Abstract
Background
Hypercoagulability may contribute to COVID‐19 pathogenicity. The role of anticoagulation (AC) at therapeutic (tAC) or prophylactic doses (pAC) is unclear.
Objectives
We evaluated the impact on survival of different AC doses in COVID‐19 patients.
Methods
Retrospective, multi‐center cohort study of consecutive COVID‐19 patients hospitalized between March 13 and May 5, 2020.
Results
A total of 3480 patients were included (mean age, 64.5 years [17.0]; 51.5% female; 52.1% black and 40.6% white). 18.5% (n = 642) required intensive care unit (ICU) stay. 60.9% received pAC (n = 2121), 28.7% received ≥3 days of tAC (n = 998), and 10.4% (n = 361) received no AC. Propensity score (PS) weighted Kaplan‐Meier plot demonstrated different 25‐day survival probability in the tAC and pAC groups (57.5% vs 50.7%). In a PS–weighted multivariate proportional hazards model, AC was associated with reduced risk of death at prophylactic (hazard ratio [HR] 0.35 [95% confidence interval {CI} 0.22‐0.54]) and therapeutic doses (HR 0.14 [95% CI 0.05‐0.23]) compared to no AC. Major bleeding occurred more frequently in tAC patients (81 [8.1%]) compared to no AC (20 [5.5%]) or pAC (46 [2.2%]) subjects.
Conclusions
Higher doses of AC were associated with lower mortality in hospitalized COVID‐19 patients. Prospective evaluation of efficacy and risk of AC in COVID‐19 is warranted.
Keywords: anticoagulation, COVID‐19, heparin, novel coronavirus
Novelty Statements.
We retrospectively compared survival of patients treated with different anticoagulation doses in a large cohort.
Higher doses of anticoagulation were associated with prolonged survival, especially in critically ill patients, but this larger effect size came at the cost of excess non‐disabling bleeding.
Anticoagulation may be considered in the treatment of hospitalized COVID‐19 patients, but prospective studies are required to further assess the benefit and bleeding risk.
1. INTRODUCTION
Hypercoagulability has emerged as an important component in the pathogenesis of severe coronavirus disease 2019 (COVID‐19) caused by the novel pathogen designated severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Studies have reported coagulation abnormalities such as elevated D‐dimer and fibrinogen in conjunction with low anti‐thrombin levels, 1 evidence of endothelial dysfunction, 2 as well as a markedly abnormal coagulation profile on thromboelastography suggestive of hypercoagulability in the context of severe systemic inflammation. 3 , 4 Furthermore, marked elevations in D‐dimer level and markers of endothelial dysfunction (von Willebrand factor antigen, soluble thrombomodulin) have been correlated with worse outcomes. 2 , 5 , 6 One case series of critically ill COVID‐19 patients reported a high incidence of thrombotic complications (31%) 7 and numerous autopsy case series have described pulmonary and other visceral microthromboses suggesting that coagulation abnormalities are not simply an epiphenomenon but are likely major pathogenic components. 8 , 9 , 10 , 11
A small number of retrospective studies have observed that thromboprophylactic‐dose anticoagulation is associated with improved outcomes in patients with COVID‐19, but a growing body of evidence suggests a possible advantage with using more intense regimens. Our group found a dose‐ and duration‐dependent delay in death in a cohort of 127 deceased patients with severe COVID‐19. 12 Others have also explored the effect of AC in a large cohort of hospitalized COVID‐19 patients and found superior outcomes for those treated with tAC, but the comparison group consisted of patients who either received pAC or no AC, and no conclusions could be reached regarding optimal dosing. 13 Notably, in critically ill patients, a significant association with improved survival in subjects treated with tAC compared to pAC was observed. 14 A subsequent analysis of the same cohort again found superior outcomes with the use of AC, but a direct comparison between tAC and pAC failed to show a significant difference. 11
There is a lack of large observational data examining outcomes among patients with COVID‐19 receiving tAC compared with pAC or no AC and available results are conflicting. We hypothesized that tAC and pAC may be associated with improved outcomes in a dose‐dependent manner relative to no AC among hospitalized patients with COVID‐19. We further hypothesized that these associations may be strongest among critically ill patients receiving mechanical ventilation. We also sought to determine the risk of major bleeding. Accordingly, we performed an observational cohort study in the largest hospital network in Southeast Michigan, USA, examining survival relative to anticoagulation dose in hospitalized patients with COVID‐19.
2. METHODS
2.1. Study design
We conducted a retrospective analysis of a large cohort of consecutive COVID‐19 patients hospitalized within the largest academic healthcare system comprised of eight hospitals located in Southeast Michigan, USA. Patients aged 18 years or older who tested positive for SARS‐CoV‐2 on nucleic amplification testing of nasopharyngeal secretions between March 13, 2020, and May 5, 2020, were retrospectively identified from electronic medical records. To ensure the main reason for hospitalization was COVID‐19, subjects who had tested positive for SARS‐CoV‐2 prior to the admission date or after the third day of hospitalization were excluded. Data were obtained automatically from electronic records. The study was approved by the Institutional Review Board (IRB # 2020‐125). In the absence of evidence‐based criteria for initiating AC in COVID‐19 patients, our institution published internal recommendations for the use of tAC for disease requiring mechanical ventilation, worsening kidney failure and/or a D‐dimer >6‐fold the upper limit of normal (>3000 ng/mL fibrinogen‐equivalent‐units), but tAC could also be initiated at the discretion of the clinician and as salvage therapy. The recommended duration of tAC was 5 days based on expert consensus, but treatment could be extended in the presence of a clear indication or by clinician choice.
Therapeutic anticoagulation was defined as a minimum 3‐day course of either: (a) intravenous unfractionated heparin (UFH) with at least one documented activated partial thromboplastin time in the anticoagulation range (≥45 seconds); (b) subcutaneous enoxaparin at doses of 1 mg/kg twice daily or 1.5 mg/kg once daily (while allowing for dose adjustment based on creatinine clearance); (c) intravenous argatroban infusion; (d) subcutaneous fondaparinux at doses of 5‐10 mg once daily (weight‐based dosing); or (e) oral anticoagulants (warfarin, apixaban, rivaroxaban, dabigatran) prescribed prior to and continued throughout hospitalization. Prophylactic anticoagulation (pAC) was defined as one of the following administrations on most days of hospitalization: 1) subcutaneous injection of UFH at doses of 5000 units twice or three times daily; subcutaneous enoxaparin injection at doses of 30‐40 mg once daily; or 3) subcutaneous fondaparinux at a dose of 2.5 mg once daily. Patients who received therapeutic anticoagulation for less than 3 days were also included in the pAC group. Because many patients transitioned between anticoagulants depending on ability to ingest oral medication and the safety profile of the drug, the specific anticoagulant agent administered in the most doses was considered the primary anticoagulant for a given patient. Immunosuppressive corticosteroid therapy was defined as at least one dose of greater than 15 mg methylprednisolone or equivalent dose of other corticosteroid. For the purposes of analysis, intensive care unit (ICU) stay was defined as need for mechanical ventilation to maintain adequate oxygenation.
Major bleeding was defined as either: (a) transfusion of five or more units of packed red blood cells within 48 hours, regardless of hemoglobin level; (b) hemoglobin <7 g/dL and any red blood cell transfusion; (c) a diagnosis code for major bleeding during the hospitalization (gastrointestinal hemorrhage, intracranial hemorrhage etc); or (d) evidence of intracranial hemorrhage obtained from reports of head computed tomography scans. The higher cutoff of five units of packed red blood cells within 48 hours was chosen based on the observation that critically ill patients received a high number of transfusions even in the absence of major bleeding.
2.2. Outcomes and statistical analysis
Categorical variables are reported with counts (percentages). Numerical variables are reported as either mean (sd = standard deviation), if normal or approximately normal variables, or median (interquartile range [IQR]) for all other numerical variables. To assess normality of numerical variables, the Lilliefors‐Kolmogorov‐Smirnov test was used together with the variable boxplot. Comparisons by groups were performed using chi‐square tests (or Fisher tests if cell count <5). ANOVA tests were used for comparison of means for normally distributed variables. Kruskal‐Wallis tests were applied for comparison of the distribution of non‐normal numerical variables. Pairwise comparisons between the groups were performed utilizing either Tukey's adjustment for approximately normal distributions or Dunn's test for the comparison for non‐normal variables with Hommel's adjustment. All P‐values were 2‐sided and a P < .05 was considered to indicate statistical significance. Adjustment for multiple testing is presented in Table 1 using the method of false discovery rate of Benjamini, Hochberg and Yekutieli. 15
Table 1.
Baseline characteristics and comorbid conditions in overall COVID‐19 study population
| All patients (n = 3480) | No AC (n = 361) | pAC (n = 2121) | tAC (n = 998) | P‐value adj. P‐value | |
|---|---|---|---|---|---|
| Age in years | 64.5 (±17.0) | 55.0 (±21.7) | 64.4 (±16.9) | 68.2 (±14.6) |
<.001 a <.001 |
| Female | 1796 (51.5%) | 207 (57.3%) | 1138 (53.7%) | 448 (44.9%) | <.001 |
| Male | 1687 (48.5%) | 154 (42.7%) | 983 (46.3%0 | 550 (55.1%) | <.001 |
| Race | |||||
| African‐American | 1814 (52.1%) | 197 (54.6%) | 1149 (54.2%) | 468 (46.9%) | |
| Caucasian | 1413 (40.6%) | 149 (41.3%) | 827 (39.0%) | 437 (43.8%) | <.001 |
| Asian | 69 (2.0%) | 2 (0.6%) | 45 (2.1%) | 22 (2.2%) | <.001 |
| Other | 184 (5.3%) | 13 (3.6%) | 100 (4.7%) | 71 (7.1%) | |
| BMI b (kg/m2) b | 30.4 (12.9, 103.9) | 30.5 (15.4, 66.7) | 30.4 (12.9, 103.9) | 30.4 (14.5, 73.3) |
.285 .285 |
| BMI (kg/m2) b | |||||
| <18.5 | 81 (2.4%) | 7 (2.1%) | 55 (2.7%) | 19 (1.9%) | |
| 18.5‐30 | 1521 (45.2%) | 154 (45.4%) | 920 (44.9%) | 447 (45.5%) | .279 |
| 30‐40 | 1260 (37.4%) | 136 (40.1%) | 774 (37.8%) | 350 (35.6%) | .285 |
| ≥40 | 507 (15.0%) | 42 (12.4%) | 299 (14.6%) | 166 (16.9%) | |
| Hypertension | 1812 (52.1%) | 120 (33.2%) | 1086 (51.2%) | 606 (60.7%) |
<.001 <.001 |
| Diabetes | 1008 (29.0%) | 67 (18.6%) | 595 (28.1%) | 346 (34.7%) |
<.001 <.001 |
| Coronary artery disease | 425 (12.2%) | 29 (8.0%) | 231 (10.9%) | 165 (16.5%) |
<.001 <.001 |
| Heart failure | 272 (7.8%) | 25 (6.9%) | 134 (6.3%) | 113 (11.3%) |
<.001 <.001 |
| Atrial fibrillation | 195 (5.6%) | 18 (5.0%) | 62 (2.9%) | 115 (11.5%) |
<.001 <.001 |
| Ischemic stroke or TIA | 312 (9.0%) | 23 (6.4%) | 171 (8.1%) | 118 (11.8%) |
<.001 <.001 |
| CKD grade 3 and above | 203 (5.8%) | 15 (4.2%) | 105 (5.0%) | 83 (8.3%) |
<.001 <.001 |
| Dialysis dependent | 97 (2.8%) | 10 (2.8%) | 50 (2.4%) | 37 (3.7%) |
.016 .019 |
| History of VTE | 203 (5.8%) | 15 (4.2%) | 75 (3.5%) | 113 (11.3%) |
<.001 <.001 |
| Chronic lung disease | 760 (21.8%) | 78 (21.6%) | 433 (20.4%) | 249 (24.9%) |
.017 .019 |
| History of malignancy | 278 (8.0%) | 19 (5.3%) | 157 (7.4%) | 102 (10.2%) |
.003 .004 |
| Ever smoker c | 1040 (38.4%) | 95 (33.6%) | 622 (37.8%) | 323 (41.4%) | ‐ |
| D‐dimer > 3000 ng/mL d | 831 (35.2%) | 15 (14.3%) | 281 (20.7%) | 535 (59.6%) | ‐ |
| Mechanical ventilation | 642 (18.5%) | 18 (5.0%) | 207 (9.8%) | 417 (41.8%) | <.001 |
| No mechanical ventilation | 2838 (81.5%) | 343 (95.0%) | 1914 (90.2%) | 581 (58.2%) | |
| AKI requiring dialysis | 214 (6.2%) | 8 (2.2%) | 67 (3.2%) | 139 (13.9%) | <.001 |
| No new dialysis requirement | 3266 (93.8%) | 353 (97.8%) | 2054 (96.8%) | 859 (86.1%) | |
| Corticosteroid treatment | 1825 (52.4%) | 64 (17.7%) | 1003 (47.3%) | 758 (76.0%) | <.001 |
| No corticosteroid treatment | 1655 (47.6%) | 297 (82.3%) | 1118 (52.7%) | 240 (24.0%) | |
| HQ and Azithromycin | |||||
| None | 592 (17.0%) | 171 (47.4%) | 332 (15.7%) | 89 (8.9%) | |
| HQ only | 356 (10.2%) | 17 (4.7%) | 224 (10.6%) | 115 (11.5%) | <.001 |
| Azithromycin only | 332 (9.6%) | 63 (17.5%) | 232 (10.9%) | 37 (3.7%) | |
| HQ and Azithromycin | 2200 (63.2%) | 110 (60.5%) | 1333(62.8%) | 757 (75.9%) | |
Age is presented as mean (standard deviation). BMI is presented as median (range). Other numbers represent n (%).The adjusted P‐value uses Benjamini, Hochberg and Yekutieli 20 , 21 method that controls the false discovery rate.
Bold values indicates P‐value < .05.
Abbreviations: AC, anticoagulation; AKI, acute kidney injury; BMI, body mass index; CKD, chronic kidney disease; HQ, hydroxychloroquine; pAC, prophylactic anticoagulation; tAC, therapeutic anticoagulation; TIA, transient ischemic attack; VTE, venous thromboembolism.
All means significantly different (P < .001) using post hoc Tukey's HSD.
Data for BMI available for N = 3369.
Data for smoker status available for N = 2710.
Data for D‐dimer available for N = 2363.
Multivariate Cox regression was used to evaluate the survival time of COVID‐19 patients compared between those who received tAC, Pac, and no AC. Time zero was the time of admission and patients were right‐censored at the time of discharge or at the end of the study period if they remained hospitalized. Propensity score (PS) weights were used as a summary adjustment variable to address the issue of possible bias introduced by the retrospective nature of the study. This approach was preferred to PS matching to preserve the sample size. The PS was calculated using a logistic regression model with categorical AC group as dependent variable and it was adjusted for the following covariables: age (years), sex, race with 4 levels (Caucasian, African‐American, Asian, Other), body mass index (BMI) with 4 levels (<18.5, 18.5‐30, 30‐40, >40 kg/m2), as well as comorbid conditions (hypertension, hyperlipidemia, coronary artery disease, peripheral artery disease, heart failure, cerebral vascular attack/transient ischemic attack, atrial fibrillation, chronic kidney disease grade 3 or above, hemodialysis dependence, history of malignancy, history of venous thromboembolism, immunocompromised status, connective tissue disease, chronic lung disease). The balance of the propensity scores was examined using the standardized effect size. Schoenfeld, deviance residuals, and Grambsch‐Therneau test evaluated the fit of the multivariate Cox model and the proportionality of hazards assumptions, respectively. PS‐weighted Kaplan‐Meier curves were plotted to compare in‐hospital mortality between groups and groups were compared using the log‐rank test. A Cox Proportional Hazard model with propensity score (PS) weights was fitted to the data to assess the effect of covariates on mortality. Univariate Cox regression guided the selection of candidate factors and variables with P‐values of .1 were included for stepwise AIC variable selection.
Statistical analysis was performed using JMP (software version 14.0.0) and R statistical software (software version 4.0.0). The package TWANG (version 1.6) was employed for calculation of the PS weights. 16
3. RESULTS
3.1. Study population and baseline characteristics
Between March 13, 2020, and May 5, 2020, 3717 adults tested positive for SARS‐CoV‐2 in our healthcare system. Of these, 3480 tested positive on the first 3 days of a hospital admission. The majority (2838 [81.5%]) were treated in a non‐ICU setting, whereas the remainder (642 [18.5%]) required mechanical ventilation and were included in the ICU group. Figure 1 provides an outline of the study population. The mean age of the overall population was 64.5 years (17.0) and the sex distribution was balanced with 51.5% females and 48.5% males. Baseline characteristics of the overall study population are presented in Table 1.
Figure 1.
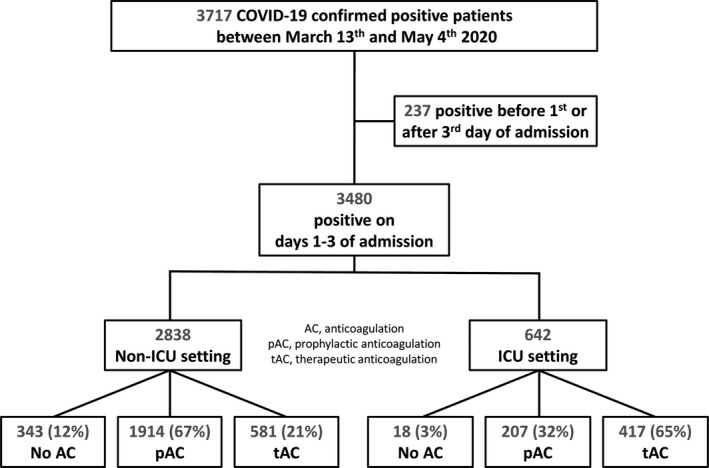
Outline of study population. AC, anticoagulation; pAC, prophylactic anticoagulation; tAC, therapeutic anticoagulation
Most patients received pAC (2121 [60.9%]) and almost one third received tAC courses of 3 days or more (998 [28.7%]). Only 361 (10.4%) did not receive any dose of anticoagulant. Of the 3119 patients who received any dose of AC, 554 (17.8%) switched between different anticoagulant agents of the same dose intensity. The most common primary anticoagulant in the pAC group was enoxaparin (1156 [54.5%]), followed by UFH (699 [33.0%]) and fondaparinux (7 [0.3%]). Patients who received less than 3 days of tAC (259) were included in the pAC group. Agents used for tAC had a similar distribution: enoxaparin (424 [42.5%]), UFH (295 [29.6%]), and fondaparinux (6 [0.6%]). Remaining tAC patients (273 [10.4%]) received primarily oral agents: apixaban (183 [18.3%]), warfarin (42 [4.2%]), rivaroxaban (46 [4.6%]), and dabigatran (2 [0.2%]). The median time from admission to initiation of tAC was 2 days (IQR 1‐6 days) and the median duration of tAC was 8 days (IQR 5‐12 days).
Analysis of demographics and medical history (Table 1) revealed that the tAC group was comprised, on average, of an older population (68.2 years [14.6] vs 64.4 [16.9] in the pAC and 55.0 [21.7] in the no AC groups), with a higher frequency of common comorbid conditions. Rates of therapeutic interventions are presented in Table 1.
3.2. Anticoagulation and in‐hospital mortality
In‐hospital mortality among the groups was statistically different (11.4% for no AC, 10.8% for pAC and 23.6% for tAC; P < .001). Median survival times were 25 days for pAC and 30 days for tAC. The median survival time was not reached in the no AC group. PS‐weighted Kaplan‐Meier curves were statistically different for the tAC group compared to the pAC and no AC groups (Figure 2). At hospital day 25, the survival probability in the tAC group was visually higher than in the pAC group (57.5% vs 50.7%). In the no AC group, the last event occurred at 11 days at which time the survival probability was 61.0%.
Figure 2.
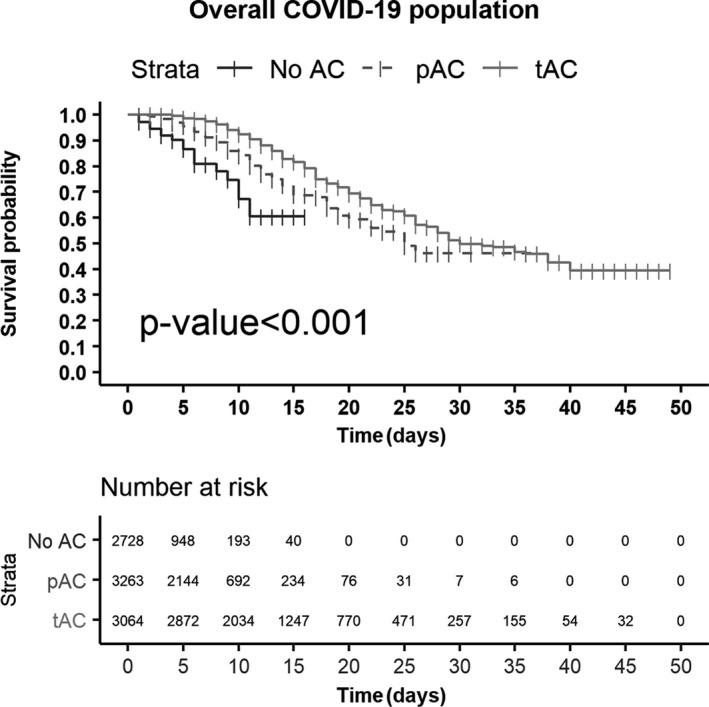
Propensity score‐weighted Kaplan‐Meier survival curves by AC dose in overall study population. AC, anticoagulation; pAC, prophylactic anticoagulation; tAC, therapeutic anticoagulation. Numbers at risk represent estimates obtained through PS weighting and not the actual number of cases; this approach ensures that patients who are not included will account for their share of the population and adjust the survival probability for possible sources of bias
In the ICU group, 310 (48.3%) patients died during their hospital stay (median survival time 22 days) compared to 196 (6.9%) in the non‐ICU population (median survival time not reached). PS‐weighted Kaplan‐Meier curves stratified by disease severity are shown in supplemental Figure S1 panels A and B. Different survival probabilities at 25 days postadmission were seen in patients receiving tAC compared to those receiving only pAC in both the ICU (56.3% vs 22.5%) and non‐ICU (78.5% vs 65.7%) populations. Baseline characteristics of these two populations are presented in Tables S1 and S2.
A preliminary PS‐weighted univariate Cox regression identified the following potential predictors of death (significant at the 0.1 level): age, BMI, race, categorical AC group, corticosteroid treatment, ICU stay, acute kidney injury (AKI) requiring dialysis, hypertension, coronary artery disease, heart failure, and combination of hydroxychloroquine (HQ) and azithromycin. D‐dimer values and smoking status were not included in the final model due to missing values and to preserve sample size. The final multivariate Cox regression model is shown in Table 2. Independent predictors of mortality were age (P < .001, hazard ratio [HR] 1.6 per 10‐year increase [95% confidence interval {CI} 1.4‐1.8]), ICU stay (P < .001, HR 5.2 [95% CI 3.5‐7.8]), and poor nutritional status defined as BMI < 18.5 (P = .001, HR 3.0 [95% CI 1.5‐6.0]); AKI requiring dialysis was linked to a poor prognosis (HR 1.3 [95% CI 0.96‐1.8]), but did not reach significance (P = .095). AC was associated with a reduced risk of death in the multivariate model. The effect was dose‐dependent: compared to no AC, pAC was associated with a 65% decrease in the risk of death (HR 0.35 [95% CI 0.22‐0.54]) and tAC with 86% decrease (HR 0.14 [95% CI 0.05‐0.23]). In both the raw and PS‐weighted multivariate analyses, corticosteroids did not significantly impact mortality risk. The effect of tAC prior to admission for pre‐existing indications (VTE, atrial fibrillation etc) and that of increasing experience with COVID‐19 (the week of diagnosis) did not impact outcomes in the model.
Table 2.
Propensity score‐weighted multivariate Cox proportional hazards model
| Hazard ratio | Confidence interval | Significance | |
|---|---|---|---|
| Age (years) | 1.6 a | 1.4‐1.8 | <.001 |
| BMI (kg/m2) b | |||
| <18.5 kg/m2 | 3.0 | 1.5‐6.0 | .001 |
| 30‐40 kg/m2 | 0.8 | 0.6‐1.1 | .214 |
| ≥40 kg/m2 | 1.1 | 0.7‐1.6 | .779 |
| ICU stay | 5.2 | 3.5‐7.8 | <.001 |
| Prophylactic anticoagulation c | 0.35 | 0.22‐0.54 | <.001 |
| Therapeutic anticoagulation c | 0.14 | 0.08‐0.23 | <.001 |
| AKI requiring dialysis | 1.3 | 0.96‐1.8 | .095 |
| HQ and Azithromycin | |||
| HQ | 0.7 | 0.4‐1.2 | .29 |
| Azithromycin | 1.4 | 0.6‐3.1 | .41 |
| HQ and Azithromycin | 0.7 | 0.4‐1.2 | .27 |
Abbreviations: AKI, acute kidney injury; Azithro, azithromycin; HQ, hydroxychloroquine; ICU, intensive care unit.
Per 10‐year increase
Reference is BMI between 18.5‐30 kg/m2
Reference is no AC
3.3. Complications of anticoagulant treatment
Major bleeding events defined by composite criteria occurred in 147 subjects: 81 (8.1%) patients treated with tAC compared to 20 (5.5%) among those who received no AC and 46 (2.3%) in those who received pAC (Table 3). Patients requiring mechanical ventilation had a markedly higher rate of bleeding (80 [12.5%] vs 67 [2.4%]). When comparing the frequency of intracranial bleeding, 13 patients (1.3%) had an event in the tAC group, compared to 4 (1.11%) in the no AC and 10 (0.5%) in the pAC groups (P = .028).
Table 3.
Complications of AC in the overall COVID‐19 population
| All patients (n = 3480) | No AC (n = 361) | pAC (n = 2121) | tAC (n = 998) | Significance | |
|---|---|---|---|---|---|
| Major bleeding | 147 (4.2%) | 20 (5.5%) | 46 (2.2%) | 81 (8.1%) | <.001 |
| No major bleeding | 3333 (95.8%) | 341 (94.5%) | 2075 (97.8%) | 917 (91.9%) | |
| ≥5 units PRBC in 48 h | 70 (2.0%) | 9 (2.5%) | 18 (0.9%) | 43 (4.3%) | <.001 |
| <5 units PRBC in 48 h | 3410 (98.0%) | 352 (97.5%) | 2103 (99.1%) | 955 (95.7%) | |
| Intracranial hemorrhage | 27 (0.8%) | 4 (1.1%) | 10 (0.5%) | 13 (1.3%) | .028 |
| No intracranial hemorrhage | 3453 (99.2%) | 357 (98.9%) | 2111 (99.5%) | 985 (98.7%) | |
| Severe thrombocytopenia | 71 (0.2%) | 10 (2.8%) | 27 (1.3%) | 34 (3.4%) | <.001 |
| No severe thrombocytopenia | 3406 (97.8%) | 349 (97.2%) | 2093 (98.7%) | 964 (96.6%) |
Abbreviations: AC, anticoagulation; AKI, acute kidney injury; HQ, hydroxychloroquine; pAC, prophylactic anticoagulation; tAC, therapeutic anticoagulation.
Bold values indicates P‐value < .05.
The presence of severe thrombocytopenia (platelet count <50.000/µL on at least two occasions 24 hours apart) was reported in 34 patients (3.4%) in the tAC group, 10 (2.8%) in the no AC and 27 (1.3%) in the pAC groups (P < .001). Eleven cases (1.1%) who received tAC had confirmed heparin‐induced thrombocytopenia (HIT), whereas only 1 (0.05%) patient treated with pAC developed HIT. Half the patients diagnosed with HIT (6/12) had severe thrombocytopenia.
4. DISCUSSION
The biological basis for the potential benefit of anticoagulation in COVID‐19 is derived from reports of hypercoagulability and endothelial dysfunction 2 , 6 , 17 , 18 , 19 with resulting formation of micro‐ and macrothrombi. 8 , 9 , 20 Ours is among the first and the largest studies to evaluate the effect of AC on survival in COVID‐19. Our main findings are as follows: 1) both prophylactic and therapeutic AC were associated with decreased mortality in COVID‐19; 2) patients receiving therapeutic doses had higher survival probability compared to those receiving prophylactic doses, and the greatest effect was observed in critically ill patients; and 3) major bleeding events occurred more frequently in patients receiving tAC.
Our study population was derived from a large US cohort (3480 subjects), of which nearly one fifth required care in an intensive care setting. Most (90%) received AC, with nearly two thirds receiving prophylactic doses and one third receiving therapeutic doses. For the remainder of the population, AC was likely withheld because of active or potential bleeding complications, low baseline hemoglobin or platelet count, and individual physician practice patterns. The precise indication for the initiation of tAC was not available for analysis, but certain observations suggest that COVID‐19‐associated hypercoagulability was a frequent indication: (a) the number of patients who continued oral anticoagulant therapy inpatient was relatively small (25.7%) corresponding to a low prevalence of pre‐existing comorbid conditions requiring AC; (b) the true inpatient incidence of VTE was unknown due to limitation of diagnostic imaging in an effort to decrease exposure and, as such, these were less likely to constitute AC indications; (c) among deceased patients in our institution, COVID‐19‐associated hypercoagulability was the sole indication in 55% of subjects 12 ; and (d) institutional guidelines recommended use of tAC in a sicker patient population.
A propensity score‐weighted multivariate proportional hazards model found that AC was associated with a decreased risk of mortality and the effect appeared to be dose‐dependent, with prophylactic doses conferring a 65% decrease in risk (HR of 0.35 [95% CI 0.22‐0.54]) and therapeutic doses an 86% decrease (HR of 0.14 [95% CI 0.08‐0.23]) compared to patients who did not receive AC. The sizeable effect of pAC on mortality in COVID‐19 was surprising as studies of pAC in the non‐surgical acutely ill population have generally failed to demonstrate a survival benefit. 21 The benefit of AC, although more prominent in the ICU population, was evident in all hospitalized patients in our cohort. In line with institutional recommendations, patients with severe organ failure and those with evidence of coagulation abnormalities (high D‐dimer) were most likely to receive tAC, but even after adjusting for multiple factors (including critical illness), the higher benefit of tAC remained evident. Due to a large number of missing D‐dimer values and recognizing that available measurements would likely have been obtained in more severe disease and, hence, would not satisfy the missing at random assumption, we chose to forego analysis of the interaction between D‐dimer level and AC in an effort to avoid confounding.
Our results supplement those of recent investigations focused on the use of AC in COVID‐19 conducted on a large cohort of hospitalized patients in the Northeastern United States. The first study by Paranjpe et al found similar in‐hospital mortality rates between patients treated with tAC and those who were not (22.5% vs 22.8%), a longer median survival time in the tAC group (21 days vs 14 days), and an association between tAC duration and reduced risk of death (HR, 0.86 per day [95% CI 0.82‐0.89]). 13 Subsequently, Trinh et al reported a 79% decrease in the risk of death in patients treated with at least 5 days of tAC compared to pAC (HR, 0.209 [95% CI 0.1‐0.46]) in critically ill patients belonging to the same cohort and. 14 Most recently, Nadkarni et al expanded the existing cohort to nearly 4400 subjects and specifically investigated the impact of tAC and pAC compared to patients who did not receive AC. In a multivariate model adjusting for critical illness, the authors found a similar reduction in in‐hospital mortality with both tAC (HR, 0.53 [95% CI 0.45‐0.62]) and pAC (HR, 0.50 [95% CI 0.45‐0.57]). In a direct comparison of tAC and pAC initiated in the first 48 hours of hospitalization, the risk was lower with higher doses but did not reach significance (HR, 0.86 [95% CI 0.73‐1.02]).
Overall, AC had a consistently demonstrated survival benefit, but the effect size was highly variable owing to differences in the definition of AC duration, dosing, and statistical methods employed for adjustment. Nadkarni et al study found a more modest risk reduction (50% compared to 86% in our analysis), which may be explained by use of competing risk analysis and the exclusion of patients who received both pAC and tAC throughout their hospital stays. We believe the latter choice, intended to simplify exposures, may have biased results as subjects whose clinical status worsened throughout their hospital stay (and, thus, for whom escalating AC dose may have been reasonable) would be lost to the analysis; following the same rationale, this same group may have included a high number of critically ill patients. Another conspicuous aspect of the Nadkarni et al study is the large number of hospitalized patients not receiving any AC (1530 [35%]) which represents a departure from current guidelines on venous thromboembolic prophylaxis in hospitalized patients.
Due to conflicting results and significant bias in available analyses, the question of optimal AC dosing remains unanswered. The importance of dosing is highlighted by increased major bleeding in the tAC group (as high as 8% of patients) according to definitions set in our study, which were largely more conservative compared to those of others. Surprisingly, a greater number of patients who did not receive AC (5.5%) experienced bleeding compared to 2.2% of those treated with pAC. This suggests that AC was withheld for those with active bleeding or an increased propensity to bleed. Frequencies in our cohort are higher, but comparable to those reported by Nadkarni et al (3% and 1.7% for those receiving tAC and pAC respectively, compared to 1.9% in the no AC group). 11 Trinh et al reported markedly higher rates of bleeding events in critically ill patients, especially in the tAC vs pAC groups (31.7% vs 20.5%; P = .07). 14 While the benefit of tAC came at the cost of more bleeding, the rate of intracranial bleed as evidenced on cranial imaging was similar between the tAC and no AC groups (1.3% vs 1.1%). If the considerable survival advantage of tAC is replicated in prospective studies, it may be reasonable to accept a higher risk of non‐disabling bleeding or transfusion requirement given the potentially fatal course of the disease and lack of other proven therapeutic interventions. Several randomized clinical trials (eg, NCT04372589, NCT04409834, NCT04406389, and NCT04359277) are ongoing to test whether higher dose AC improves outcomes among patient with COVID‐19.
4.1. Limitations
This retrospective, observational study has several important limitations. By design, the study can only report associations, cannot investigate causality, and is susceptible to multiple sources of bias such as indication bias and hidden confounders. We attempted to control for these using a PS‐weighted multivariate model. A high proportion of missing data as can be expected with automatically extracted information from electronic medical records was a particular challenge in our cohort and made it difficult to investigate the value of laboratory coagulation studies (chiefly D‐dimer) that have been identified as candidate measurements to identify patients to most likely benefit from AC. Imputation of missing data could not be used because of violation of the missing at random assumption and difficulty in reconciling imputation with the PS weighting method. One potential limitation was the inclusion of patients with less than three days of tAC in the pAC group. The reasons for shorter duration of tAC (bleeding, discharge, death) may have skewed results slightly, but their number was relatively small and analyzing these patients in the tAC group would have introduced its own type of bias as others have pointed out that AC is infrequently truly therapeutic in this timeframe. 14 More granular analyses to compare the benefit of heparin and non‐heparin anticoagulants or to identify the precise cause of death were not possible. Another source of bias is heterogeneity resulting from the creation of institutional recommendations pertaining to AC throughout the study period as new data emerged about the benefits of AC. An example is the higher attrition rate of patients who did not receive AC or who received pAC, which may lead to bias in comparisons; these represent a mixed population who were either admitted prior to the institutional recommendation to use tAC or had less severe disease and were discharged early. Similarly, the introduction of these recommendations at our institution likely coincided with an increase in our clinical experience in the management of COVID‐19, and confounding may influence our observations. We did attempt to control for the latter by introducing a time variable representing the week of the pandemic in which patients were diagnosed into the multivariate models, but this did not impact outcomes.
5. CONCLUSIONS
Hypercoagulability is emerging as an important component of COVID‐19 pathogenicity and evidence for the beneficial effect of anticoagulation on survival is increasing. We report a statistically significant association between anticoagulant treatment and decreased mortality in a large US cohort of COVID‐19 patients. The effect of anticoagulation appears to be dose‐dependent, with a stepwise increase in survival benefit observed with the use of prophylactic regimens and a three‐day course of therapeutic anticoagulation compared to no anticoagulation. The greatest impact is seen primarily in patients with critical illness, but benefit was also observed in hospitalized non‐ICU patients. Bleeding was more frequent with the use of therapeutic anticoagulation, but the prevalence of disabling intracranial bleed was similar which may tip the risk‐benefit discussion in the favor of anticoagulation. Prospective, randomized controlled trials are required to establish the true effect of tAC on survival and to identify patients with COVID‐19 most likely to benefit from this intervention.
CONFLICT OF INTEREST
PR Lawler receives research funding from the Canadian Institutes of Health Research, the Peter Munk Cardiac Centre, the Ted Rogers Centre for Heart Research, the LifeArc Foundation, and the Thistledown Foundation. He reports consultant fees from Brigham and Women's Hospital (moderate) and Corrona LLC (moderate) and royalties from McGraw‐Hill Publishing moderate). PR Lawler is co‐principal investigator of the Antithrombotic Therapy to Ameliorate Complications of COVID‐19 (ATTACC) trial (NCT04372589). GB Nair has received research funding from Beaumont Research Institute and has consulted for Genentech and Boehringer Ingelheim. GB Nair is the Beaumont site primary investigator for ATTACC trial (NCT04372589). The remaining authors have nothing to disclose.
AUTHOR’S CONTRIBUTION
Ionescu F performed literature search, designed the study, analyzed and processed the data, provided figures, and wrote, critically revised, and finally approved the manuscript. Jaiyesimi I, Lawler PR, Abbas AE, Konde A, Narasimhan M, and Castillo E critically revised and finally approved the manuscript. Petrescu I wrote, critically revised, and finally approved the manuscript. Munoz‐Maldonado Y analyzed the data, provided figures, and critically revised and finally approved the manuscript. Imam Z processed the data and critically revised and finally approved the study. Nair GB literature search, designed the study, analyzed and processed the data, and wrote, critically revised, and finally approved the manuscript.
Supporting information
Fig S1A
Fig S1B
Supplementary Material
Ionescu F, Jaiyesimi I, Petrescu I, et al. Association of anticoagulation dose and survival in hospitalized COVID‐19 patients: A retrospective propensity score‐weighted analysis. Eur J Haematol. 2021;106:165–174. 10.1111/ejh.13533
DATA AVAILABILITY STATEMENT
Raw data were generated within the Beaumont Health System. Derived data supporting the findings of this study are available from the corresponding author, GBN, on request.
REFERENCES
- 1. Terpos E, Ntanasis‐Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID‐19. Am J Hematol. 2020;95(7):834‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goshua G, Pine AB, Meizlish ML, et al. Endotheliopathy in COVID‐19‐associated coagulopathy: evidence from a single‐centre, cross‐sectional study. Lancet Haematol. 2020;7(8):e575‐e582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Panigada M, Bottino N, Tagliabue P, et al. Hypercoagulability of COVID‐19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost JTH. 2020;18(7):1738‐1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ranucci M, Ballotta A, Di Dedda U, et al. The procoagulant pattern of patients with COVID‐19 acute respiratory distress syndrome. J Thromb Haemost JTH. 2020;18(7):1747‐1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang L, Yan X, Fan Q, et al. D‐dimer levels on admission to predict in‐hospital mortality in patients with Covid‐19. J Thromb Haemost. 2020;18(6):1324‐1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:145‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dolhnikoff M, Duarte‐Neto AN, de Almeida Monteiro RA, et al. Pathological evidence of pulmonary thrombotic phenomena in severe COVID‐19. J Thromb Haemost. 2020;18(6):1517‐1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fox SE, Akmatbekov A, Harbert JL, Li G, Brown JQ, Heide RSV. Pulmonary and cardiac pathology in African American patients with COVID‐19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8(7): 681‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID‐19 infection: A report of five cases. Transl Res. 2020;220:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nadkarni GN, Lala A, Bagiella E, et al. Anticoagulation, mortality, bleeding and pathology among patients hospitalized with COVID‐19: a single health system study. J Am Coll Cardiol. 2020;76(16):1815‐1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ionescu F, Grasso‐Knight G, Castillo E, et al. Therapeutic anticoagulation delays death in COVID‐19 patients: cross‐sectional analysis of a prospective cohort. TH Open. 2020;04(3):e263‐e270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Paranjpe I, Fuster V, Lala A, et al. Association of Treatment Dose Anticoagulation with In‐Hospital Survival Among Hospitalized Patients with COVID‐19. J Am Coll Cardiol [Internet]. 2020;76(1):122‐124. 10.1016/j.jacc.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Trinh M, Chang DR, Govindarajulu US, et al. Therapeutic anticoagulation is associated with decreased mortality in mechanically ventilated COVID‐19 patients. medRxiv. 2020. https://www.medrxiv.org/content/10.1101/2020.05.30.20117929v1 [Google Scholar]
- 15. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57(1):289‐300. [Google Scholar]
- 16. Ridgeway G, McCaffrey D, Morral A, Griffin BA, Burgette L, Cefalu M. twang: Toolkit for Weighting and Analysis of Nonequivalent Groups. R package version 1.6. Accessed August 10, 2020. https://CRAN.R‐project.org/package=twang
- 17. Arachchillage DRJ, Laffan M. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(5):1233‐1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid‐19. N Engl J Med. 2020;382(17):e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Connors JM, Levy JH. COVID‐19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033‐2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID‐19 infection: A report of five cases. Transl Res J Lab Clin Med. 2020;220:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lederle FA, Zylla D, MacDonald R, Wilt TJ. Venous thromboembolism prophylaxis in hospitalized medical patients and those with stroke: a background review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2011;155(9):602‐615. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1A
Fig S1B
Supplementary Material
Data Availability Statement
Raw data were generated within the Beaumont Health System. Derived data supporting the findings of this study are available from the corresponding author, GBN, on request.


