Abstract
Purpose:
To characterize outcomes of TaT1 UCB patients stratified by the EAU categories and to compare them with EORTC risk-groups to assess the rate and effect of reclassification.
Patients and methods:
A multi-institutional database of 5122 patients with TaT1 UCB who underwent TURB with or without adjuvant therapy at eight institutions between 1996 and 2007. Multivariable Cox regression analyses addressed factors associated with disease recurrence and progression. The net reclassification index was used to compare the performance of the EAU categories with the EORTC scoring system.
Results:
Of 5122 patients, 632 (12.3%), 2302 (45.0%) and 2188 (42.7%) were assigned to the low-, intermediate-, and high-risk EAU category, respectively. Within a median follow-up of 62 months (Interquartile range 27–97), 2365 (46.2%) and 516 (10.1%) patients experienced disease recurrence and progression, respectively. In multivariable Cox regression analyses, EAU intermediate- and high-risk categories were associated with a higher risk of disease recurrence (p<0.001) and progression (p<0.001) compared to low-risk patients. Application of the EAU categories reclassified 1940 (37.9%) patients into a higher risk group for recurrence. Likewise, 602 (11.8%) patients were reclassified to a higher and 278 (5.4%) to a lower risk group for progression. The net reclassification index of the EAU risk stratification was 0.1% (95% CI −3.1% – 3.2%) for recurrence and 10.1% (95% CI −8.0% – 12.0%) for progression, respectively.
Conclusions
Compared to EORTC risk stratification, the EAU categories reclassifies 37.9% patients into a higher risk group of recurrence and 11.8% into a higher risk of progression. However, the novel risk stratification assigns the majority of patients to the same treatment as the more complex EORTC tables and can be regarded as an alternative tool for treatment decision-making.
Introduction
Urothelial carcinoma of the bladder (UCB) is a highly aggressive malignancy that causes significant morbidity and mortality (1) with an estimated 74,960 new cases and 16,390 deaths in the US in 2016 only (2). At initial diagnosis, over 70% of the patients have non-muscle invasive bladder cancer (NMIBC), which in most of the cases is treated with transurethral resection of the bladder (TURB) with or without intravesical therapy (3).
The probabilities of disease recurrence and progression in patients with UCB differ significantly (4). To predict an individual NMIBC patients’ risk of disease recurrence and progression, the European Organization for Research and Treatment of Cancer (EORTC) developed a scoring system (5). The probability of disease recurrence and progression can be calculated (5). Based on the EORTC recurrence and progression score, the guidelines panel of the European Association of Urology (EAU) stratified patients into low-, intermediate-, and high-risk of recurrence and progression (4). The treatment and surveillance recommendations of the EAU for an individual patient were based on this risk group stratification (4).
The guidelines panel of the European Association of Urology (EAU) introduced a simplified risk group stratification, which is based on well-established prognostic factors and the EORTC risk tables (3). According to clinical and pathological features, patients with NMIBC are stratified into three risk groups with the intent to facilitate treatment recommendations (3). The aim of our study was to characterize disease specific outcomes of NMIBC patients stratified by the EAU categories. We also aimed to compare EAU to EORTC risk-groups to assess the rate and effect of reclassification. To this end, we used a large multi-institutional database of 5122 patients with NMIBC. We hypothesized that disease specific outcomes of patients stratified by the EAU risk stratification are comparable to the ones from EORTC risk tables and that the application of the novel EAU risk groups does not lead to substantial changes in treatment recommendation.
Patients and Methods
Patient selection and data collection
This was an institutional review board approved study with all participating sites providing the necessary data-sharing agreements prior to initiation of the study. The patients were extracted from a multi-institutional database of 5122 patients with TaT1 UCB who underwent TURB according to guidelines recommendations at eight institutions between 1996 and 2007. A re-TURB was performed according to guideline recommendations and at surgeons’ discretion within 2–6 weeks after initial treatment based on pathologic and intraoperative findings. Immediate postoperative instillation of chemotherapy (IPIC; 40 mg mitomycin, 80 mg epirubicin or 50 mg doxorubicin), adjuvant intravesical chemotherapy or adjuvant BCG was administered at the operating surgeons’ discretion as well as according to guideline recommendations. The first adjuvant instillation was given within 7–21 days after TURB and repeated weekly for 6 weeks. All BCG patients were proposed some form of maintenance therapy. None of the patients had upper tract urothelial carcinoma, prostatic stroma invasion or metastatic UCB at diagnosis.
Pathologic Evaluation
All surgical specimens were processed according to standard pathologic procedures. Genitourinary pathologists assigned tumor grade according to the 1973 World Health Organization (WHO) grading system. Pathological stage was reassigned according to the 2009 American Joint Committee on Cancer TNM staging system (6). The presence of concomitant CIS was defined as presence of CIS in conjunction with another tumor.
Follow-up
Patients were generally followed every 3–6 months for the first 2 years after TURB, biannually up to 5 years, and annually thereafter (4). Follow-up consisted of a history, physical examination, urinary cytology, standard white light cystoscopy, and biopsy of suspicious lesions. Radiographic evaluation of the upper urinary tract was generally done at diagnosis and yearly thereafter or in case of disease recurrence or suspicion, such as positive urine cytology without a bladder lesion during follow-up. When disease recurrence was detected, the tumor was resected. In case of positive cytology, mapping bladder biopsies, prostatic urethra resection, and upper urinary tract workup were performed. Disease recurrence was defined as first histologically documented tumor relapse in the bladder or prostatic urethra regardless of tumor stage. Disease progression was defined as tumor relapse at tumor stage ≥T2 in the bladder or prostatic urethra. Cause of death was determined by treating physicians, by chart review corroborated by death certificates, or by death certificates alone (7). Tumor occurrence in the upper urinary tract was not considered as tumor recurrence but as new primary tumor.
Statistical analyses
According to the EAU guidelines, patients with NMIBC were stratified into three risk categories (3).
Low-risk tumors: Primary, solitary tumor, Ta tumor, G1 tumor, size < 3 cm, no Carcinoma in situ (CIS)
Intermediate-risk tumors: All tumors between the category of low- and high-risk
High-risk tumors: T1 tumor, G3 tumor, Carcinoma in situ (Cis), multiple and recurrent and large (> 3 cm) Ta G1/2 tumors
Disease recurrence-free and disease progression-free survival curves for patients in each EAU risk-group were generated using the Kaplan-Meier method; the log rank test was applied to compare survival between groups of patients. Univariable and multivariable Cox regression models addressed the association of clinicopathologic feature and risk-group with disease recurrence and disease progression.
To analyze the percentage of NMIBC patients who are reclassified into lower or higher risk groups of disease recurrence and progression when the EAU stratification is applied, all NMIBC patients in this study were also stratified according to the previously published EORTC model (5). The model incorporates the number of tumors (single, 2–7, ≥ 8), tumor size (< 3 cm, ≥ 3 cm), prior recurrence rate (primary, ≤ 1 recurrence/year, > 1 recurrence/year), T category (Ta, T1), CIS (no/yes), and tumor grade (G1–3, WHO 1973) (5). Each of these variables received a scoring, and according to the recurrence and progression score, patients were assigned to different risk-categories (4).
For disease recurrence:
Recurrence score 0: Low-risk
Recurrence score 1–9: Intermediate-risk
Recurrence score 10–17: High-risk
For disease progression:
Progression score 0: Low-risk
Progression score 2–6: Intermediate-risk
Progression score 7–23: High-risk
The net reclassification index was used to compare the performance of the EAU categories with the EORTC scoring system. All p-values were two-sided and statistical significance was defined as p<0.05. Statistical analyses were performed using SPSS Statistics® 20 (SPSS®, IBM Corp, Armonk, NY, USA) and SAS Version 9.2.
Results
Patients’ clinicopathologic characteristics
Table 1 shows clinicopathologic characteristic of the 5122 patients included in the study. Immediate postoperative instillation, adjuvant intravesical chemotherapy, and adjuvant treatment with BCG were administered in 2661 (52.0%), 89 (1.7%), and 622 (12.1%) patients. Of the patients, 632 (12.3%), 2303 (45.0%), and 2188 (42.7%) were stratified into EAU low-, intermediate-, and high-risk category, respectively.
Table 1:
Clinicopathologic characteristics of 5122 patients with NMIBC
| Characteristics | Total |
|---|---|
| Gender (n, %) | |
| Male | 4049 (79.1) |
| Female | 1073 (20.9) |
| Age (years) | |
| Mean (SD) | 65.8 (11.4) |
| Median (IQR) | 67.0 (15) |
| Tumor stage (n, %) | |
| Ta | 3254 (63.5) |
| Tis | 76 (1.5) |
| T1 | 1792 (35.0) |
| Tumor grade, WHO 1973 (n, %) | |
| G1 | 1552 (30.3) |
| G2 | 1518 (29.6) |
| G3 | 2052 (40.1) |
| Concomitant CIS (n, %) | |
| No | 4822 (94.1) |
| Yes | 300 (5.9) |
| Prior recurrence rate (n, %) | |
| Primary | 3569 (69.7) |
| ≤ 1 recurrence/year | 824 (16.1) |
| > 1 recurrence/year | 729 (14.2) |
| Tumor size (n, %) | |
| < 3 cm | 4058 (79.2) |
| ≥ 3 cm | 1064 (20.8) |
| Number of tumors (n, %) | |
| Single | 3141 (61.3) |
| 2–7 | 1971 (38.5) |
| ≥ 8 | 10 (0.2) |
| Postoperative instillation (n, %) | |
| No | 1750 (34.2) |
| Immediate postoperative | 2661 (52.0) |
| Adjuvant instillation | 89 (1.7) |
| BCG | 622 (12.1) |
| Recurrence (n, %) | |
| No | 2757 (53.8) |
| Yes | 2365 (46.2) |
| Progression to muscle-invasion (≥T2) (n, %) | |
| No | 4606 (89.9) |
| Yes | 516 (10.1) |
Disease recurrence of patients stratified by EAU categories
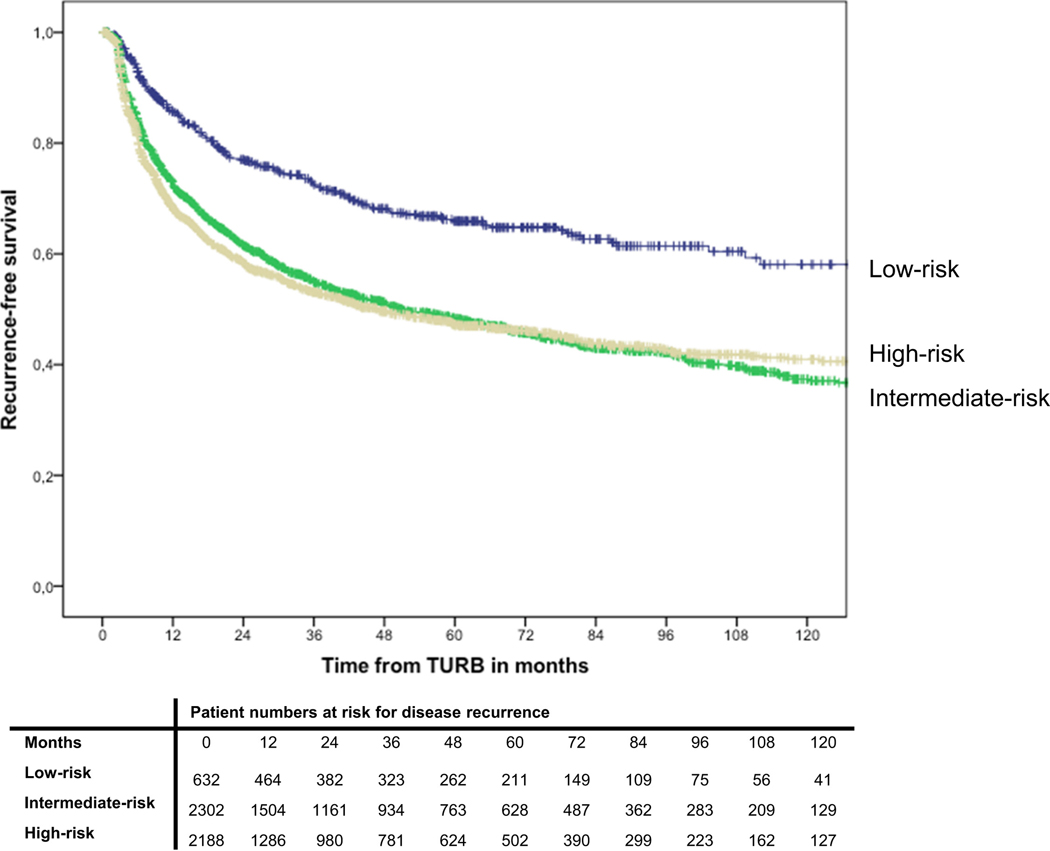
Within a median follow-up of 61.5 months (Interquartile range 26.5–96.5), 2365 patients (46.2%) experienced disease recurrence. Actuarial estimates of 2-year recurrence-free survival were 77% (standard error: ± 1) for low-risk patients, 62% ±1 for intermediate-risk patients, and 59% ±1 for high-risk patients, respectively (Figure 1). Recurrence-free survival was not significantly different between intermediate- and high-risk patients (p=0.39). In multivariable Cox-regression analysis that adjusted for the effects of age and type of intravesical therapy, intermediate and high-risk patients were at a significantly higher risk of disease recurrence than low-risk patients (p<0.001) (Table 2).
Fig. 1.
Kaplan-Meier curves depicting recurrence-free survival in 5,122 patients with TaT1 urothelial carcinoma of the bladder, stratified by EAU risk group.
Table 2.
Multivariable Cox regression analyses predicting disease recurrence, and disease progression5122 patients with non-muscle invasive bladder cancer (NMIBC)
| Disease recurrence | Disease progression | |||||
|---|---|---|---|---|---|---|
| Characteristics | HR | 95% CI | p value | HR | 95% CI | p value |
| EAU risk category | ||||||
| Low-risk | - | Referent | - | - | Referent | - |
| Intermediate-risk | 1.86 | 1.59–2.17 | < 0.001 | 3.55 | 1.92–6.54 | < 0.001 |
| High-risk | 2.02 | 1.72–2.36 | < 0.001 | 9.36 | 5.12–17.11 | < 0.001 |
Analysis adjusted for age (continuous) and type of intravesical therapy (no therapy vs. immediate postoperative instillation, adjuvant intravesical chemotherapy, BCG)
Disease progression of patients stratified by EAU categories
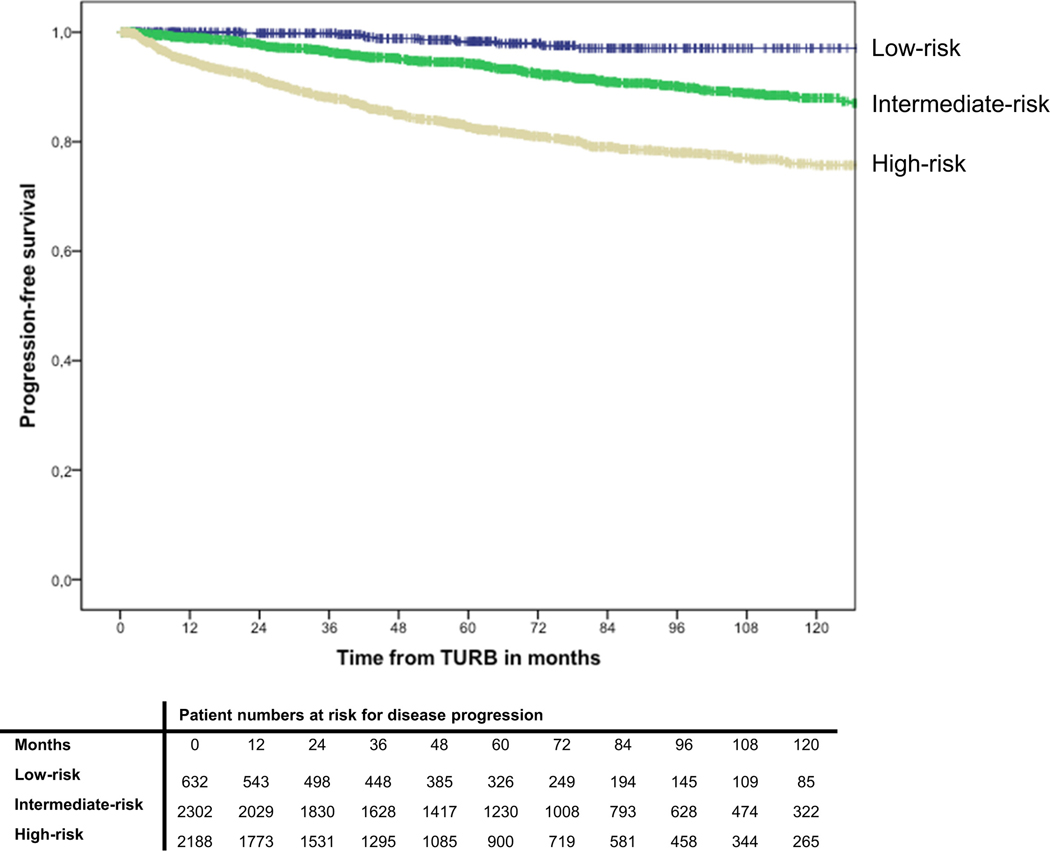
During follow-up, 516 patients (10.1%) experienced disease progression to muscle-invasive disease. Actuarial estimates of 2-year progression-free survival were 100% ±0 for low-risk patients, 98% ±0 for intermediate-risk patients, and 92% ±1 for high-risk patients, respectively (Figure 2). In multivariable Cox-regression analysis, intermediate and high-risk patients were at a significantly higher risk of disease recurrence than low-risk patients (p<0.001) (Table 2).
Fig. 2.
Kaplan-Meier curves depicting progression-free survival in 5,122 patients with TaT1 urothelial carcinoma of the bladder, stratified by EAU risk group.
Reclassification of patients stratified by EORTC recurrence and progression risk categories
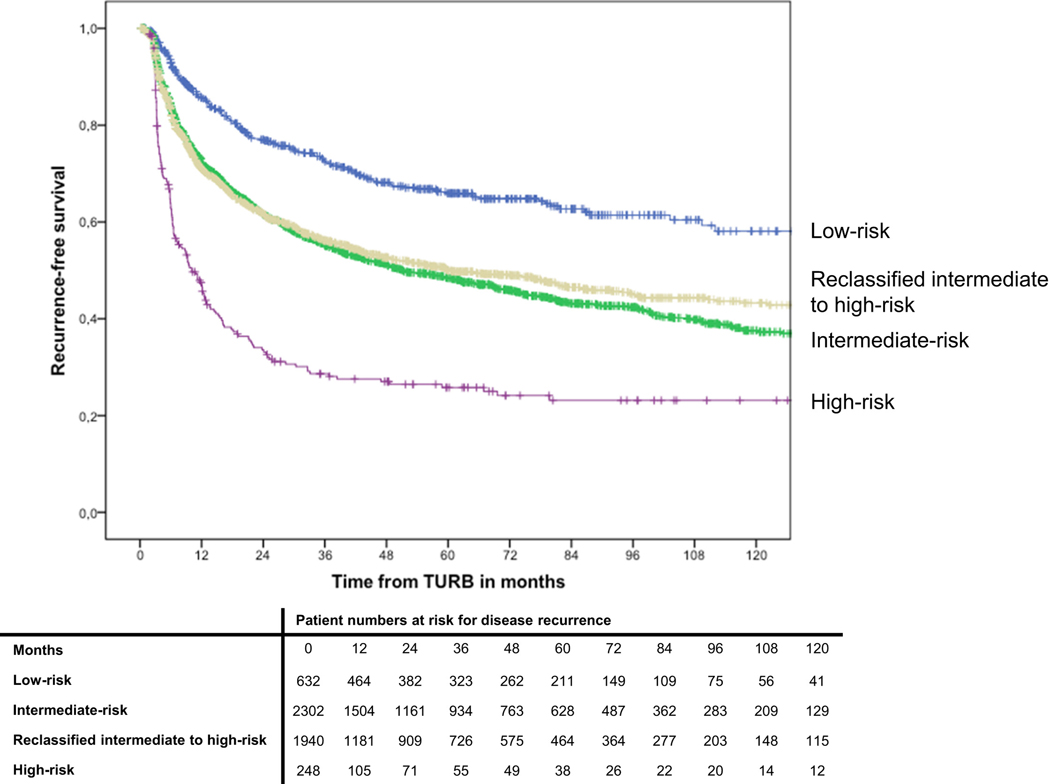
The application of the EAU categories on patients stratified by EORTC categories lead to a reclassification of 1940 (45.7%) EORTC recurrence intermediate-risk patients to EAU high-risk category (Table 3). Recurrence-free survival was comparable between EAU intermediate risk and EAU high-risk patients, which were reclassified from EORTC intermediate risk (p=0.28) (Figure 3). The net reclassification index of the EAU risk stratification for recurrence was 0.1% (95% CI − 3.1% – 3.2%).
Table 3:
Reclassification of risk of recurrence in 5122 patients with NMIBC assigned by EORTC categories and EAU categories
| EAU Categories | |||||
|---|---|---|---|---|---|
| Low-risk | Intermediate-risk | High-risk | Total | ||
| EORTC Categories | Low-risk | 632 (100%) | 0 | 0 | 632 |
| Intermediate-risk | 0 | 2302 (54.3%) | 1940 (45.7%) | 4242 | |
| High-risk | 0 | 0 | 248 (100%) | 248 | |
Fig. 3.
Kaplan-Meier curves depicting recurrence-free survival in 5,122 patients with TaT1 urothelial carcinoma of the bladder, stratified by EAU risk group and reclassification in comparison to EORTC tables.
Likewise, the application of the EAU categories on patients stratified by EORTC progression categories lead to a reclassification of 510 out of 1142 (45.7%) EORTC low-risk patients to EAU intermediate-risk category (Table 4). Furthermore, 92 (5.7%) EORTC intermediate risk patients were reclassified to high-risk patients and 278 (11.7%) EORTC high-risk patients were reclassified to intermediate-risk (Table 4). Progression-free survival was comparable between low-risk patients and those reclassified from low- to intermediate risk (p=0.30), intermediate-risk patients and those reclassified from intermediate-risk to high-risk (p=0.57) and high-risk patients and those reclassified from high-risk to intermediate-risk (p=0.15) (Figure 4). The net reclassification index of the EAU risk stratification was 10.1% (95% CI 8.0% – 12.0%) for progression.
Table 4:
Reclassification of risk of progression in 5122 patients with NMIBC assigned by EORTC categories and EAU categories
| EAU Categories | |||||
|---|---|---|---|---|---|
| Low-risk | Intermediate-risk | High-risk | Total | ||
| EORTC Categories | Low-risk | 632 (55.3%) | 510 (44.7%) | 0 | 1142 |
| Intermediate-risk | 0 | 1514 (94.3%) | 92 (5.7%) | 1606 | |
| High-risk | 0 | 278 (11.7%) | 2096 (88.3) | 2374 | |
Fig. 4.
Kaplan-Meier curves depicting progression-free survival in 5,122 patients with TaT1 urothelial carcinoma of the bladder, stratified by EAU risk group and reclassification in comparison to EORTC tables.
Impact of reclassification on treatment recommendation
In order to assess, whether the reassignment of patients has consequences on treatment recommendation, we analyzed the corresponding EORTC recurrence and progression category in all reclassified patients and accounted for the EAU treatment recommendation in either risk group. All 1940 upclassified EORTC recurrence intermediate risk patients were EORTC progression high-risk patients. In addition, all patients upclassified from EORTC progression low-risk to EAU intermediate risk were EORTC recurrence intermediate-risk. In all cases the upclassification in recurrence or progression risk by the application of the EAU categories eventually did not lead to any change in treatment recommendation. A change in treatment concept may have occurred in 370 (7.2%) patients, who were either down- or upclassified between intermediate- and high-risk categories.
Subgroup analysis in patients treated with BCG
Of 622 patients who received adjuvant BCG treatment, 24 (3.9%), 159 (25.9%), and 439 (70.6%%) were stratified into EAU low-, intermediate-, and high-risk category, respectively. During follow-up, 250 (40.2%) patients experienced disease recurrence and 51 (8.2%) patients experienced disease progression, respectively. Recurrence- and progression free survival did not differ significantly between EAU low- intermediate- and high-risk patients. Similar to the whole cohort, 428 (72.95) EORTC recurrence score intermediate-risk patients were reclassified into EAU high-risk patients. Regarding the progression risk group, 44 (64.7%) were reclassified from low- to intermediate-risk, 14 patients were reclassified from intermediate- to high-risk and 6 patients were reclassified from high-risk to intermediate risk. The net reclassification index of the EAU risk stratification in BCG-treated patients was 2.9% (95% CI: −5.8% – 10.5%) for recurrence and 6.0% (95% CI −3.5% – 11.8%) for progression, respectively.
Discussion
Non-muscle invasive bladder cancer is characterized by a highly variable risk of disease recurrence and progression. To allow an individualized prediction of cancer-specific outcomes, the prognostic EORTC model was developed (5). Based on the recurrence and progression score, patients are assigned to risk groups, which tailor recommendations for treatment and follow-up scheduling (4). However, several studies have shown a significant non-adherence to guideline recommendations both in Europe and North America (8–11), which appears to translate into worse clinical outcomes of patients with NMIBC (12). To facilitate risk group assignment as well as treatment and follow-up recommendations, the EAU guidelines introduced a simplified risk group stratification in 2013 (3).
Up to our knowledge, this study is the first to characterize cancer-specific outcomes of patients with NMIBC stratified by EAU recommendation and to compare it to EORTC risk-groups. In the first step of our analysis, we characterized disease recurrence and progression separately. In our cohort, recurrence-free survival of low-risk patients differs significantly from intermediate- and high-risk patients. The recurrence-free rates at 1- and 5 years in EAU low-risk patients are comparable to the ones described in previous studies on patients with comparable clinicopathologic features, with a 1- and 5-year probability ranging between 6–15% and 28–31%, respectively (5, 13). In contrast, recurrence-free survival rates of EAU intermediate-and high-risk patients appear to be comparable in our study. Especially high-risk patients using the EAU classification, experience superior recurrence-free survival rates when compared to previously published studies due to reclassification (5, 13). When age and type of intravesical therapy are taken into account, intermediate- and high-risk patients are at significantly higher risk of disease recurrence compared to low-risk patients. However, the hazard ratios between EAU intermediate- and high-risk patients show no significant differences in our study. In addition, the reclassification of 45.7% of EORTC recurrence intermediate-risk patients into EAU high-risk category did not change the probability of recurrence free survival. While disease recurrence is relevant in patients with NMIBC, disease progression is associated with radical surgery in most of the cases and thus has a major impact on patients.
Progression-free survival of NMIBC patients assigned to different EAU risk groups showed significant differences. EAU intermediate- and high-risk categories were associated with significantly higher rates of disease progression than low-risk patients. While progression-free survival rates for EAU low-risk patients appear to be comparable with previously published series, EAU intermediate- and high-risk patients appear to exert a lower risk of disease progression when compared to similar patients cohorts (5, 13). One possible explanation is that progression rates of patients included to generate the EORTC model appear to be higher than progression rates in more contemporary cohorts. This may be attributed to the low numbers of patients who received adjuvant BCG instillations in EORTC trials: Of 2928 patients included, only 171 patients received adjuvant BCG, while other patients received various intravesical chemotherapy regimens (5, 14). As intravesical chemotherapy is considered to be inferior to BCG-therapy for high-risk tumors (15), this might have contributed to higher progression rates in the EORTC studies. In addition, patients in the EORTC studies received BCG induction therapy only (5). A metaanalysis of nine trials in patients with NMIBC demonstrated superiority for BCG compared with mitomycin C for the prevention of tumor progression only if BCG maintenance therapy was provided (16). In contrast, a recent critical evaluation of evidence found that individual randomized studies are generally of poor quality, and provide little evidence for a benefit of maintenance BCG to reduce progression (17).
Regardless of the limitations of the EORTC tables, these are an extremely important tool for risk stratification of patients with NMIBC. In the present study, we aimed to characterize the rate of reclassification introduced by the simplified EAU risk stratification. While none of the EORTC recurrence low- or high-risk patients was reclassified, around 46% of EORTC intermediate-risk patients were reclassified into high-risk of recurrence, when the EAU stratification was applied.The false reclassification of patients into higher risk groups of recurrence with the EAU categories may lead to a Will-Rogers Phenomenon. Similarly, the application of the EAU stratification reclassified around 45% of EORTC progression low-risk to intermediate-risk as well as 6% of intermediate-risk patients to high-risk of progression. Treatment recommendations and follow-up scheduling in patients with NMIBC account for the risk of disease recurrence and progression. However, the final choice of adjuvant treatment is determined by the risk of tumor progression. The EORTC score is calculated separately for recurrence and progression whereas the EAU classification assigned the patients to one general risk group. In our study, we clearly show that the EAU risk stratification does not lead to a change of treatment recommendation in the majority of the patients. The EAU risk stratification is a simple and easily applicable alternative to the EORTC tables, which facilitates the correct assignment of patients to risk-adapted adjuvant treatment and surveillance. The consistent application of this tool may ultimately lead to an improved in treatment of our patients with NMIBC.
Our study has several limitations. The retrospective design warrants further confirmation in prospective cohorts. As a multi-center study, there were multiple surgeons. We did not perform a central pathology review on the specimens; however, all involved urologists and pathologist were working in academic departments and were dedicated to uro-oncology. We used the WHO 1973 classification with 29.4% of the cohort were G2. This could change the results of the study if we assign them to the 2004 WHO classification. Furthermore, we did not control for treatment delay, effect of repeat TURB, and quality of TURB. We also did not have any information on completion of adjuvant intravesical therapy. In addition, we did not have any information on the smoking status of patients, which is a well known prognostic factor associated with outcomes in UCB (18). Finally, the discrepancies between the baseline characteristics of EORTC trials patients and our cohort, and the rate of BCG administration may affect the results of re-classification. Despite these limitations, this study is the first to describe outcomes with the EAU risk classification and compares outcomes to the EORTC model.
Conclusions
Compared to EORTC risk stratification, the EAU categories reclassifies 37.9% patients into a higher risk group of recurrence and 11.8% into a higher risk of progression potentially leading to a Will Rogers phenomenon. However, the novel risk stratification assigns the majority of patients to the same treatment as the more complex EORTC tables and can be regarded as an alternative and easy-to-use tool for treatment decision-making.
Highlights.
The EAU risk stratification is a simple tool to assign patients into treatment schedules.
Intermediate- and high-risk EAU categories were associated with higher risk of disease recurrence and progression.
EAU categories reclassifies 37.9% patients into a higher risk group of recurrence and 11.8% into a higher risk of progression. Only 5.4% were reclassified to a lower risk group for progression.
EAU risk stratification assign the majority of patients into the same treatment plan as the more complex EORTC tables.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stenzl A, Cowan NC, De Santis M, et al. Treatment of muscle-invasive and metastatic bladder cancer: update of the EAU guidelines. European urology. 2011;59(6):1009–18. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD & Jemal A Cancer statistics, 2016. CA. Cancer J. Clin 66, 7–30 [DOI] [PubMed] [Google Scholar]
- 3.Babjuk M, Burger M, Zigeuner R, et al. Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and CIS). Uroweb 2013. Available at: http://www.uroweb.org/gls/pdf05_TaT1_Bladder_Cancer_LR.pdf 2013. [Google Scholar]
- 4.Babjuk M, Böhle A, Burger M, Capoun O, Cohen D, Compérat EM, et al. EAU Guidelines on Non–Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. European Urology. 2016. [DOI] [PubMed] [Google Scholar]
- 5.Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. European urology. 2006;49(3):466–5; discussion 75–7. [DOI] [PubMed] [Google Scholar]
- 6.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Annals of surgical oncology. 2010;17(6):1471–4. [DOI] [PubMed] [Google Scholar]
- 7.Rink M, Fajkovic H, Cha EK, et al. Death certificates are valid for the determination of cause of death in patients with upper and lower tract urothelial carcinoma. European urology. 2012;61(4):854–5. [DOI] [PubMed] [Google Scholar]
- 8.Gontero P, Oderda M, Altieri V, et al. Are referral centers for non-muscle-invasive bladder cancer compliant to EAU guidelines? A report from the vesical antiblastic therapy Italian study. Urologia internationalis. 2011;86(1):19–24. [DOI] [PubMed] [Google Scholar]
- 9.Chamie K, Saigal CS, Lai J, et al. Compliance with guidelines for patients with bladder cancer: variation in the delivery of care. Cancer. 2011;117(23):5392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nielsen ME, Smith AB, Pruthi RS, et al. Reported use of intravesical therapy for non-muscle-invasive bladder cancer (NMIBC): results from the Bladder Cancer Advocacy Network (BCAN) survey. BJU international. 2012;110(7):967–72. [DOI] [PubMed] [Google Scholar]
- 11.Witjes JA, Palou J, Soloway M, et al. Current clinical practice gaps in the treatment of intermediate- and high-risk non-muscle-invasive bladder cancer (NMIBC) with emphasis on the use of bacillus Calmette-Guerin (BCG): results of an international individual patient data survey (IPDS). BJU international. 2013;112(6):742–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lammers RJ, Palou J, Witjes WP, Janzing-Pastors MH, Caris CT, Witjes JA. Comparison of expected treatment outcome provided by risk models and international guidelines with observed treatment outcome in a cohort of Dutch non-muscle-invasive bladder cancer patients treated with intravesical chemotherapy. BJU international. 2013. [DOI] [PubMed] [Google Scholar]
- 13.Hernandez V, De La Pena E, Martin MD, Blazquez C, Diaz FJ, Llorente C. External validation and applicability of the EORTC risk tables for non-muscle-invasive bladder cancer. World journal of urology. 2011;29(4):409–14. [DOI] [PubMed] [Google Scholar]
- 14.Witjes JA, v d Meijden AP, Collette L, et al. Long-term follow-up of an EORTC randomized prospective trial comparing intravesical bacille Calmette-Guerin-RIVM and mitomycin C in superficial bladder cancer. EORTC GU Group and the Dutch South East Cooperative Urological Group. European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Collaborative Group. Urology. 1998;52(3):403–10. [DOI] [PubMed] [Google Scholar]
- 15.Malmstrom PU, Sylvester RJ, Crawford DE, et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guerin for non-muscle-invasive bladder cancer. European urology. 2009;56(2):247–56. [DOI] [PubMed] [Google Scholar]
- 16.Bohle A, Bock PR. Intravesical bacille Calmette-Guerin versus mitomycin C in superficial bladder cancer: formal meta-analysis of comparative studies on tumor progression. Urology. 2004;63(4):682–6; discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 17.Ehdaie B, Sylvester R, Herr HW. Maintenance bacillus Calmette-Guerin treatment of non-muscle-invasive bladder cancer: a critical evaluation of the evidence. European urology. 2013;64(4):579–85. [DOI] [PubMed] [Google Scholar]
- 18.Rink M, Furberg H, Zabor EC, et al. Impact of smoking and smoking cessation on oncologic outcomes in primary non-muscle-invasive bladder cancer. European urology. 2013;63(4):724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]