To the Editor
The COVID‐19 pandemic has limited dermatologic care with >50% decrease in the number of patients seen and biopsies completed. 1 Even after this pandemic has passed, social distancing may limit the number of patients seen in‐person and telemedicine will likely play a larger permanent role.
Teletrichology describes the use of telecommunication platforms to interact with hair disorder patients remotely and to select patients who need in‐person consultation for diagnostic or therapeutical procedures. Teletrichology is excellent for both initial and follow‐up examinations of patients with different types of hair loss including telogen effluvium, androgenetic alopecia, alopecia areata and scarring alopecia. With teletrichology, dermatologist can effectively evaluate patients by instructing them to perform the pull/tug tests, measure thickness of the ponytail, measure distance from the hairline to the glabella and show daily/shampoo hair shedding. Since April 2020, one of the authors has visited 235 patients using teletrichology and teletrichoscopy.
Trichoscopy is a valuable diagnostic tool to differentiate inflammatory, infectious, scarring and non‐scarring conditions. 2 However, some concerns regarding the use of trichoscopy during the COVID‐19 pandemic have recently been raised due to the possible risk of viral contamination of hair. Although, currently, no data exist examining the presence of COVID‐19 on hair follicles or the risk of spread through the use of dermatoscopes, data exist showing that bacteria and HPV DNA can be detected on dermatoscopic lenses and the use of contact dermoscopy (which is used for trichoscopy) is not recommended. 3 , 4 , 5
To overcome these limitations, we have successfully developed teletrichoscopy by instructing patients to use smartphone applications or inexpensive imaging attachments (Table 1).
Table 1.
Summary of appropriate teletrichology clinical evaluations that can be completed during hair disorder consult
| Hair condition | Teletrichology visit evaluations | Instrument | Instrument findings |
|---|---|---|---|
| Telogen effluvium † |
Pull test Hair shedding Ponytail measure |
Macro‐imaging app/handheld microscope | Lack of hair shaft variability |
| Alopecia areata † |
Pull test SALT PGA |
Macro‐imaging app |
Yellow dots Broken hairs Exclamation mark hairs |
| Androgenic alopecia † |
Pull test Hair shedding Ponytail measure |
Macro‐imaging app/handheld microscope | Hair shaft variability |
| FFA § | Measure hairline distance from glabella | Macro‐imaging app/handheld microscope | Absence of vellus hair |
| CCCA ¶ | Clinical pattern suggests diagnosis | Macro‐imaging app/handheld microscope | Hair shaft variability |
| Other scarring alopecias † | Patchy alopecia | Macro‐imaging app/handheld microscope | Absence of follicular openings and peripilar casts |
Type of instrument patient can use remotely and findings that can be seen in each disorder. Additionally, recommendations on telemedicine or in‐person follow‐up and treatment have been provided.
CCCA, central centrifugal cicatricial alopecia; FFA, frontal fibrosing alopecia; PGA, Physician Global Assessment score; SALT, severity of alopecia score.
Treat and follow up with telemedicine. ‡Treat and follow up with telemedicine except for patients requiring intralesional corticosteroid treatment. §You can diagnose and follow up with telemedicine, in‐person visit only required to confirm diagnosis with biopsy at first visit. ¶Need to see in person for biopsy and procedures.
Patients can then, safely, take images and share with their dermatologist. Low‐cost or even free applications for macrophotography are available for both Apple and Android devices (Camera +2 for apple and Camera FV‐5 for android). Macro‐images are effective to assess presence of broken and exclamation mark hairs in alopecia areata, presence/absence of vellus hairs in frontal fibrosing alopecia, peripilar casts in scarring alopecias and hair shaft variability in androgenetic alopecia (Fig. 1a,b).
Figure 1.
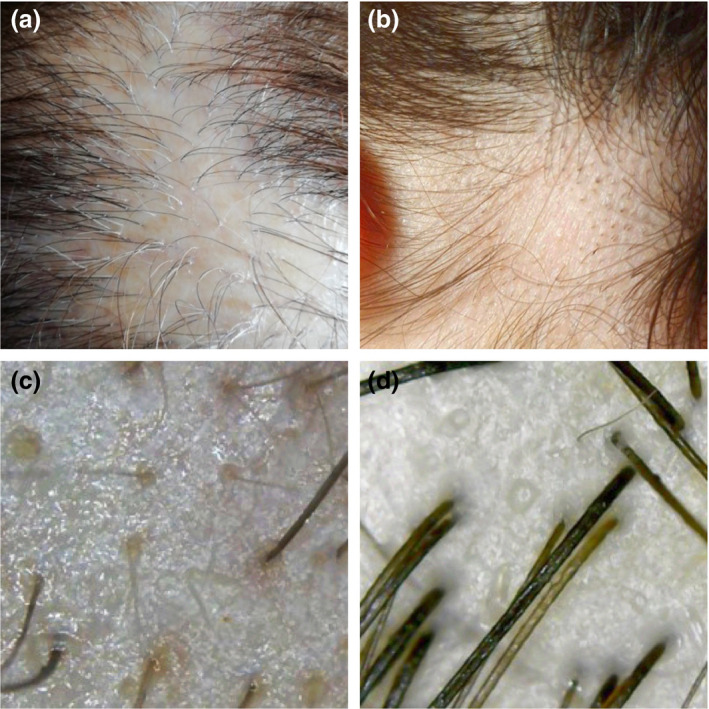
Images taken using smartphone applications at 10× magnification (a and b). (a) Peripilar casts in lichen planopilaris. (b) Broken hairs and black dots in alopecia areata. Images taken using handheld microscope at 50× magnification (c and d). (c) Yellow dots and short regrowing hairs in alopecia areata. (d) Hair shaft variability and empty follicles in female pattern hair loss.
Low‐cost handheld microscopes are another option and can be purchased for under $40. Micali et al. 6 evaluated inexpensive handheld video microscope for trichoscopy and determined they could identify hair shaft variations, but struggled to identify other features such as yellow dots, white dots and perifollicular scales when compared to medical video dermatoscopes. However, improved technology and new instruments can detect yellow dots, perifollicular scales and casts among other features (Fig. 1c,d). Instrument magnification ranges from 50× to 1000×, with 50× recommended for trichoscopy.
Successful teletrichology requires clear patient instruction on how to properly obtain the appropriate instruments/software and how to take useful images. Patients should take multiple images at specific locations on their scalp, hairline and temples. Patients should take images of the frontal and temporal hairline of the top of the scalp after central parting. Patients with patchy alopecia should take images from the centre and periphery of the patch. Even with clear instructions and multiple images, image quality may be limited due to the skill of the patient. This requires further instruction/training until useful images can be obtained.
The use of teletrichoscopy is a promising new option to maintain physician–patient contact and continue management of care.
Conflicts of interest
Michael Randolph and Ammar Al‐alola have nothing to disclose. Antonella Tosti served as a consultant or advisor for DS Laboratories, Monat Global, Almirall, Thirty Madison, Lilly, Leo Pharmaceuticals, Bristol Myers Squibb and P&G.
Funding sources
None.
Acknowledgement
The patients in this manuscript have given written informed consent to the publication of their case details.
References
- 1. Litchman GH, Rigel DS. The Immediate Impact of COVID‐19 on US. Dermatol Pract 2020; 83: 685–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miteva M, Tosti A. Hair and scalp dermatoscopy. J Am Acad Dermatol 2012; 67: 1040–1048. [DOI] [PubMed] [Google Scholar]
- 3. Jakhar D, Kaur I, Kaul S. Art of performing dermoscopy during the times of coronavirus disease (COVID‐19): simple change in approach can save the day! J Eur Acad Dermatol Venereol 2020; 34 :e242–e244. [DOI] [PubMed] [Google Scholar]
- 4. Penso‐Assathiany D, Gheit T, Prétet JL et al. Presence and persistence of human papillomavirus types 1, 2, 3, 4, 27, and 57 on dermoscope before and after examination of plantar warts and after cleaning. J Am Acad Dermatol 2013; 68: 185–186. [DOI] [PubMed] [Google Scholar]
- 5. Goldust M, Zalaudek I, Gupta A, Lallas A, Rudnicka L, Navarini AA. Performing dermoscopy in the COVID‐19 pandemic. Dermatol Ther 2020: e13506.33:e13506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Verzì AE, Lacarrubba F, Micali G. Use of low‐cost videomicroscopy versus standard videodermatoscopy in trichoscopy: a controlled. Blinded Noninferiority Trial. Ski Appendage Disord 2015; 1: 172–174. [DOI] [PMC free article] [PubMed] [Google Scholar]


