ASV and Primary Composite Outcome Across Tertiles of MAGGIC Risk Score
eTable 10. Comparison of Model Discrimination With Variability or Percent Change in Laboratory Value and MAGGIC Risk Score
eTable 11. Baseline Characteristics Across Quintiles of Sodium Variability Assessed by ASV
eTable 12. Baseline Characteristics Across Quintiles of Potassium Variability Assessed by ASV
eTable 13. Baseline Characteristics Across Quintiles of Chloride Variability Assessed by ASV
eFigure 1. The Mean Laboratory Value at Each Visit
eFigure 2. Sensitivity Analyses: Associations of ASV and Varying Variability Time Ranges and Risk of the Primary Composite Outcome.
eFigure 3. Sensitivity Analyses: Variability and Risk of Primary Composite Across Tertiles of the MAGGIC Risk Score