Abstract
Little is known on the association between clinical factors and coronavirus disease 2019 (COVID‐19) more than 15 days after diagnosis. We conducted a multicentric prospective cohort of COVID‐19 hospitalized patients to describe clinical, biological, and virological characteristics at hospital admission and over time, according to mortality up to Day 60 after admission. For the analysis of risk factors of survival, analyses assessing associations between mortality and demographic characteristics or comorbidities were performed using a Cox regression model. Between January 24 and March 15, 2020, 246 patients with reverse‐transcriptase polymerase chain reactions virologically confirmed COVID‐19 were enrolled. In multivariate analysis, mortality at Day 60 (n = 42 patients, 17.1% [95% confidence interval, 12.6–22.4]) was associated with older age (adjusted hazard ratio [aHR] for age ≥ 65 years: 5.22 [2.56–10.63, p < .001]), gender (aHR for male: 2.97 [1.47–5.99, p = .002]), chronic pulmonary disease (aHR: 4.84 [2.32–10.07, p < .001]), obesity (aHR: 3.32 [1.70–6.52, p < .001]), and diabetes (aHR: 1.98 [1.01–3.89, p = .048]). The median nasopharyngeal viral load at admission was 6.4 log10 copies/ml and was associated with mortality regardless of clinical risk factors. Viral load decreased with time elapsed since symptoms onset. Our study confirmed that mortality was associated with clinical characteristics at admission. The viral load at admission was significantly lower in patients admitted late after the onset of symptoms in both dead and alive patients. Our results could improve earlier identification of patients with increased risk of mortality and adapted management.
Keywords: coronavirus disease 2019, emerging infection diseases, prospective cohort, SARS‐CoV‐2, viral load
Highlights
Older age, male gender, chronic pulmonary disease (not asthma), obesity, and diabetes were associated with mortality in COVID‐19 hospitalized patients.
Viral load at admission decreased with the time since symptom onset, with an association with mortality adjusted on clinical risk factors.
1. INTRODUCTION
At the end of 2019, the first cases of an epidemic of viral pneumonia of unknown etiology were reported in the city of Wuhan, China. Efficient person‐to‐person transmission was rapidly demonstrated. At the beginning of 2020, the Chinese health authorities and the World Health Organization (WHO) announced the discovery of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), a novel coronavirus, and its associated disease, coronavirus disease‐2019 (COVID‐19). 1 On January 30th, WHO declared the disease as a Public Health Emergency of International Concern and, on March 11th, as a pandemic.
In France, the first confirmed case—a Chinese tourist— was reported on January 14th, 2020. 2 As an attempt to contain the virus spread, all patients with confirmed COVID‐19 were admitted to hospital, regardless of their medical condition. By March 15th, 6378 confirmed cases were reported in France, with a doubling time of 48 h. As of March 15th, admission to hospital was based on the medical condition and patients with mild symptoms were no longer hospitalized.
The national prospective French COVID cohort was initiated end‐January and enrolled hospitalized patients in various centers across mainland France and overseas territories. The cohort collects systematic clinical, biological, radiological, and virological data during hospitalization, as well as after discharge. Here, we describe the clinical, biological, and virological characteristics at admission and during the first 60 days after admission in all patients enrolled up to March 15th, 2020.
2. METHODS
2.1. Study oversight
Hospitalized patients with a virologically confirmed COVID‐19 were enrolledin the French COVID cohort (registered in clinicaltrials.gov NCT04262921). The study was conducted with the understanding and the consent of each participant or its surrogate. The study was sponsored by Inserm and Ethics approval was given by the French Ethics Committee CPP‐Ile‐de‐France VI (ID RCB: 2020‐A00256‐33). All patients enrolled until March 15th, 2020 were analyzed.
2.2. Laboratory testing for confirmation
Nasopharyngeal swabs were performed on the day of admission for SARS‐CoV‐2 testing according to WHO or French National Health Agency guidelines. 3 Viral loads were quantified by real‐time semiquantitative reverse‐transcriptase polymerase chain reactions (RT‐PCR), using either the Charité WHO protocol (testing E gene and RdRp), or the Pasteur institute assay (testing E gene and two other RdRp targets, IP2 and IP4). 4 , 5 When several assays were available, the IP4 Ct value was used as a reference. Results provided in cycle threshold (Ct) were transformed to log10 RNA copies/ml, using the relationship assessed by Pasteur Institute for both genes. 5
2.3. Data collection
Data were collected through the French‐modified version, the open‐access Case Report Form of the International Severe Acute Respiratory and Emerging Infection Consortium. 6 Baseline laboratory and radiological findings were recorded within the first 3 days of hospitalization. Clinical, biological, radiological, virological data, and outcomes were assessed during hospitalization. Patients were followed up 2/4 weeks after discharge and at 3 months postadmission.
2.4. Study definitions and outcomes
The primary objectives of this study were to describe the baseline clinical, biological, and radiological characteristics of virological‐confirmed patients with COVID‐19 at admission, to describe their evolution in the 60 days after admission, and to compare these variables between dead and alive patients.
2.5. Statistical analysis
Continuous variables were described as medians and interquartile ranges [IQR]. Categorical variables were described as numbers of patients (percentages). The number of available data for each variable was given. For the analysis of risk factors of survival, univariate analyses assessing associations between mortality and demographic characteristics or comorbidities were performed using a Cox regression model. Patients discharged alive were censored at the first date between the date of their last follow‐up (date of discharge or date of visit 2–4 weeks after discharge) and the date of administrative censoring (Day 60). Variables with less than 10% of missing values and with p < .20 in univariate analysis were tested in multivariate models. The variable selection was performed using a stepwise backward multivariate Cox regression with a p‐value cutoff point of .05. Two‐way interactions between risk factors kept in the multivariate analysis were tested. The association between viral load (available on a subset of the population) and survival was assessed in a Cox regression multivariate model adjusted on the risk factors identified in the previous multivariate model. All tests were two‐sided and analyses were performed with SAS software (SAS V9.4).
2.6. Viral load kinetic
We assessed the association between viral load at admission and the time since symptoms onset in both dead and alive patients (Spearman's correlation). To account for potential heterogeneity in the viral load detection, patients with Ct more than 37 were considered undetectable.
3. RESULTS
3.1. Demographic and clinical characteristics at admission
Among 254 eligible patients, we enrolled 246 virologically confirmed COVID‐19 patients admitted to 25 hospitals, participating in the cohort throughout France (four were outpatients not hospitalized and four others had negative SARS‐CoV‐2 RT‐PCR test results). A total of 175 (71%) patients were initially admitted to medical wards, including 27 who were subsequently transferred to the intensive care unit (ICU) or died within 60 days since admission, and 71 (29%) patients were initially admitted to ICU. The demographic and clinical characteristics of the patients at admission are shown in Table 1. The median age was 62 years (IQR, 50–73), with 106 (43%) aged 65 years or older, and the proportion of males was 57%. The proportion of patients having at least one comorbidity was 38%, in particular hypertension (30%), other chronic cardiovascular diseases (20%), obesity (17%), and diabetes (16%). The proportion of current smokers was 8%. The proportion of healthcare workers or working in a microbiology laboratory was 9%. The median number of days between the onset of clinical symptoms until hospital admission was 7 days. 5 , 6 , 7 , 8 , 9 , 10 , 11 The four most common symptoms at admission were a history of fever, faintness, shortness of breath, and myalgia. Chest x‐ray at admission was abnormal in 75% of the patients, with bilateral abnormalities in 82% of them. Laboratory findings at the admission are summarized in Table 1.
Table 1.
Demographic, clinical, radiologic, and biologic characteristics at admission of the first 246 patients enrolled in the French COVID cohort
| No. | All N = 246 | Ward at admission (n = 175) | ICU at admission (n = 71) | |
|---|---|---|---|---|
| Demographic and clinical characteristics | ||||
| Age: median (IQR), year | 246 | 62 [50–73] | 60 [49–72] | 68 [53–76] |
| Age distribution: no./total no. (%) | 246 | |||
| 15‐49 year | 59/246 (24) | 48/175 (27) | 11/71 (15) | |
| 50–64 year | 81/246 (33) | 59/175 (34) | 22/71 (31) | |
| 65–80 year | 73/246 (30) | 46/175 (26) | 27/71 (38) | |
| ≥80 year | 33/246 (13) | 22/175 (13) | 11/71 (15) | |
| Male sex: no./total no. (%) | 246 | 139/246 (57) | 88/175 (50) | 51/71 (72) |
| Ethnic group: no./total no. (%) | 162 | |||
| Arab | 14/162 (9) | 6/112 (5) | 8/50 (16) | |
| Black | 15/162 (9) | 8/112 (7) | 7/50 (14) | |
| Asian | 8/162 (5) | 4/112 (4) | 4/50 (8) | |
| White | 118/162 (73) | 88/112 (79) | 30/50 (60) | |
| Healthcare worker: no./total no. (%) | 246 | 22/246 (9) | 19/175 (11) | 3/71 (4) |
| Smoking history: no./total no. (%) | 169 | |||
| Never smoked | 113/169 (67) | 81/121 (67) | 32/48 (67) | |
| Former smoker | 43/169 (25) | 30/121 (25) | 13/48 (27) | |
| Current smoker | 13/169 (8) | 10/121 (8) | 3/48 (6) | |
| Comorbidities: no./total no. (%) | ||||
| At least one comorbidity | 239 | 148/239 (38) | 93/169 (45) | 55/70 (21) |
| Chronic cardiac disease (not hypertension) | 245 | 48/245 (20) | 32/174 (18) | 16/71 (23) |
| Hypertension | 243 | 73/243 (30) | 42/173 (24) | 31/70 (44) |
| Chronic pulmonary disease (not asthma) | 246 | 21/246 (9) | 12/175 (7) | 9/71 (13) |
| Asthma | 246 | 23/246 (9) | 14/175 (8) | 7/71 (10) |
| Chronic kidney disease | 246 | 16/246 (7) | 7/175 (4) | 9/71 (13) |
| Chronic liver disease | 245 | 9/245 (4) | 8/175 (5) | 1/70 (1) |
| Chronic neurological disorder | 246 | 18/246 (7) | 10/175 (6) | 8/71 (11) |
| Malignant neoplasm | 245 | 14/245 (6) | 11/175 (6) | 3/70 (4) |
| Chronic hematologic disease | 246 | 8/246 (3) | 6/175 (3) | 4/71 (6) |
| AIDS/HIV | 74 | 0/74 (0) | 0/50 (0) | 0/24 (0) |
| Obesity | 241 | 44/241 (18) | 17/171 (10) | 27/70 (39) |
| Diabetes | 244 | 39/244 (16) | 26/175 (15) | 13/69 (19) |
| Rheumatologic disorder | 244 | 20/244 (8) | 11/173 (6) | 9/71 (13) |
| Dementia | 246 | 5/246 (2) | 4/175 (2) | 1/71 (1) |
| Malnutrition | 244 | 1/244 (0) | 1/173 (1) | 0/71 (0) |
| Splenectomy | 245 | 0/245 (0) | 0/175 (0) | 0/70 (0) |
| Sickle cell disease | 178 | 0/178 (0) | 0/131 (0) | 0/47 (0) |
| Solid organ transplant | 242 | 3/242 (1) | 2/174 (1) | 1/68 (1) |
| Inflammatory bowel disease | 240 | 4/240 (1) | 4/172 (2) | 0/0 (0) |
| Immunosuppressive therapy: no./total no. (%) | ||||
| Corticoid | 245 | 8/245 (3) | 4/175 (2) | 4/70 (6) |
| Number of days with symptoms before admission: median (IQR) | 243 | 6 [3–8] | 5 [3–8] | 7 [4.5–9] |
| Clinical characteristics at admission: no./total no. (%) | ||||
| Cough | ||||
| With sputum production | 219 | 40/219 (18) | 29/160 (18) | 11/59 (19) |
| With hemoptysis | 221 | 2/221 (1) | 2/161 (1) | 0/60 (0) |
| Sore throat | 218 | 31/218 (14) | 30/166 (18) | 1/52 (2) |
| Runny nose | 226 | 46/226 (20) | 44/171 (26) | 2/55 (4) |
| Ear pain | 190 | 3/190 (2) | 2/153 (1) | 1/37 (3) |
| Wheezing | 229 | 15/229 (7) | 6/171 (4) | 9/58 (16) |
| Chest pain | 231 | 22/231 (10) | 18/172 (10) | 4/59 (7) |
| Myalgia | 232 | 96/232 (41) | 78/171 (46) | 18/61 (30) |
| Arthralgia | 225 | 24/225 (11) | 19/168 (11) | 5/57 (9) |
| Faintness | 227 | 124/227 (55) | 96/167 (57) | 28/60 (47) |
| Shortness of breath | 241 | 108/241 (45) | 51/173 (29) | 57/68 (84) |
| Headache | 233 | 71/233 (30) | 68/171 (40) | 3/62 (5) |
| Altered consciousness/confusion | 238 | 11/238 (5) | 6/175 (3) | 5/63 (8) |
| Seizures | 234 | 1/234 (0) | 1/170 (1) | 0/64 (0) |
| Abdominal pain | 235 | 14/235 (6) | 8/172 (5) | 6/63 (10) |
| Vomiting/nausea | 238 | 26/238 (11) | 19/174 (11) | 7/64 (11) |
| Diarrhea | 239 | 45/239 (19) | 34/173 (20) | 11/66 (17) |
| Conjunctivitis | 237 | 4/237 (2) | 4/173 (2) | 0/64 (0) |
| Skin rash | 239 | 4/239 (2) | 2/175 (1) | 2/64 (3) |
| Skin ulcers | 201 | 0/201 (0) | 0/155 (0) | 0/46 (0) |
| Lymphadenopathy | 235 | 3/235 (1) | 2/172 (1) | 1/63 (2) |
| Anosmia | 109 | 5/109 (5) | 4/76 (5) | 1/33 (3) |
| Ageusia | 110 | 4/110 (4) | 3/77 (4) | 1/33 (3) |
| Hemorrhage | 238 | 1/238 (0) | 1/174 (1) | 0/64 (0) |
| Fever: no./total no. (%) | 235 | 84/235 (36) | 46/170 (27) | 38/65 (58) |
| Heart rate: median (IQR), beats per min | 221 | 85 [77–97] | 84 [75.25–98.75] | 87 [80–95.5] |
| Respiratory rate: median (IQR), breaths per min | 108 | 20 [16–26] | 19 [16–20] | 25 [22–30] |
| Systolic blood pressure: median (IQR), mmHg | 224 | 130 [118–140] | 130 [120–140] | 128 [113–138] |
| Diastolic blood pressure: median (IQR), mmHg | 224 | 77 [65–85] | 78 [68–86] | 70 [62–81] |
| Severe dehydration: no./total no. (%) | 201 | 7/201 (3) | 5/145 (3) | 2/56 (4) |
| Oxygen saturation on room air: median (IQR), % | 152 | 96 [94–98] | 96 [95–98] | 90 [81.5–93.75] |
| Oxygen therapy: no./total no. (%) | 214 | 61/214 (29) | 14/148 (9) | 47/66 (71) |
| Oxygen flow: median (IQR), L/min | 15 | 2 [0–8] | 0 [0–1.125] | 8 [4–15] |
| Coinfections: no./total no. (%) | ||||
| Influenza | 189 | 3/189 (2) | 2/127 (2) | 1/62 (2) |
| Adenovirus | 212 | 0/162 (0) | 0/103 (0) | 0/59 (0) |
| Bacteria | 168 | 10/168 (6) | 4/106 (4) | 6/62 (10) |
| Viral load (log10 copies/ml) | 184 | 6.6 [4.2–8.6] | 6.7 [4.4–8.6] | 6.2 [4.1–8.4] |
| Radiologic findings in the first 3 days following first positive SARS‐Cov‐2 sample | ||||
| Abnormalities on chest radiograph: no./total no. (%) | 239 | 103/131 (78.6) | 44/71 (62) | 59/60 (98.3) |
| Alveolar opacity | 238 | 49/95 (51.6) | 20/44 (45.5) | 29/51 (56.9) |
| Interstitial infiltration | 243 | 90/100 (90) | 40/44 (90.9) | 50/56 (89.3) |
| Pleural effusion | 239 | 3/96 (3.1) | 2/43 (4.7) | 1/53 (1.9) |
| Pneumothorax | 238 | 2/95 (2.1) | 1/43 (2.3) | 1/52 (1.9) |
| Bilateral | 241 | 83/98 (84.7) | 32/44 (72.7) | 51/54 (94.4) |
| Laboratory findings in the first 3 days following first positive SARS‐Cov‐2 sample | ||||
| Hemoglobin: median (IQR), g/dL | 88 | 13.4 [12.5–14.5] | 13.9 [13–14.5] | 12.5 [11.6–13.5] |
| WBC count: median (IQR), ×109/L | 92 | 5.6 [4.3–7.5] | 5.4 [4.1–6.7] | 6.4 [5–8.9] |
| Lymphocyte count, median (IQR): ×109/L | 117 | 1.1 [0.8–1.5] | 1.2 [0.9–1.6] | 0.8 [0.5–1.1] |
| Platelet count: median (IQR): ×109/L | 88 | 187.5 [152.2–227.8] | 190 [156–225.5] | 186.5 [150.8–251.5] |
| Creatininemia: median (IQR): µmol/L | 85 | 6541.6 [5304–8044.4] | 6470.9 [5321.7–8044.4] | 6718.4 [4817.8–8398] |
| ALT/SGPT: median (IQR), U/L | 107 | 32 [23.5–53.5] | 29 [22–42.8] | 40 [25–70] |
| AST/SGOT: median (IQR), U/L | 115 | 36 [26.5–53.5] | 32 [25–42.2] | 49 [35–118.5] |
| Total bilirubin: median (IQR), µmol/L | 135 | 10 [6–12] | 7 [6–11.2] | 10 [6.4–12] |
| Procalcitonine, median (IQR), ng/ml | 174 | 0.1 [0.1–0.7] | 0.1 [0.1–0.1] | 0.5 [0.2–1.4] |
| C‐reactive protein (CRP), median (IQR), mg/L | 103 | 52.7 [12.4–132] | 25 [8.6–72.5] | 132 [71.8–177] |
| Blood urea nitrogen (urea): median (IQR), mmol/L | 238 | 10.3 [8–10.6] | 10.1 [8–10.2] | 10.6 [8.3–10.6] |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; COVID‐19, coronavirus disease‐2019; ICU, intensive care unit; IQR, interquartile range; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
3.2. Follow‐up and outcome at Day 60 postadmission
Figure 1 shows the evolution of all patients during the first 60 days after admission. At Day 60, 42 patients had died (17.1% [95% confidence interval [CI], 12.6–22.4]), 192 had been discharged alive (78.0% [72.3–83.1]), eight were still hospitalized in medical wards (3.3% [1.4–6.3]), and four were still hospitalized in ICU (1.6% [0.4–4.1]). The survival curve is shown in Figure S2. Of the 175 patients initially admitted to medical wards, 27 patients worsened and were subsequently transferred to ICU or died (15.4% [10.4–21.6]). Overall, 12 patients initially admitted to medical wards died (6.9% [3.6%–11.7%]) and 30 patients initially admitted to ICU died (42.3% [30.6%–54.6%]; Figure S1). Of note, no patient initially admitted to medical wards was transferred to ICU after Day 14; however, the mortality rate was different between Day 14 (25/246, 10.2% [6.7–14.6]) and Day 60 (42/246, 17.1% [12.6–22.4]).
Figure 1.
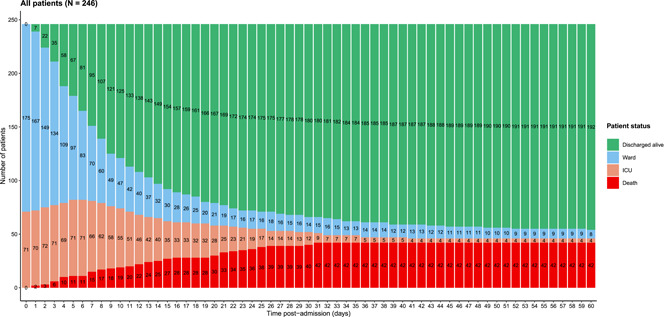
Follow‐up and outcome until Day 60 postadmission. Figure 1 shows the evolution of all patients during the first 60 days after admission. At Day 60, 42 patients had died (17.1% [95% CI: 12.6–22.4]), 192 had been discharged alive (78.0% [72.3–83.1]), eight were still hospitalized in medical wards (3.3% [1.4–6.3], and four were still hospitalized in ICU (1.6% [0.4–4.1]). CI, confidence interval; ICU, intensive care unit
During hospitalization, patients were treated at the doctor's discretion as follows: 9/241 (4%) with remdesivir (7/200 in alive patients at D60 and 2/41 in dead patients at D60), 5/194 (3%) with hydroxychloroquine (3/156 in alive patients at D60 and 2/38 in dead patients at D60), and 68/241 (27%) with lopinavir/ritonavir (50/200 in alive patients at D60 and 18/41 in dead patients at D60). Figure 2 shows the evolution of selected clinical and biological parameters. Patients who subsequently died had higher heart and respiratory rates at admission, and these values continued to increase during follow‐up as compared with alive patients. Likewise, C‐reactive protein (CRP), blood urea nitrogen, and white blood cells count increased more rapidly during the first week of hospitalization in patients who subsequently died than in those who survived. Hemoglobin decreased rapidly in the first week of follow‐up while platelet count sharply dropped later on in patients who subsequently died compared to those who survived.
Figure 2.
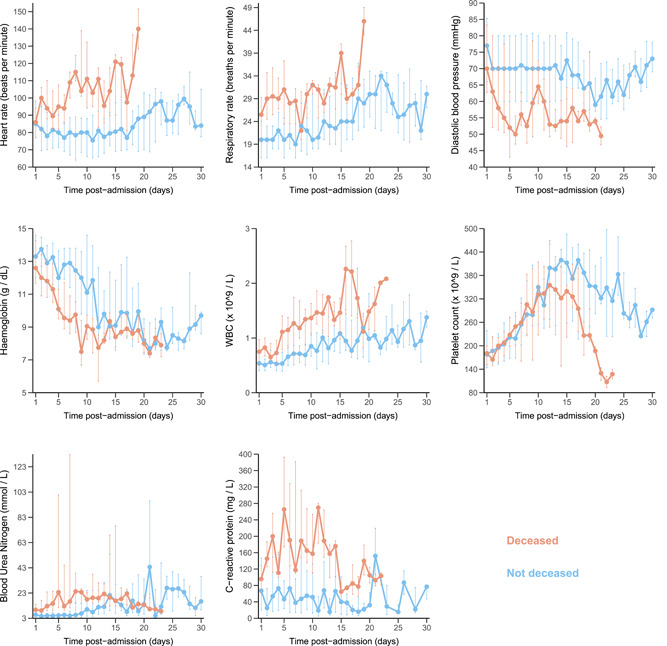
Evolution of clinical measures and biological parameters from admission to Day 30 after the admission of the first 246 patients enrolled in the French COVID cohort. The median values are described. Vertical bars represent 1st and 3rd percentiles at each time‐point. Light blue: not deceased patients; orange: deceased patients. COVID, coronavirus disease
3.3. Comparison of demographic and comorbidities at admission between dead and alive patients
We compared baseline demographic and comorbidities between dead (n = 42) and alive (n = 204) patients. In univariate analysis (Table 2), older age, male gender, chronic cardiac disease, hypertension, chronic pulmonary disease, chronic kidney disease, chronic neurological disorder, malignant neoplasm, obesity, and diabetes were significantly associated with severe disease. In multivariate analysis, older age (aHR for age ≥ 65 years: 5.22 [2.56–10.63, p < .001]), gender (aHR for male: 2.97 [1.47–5.99, p = .002]), chronic pulmonary disease (aHR: 4.84 [2.32–10.07, p < .001]), obesity (aHR: 3.32 [1.70–6.52, p < .001]), and diabetes (aHR: 1.98 [1.01–3.89, p = .048]) were independent risk factors associated with mortality. There was no significant two‐way interaction among those factors. Viral load at admission was associated with mortality adjusted on the previous five clinical risk factors (Table S1).
Table 2.
Demographic and baseline comorbidities associated with time to death until Day 60, in the first 246 patients enrolled in the French COVID cohort
| Alive | Dead | Bivariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|
| Missing n (%) | (n = 204) | (n = 42) | HRa (95%CI) | p | aHRb (95% CI) (n = 239–197 alive/42 dead) | p | |
| Demographic and clinical characteristics | |||||||
| Age: median (IQR), year | 0 (0) | 59 [47.75–70] | 74 [65.5–80.75] | 1.06 (1.04–1.09) | <.001 | ||
| Age distribution: no./total (%) | 0 (0) | ||||||
| 15–49 year | 62/204 (30) | 2/42 (5) | 1 | 1 | |||
| 50–64 year | 73/204 (36) | 7/42 (17) | 2.67 (0.55–12.85) | .22 | |||
| 65–80 year | 53/204 (26) | 21/42 (50) | 9.3 (2.18–39.65) | .003 | 5.22 (2.56–10.63) | <.001 | |
| ≥80 year | 16/204 (8) | 12/42 (29) | 18.08 (4.04–80.84) | <.001 | |||
| Male sex: no./total no. (%) | 0 (0) | 108/204 (53) | 31/42 (74) | 2.32 (1.17–4.62) | .02 | 2.97 (1.47–5.99) | 0.002 |
| Ethnic group: no./total no. (%) | 84 (34) | ||||||
| Arab | 9/128 (7) | 5/34 (15) | 2.18 (0.82–5.77) | .12 | |||
| Black | 12/128 (9) | 3/34 (9) | 0.99 (0.3–3.32) | .99 | |||
| Asian | 5/128 (4) | 3/34 (9) | 1.92 (0.57–6.47) | .29 | |||
| White | 96/128 (75) | 22/34 (65) | 1 | ||||
| Healthcare worker: no./total no. (%) | 0 (0) | 21/204 (10) | 1/42 (2) | 0.21 (0.03–1.53) | .12 | ||
| Smoking history: no./total no. (%) | 77 (31) | ||||||
| Never smoked | 95/140 (68) | 18/29 (62) | 1 | ||||
| Former smoker | 34/140 (24) | 9/29 (31) | 1.44 (0.65–3.22) | .37 | |||
| Current smoker | 11/140 (8) | 2/29 (7) | 1.36 (0.31–5.86) | .68 | |||
| Comorbidities: no./total no. (%) | |||||||
| Chronic cardiac disease (not hypertension) | 1 (0.4) | 31/203 (15) | 17/42 (40) | 3.65 (1.97–6.77) | <.001 | ||
| Hypertension | 3 (1) | 48/201 (24) | 25/42 (60) | 3.77 (2.04–6.99) | <.001 | ||
| Chronic pulmonary disease (not asthma) | 0 (0) | 10/204 (5) | 11/42 (26) | 5.33 (2.67–10.62) | <.001 | 4.84 (2.32–10.07) | <.001 |
| Asthma | 0 (0) | 17/204 (8) | 4/42 (10) | 1.17 (0.42–3.28) | .77 | ||
| Chronic kidney disease | 0 (0) | 8/204 (4) | 8/42 (19) | 4.7 (2.17–10.16) | <.001 | ||
| Chronic liver disease | 1 (0.4) | 7/203 (3) | 2/42 (5) | 1.28 (0.31–5.28) | .74 | ||
| Chronic neurological disorder | 0 (0) | 10/204 (5) | 8/42 (19) | 4.11 (1.9–8.89) | <.001 | ||
| Malignant neoplasm | 1 (0.4) | 9/204 (4) | 5/41 (12) | 3.05 (1.19–7.78) | .02 | ||
| Chronic hematologic disease | 0 (0) | 6/204 (3) | 4/42 (10) | 2.49 (0.89–6.99) | .08 | ||
| Obesity | 5 (2) | 30/199 (15) | 14/42 (33) | 2.34 (1.23–4.44) | .009 | 3.32 (1.70–6.52) | <.001 |
| Diabetes | 2 (0.8) | 26/202 (13) | 13/42 (31) | 2.43 (1.26–4.67) | .008 | 1.98 (1.01–3.89) | .048 |
| Rheumatologic disorder | 2 (0.8) | 15/202 (7) | 5/42 (12) | 1.66 (0.65–4.23) | .29 |
Abbreviation: COVID, coronavirus disease.
HR: hazard ratios of the association of each factor with mortality.
aHR: adjusted hazard ratios of the association with mortality.
3.4. Viral loads
A total of 351 nasopharyngeal viral load data were obtained from 159 patients, with 35 dead patients and 124 alive patients at Day 60 (Figure 3A). The median viral load at admission was 6.4 log10 copies/ml [IQR, 4.2–8.6]. The viral load at admission was significantly lower in patients admitted late after the onset of symptoms in both dead and alive patients (p = .06 and P < 10−4, respectively, Figure 3B).
Figure 3.
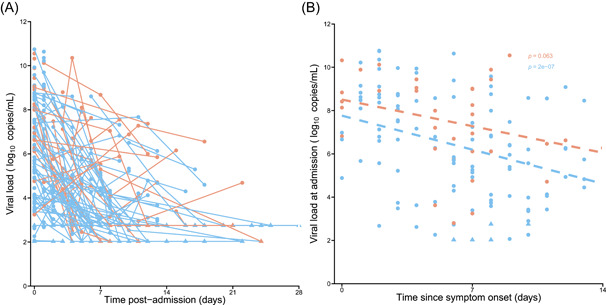
Viral load kinetics in 159 patients out of the first 246 of the French COVID cohort in which at least one viral load was available. (A) Nasopharyngeal viral load. (B) Viral load at admission according to the time since symptom onset. Light blue: not deceased patients; Orange: deceased patients. COVID, coronavirus disease
4. DISCUSSION
In this prospective clinical cohort, we described the clinical, biological, and virological characteristics at admission and during the first 60 days of hospitalization in all patients enrolled up to March 15th, 2020. Other studies comparing characteristics of patients with and without severe diseases have been published recently in larger populations, in particular in the United States and in China. 7 , 8 However, these studies were mostly retrospective or studies in which data were collected retrospectively, and many of these data were restricted to critical or severe patients, with a short‐term follow‐up and no data on viral load kinetics. For example, a retrospective report was published on more than 1500 patients in Lombardia, Italy, but this study only included ICU patients. 9 In the present study, we prospectively enrolled patients with a wide range of disease severity status: patients who did not medically require hospitalization but who were hospitalized to avoid disease transmission in the community, patients who required hospitalization but who were not admitted to ICU, and those admitted to ICU. This allows us to evaluate characteristics associated with mortality and with a preplanned and prospective collection of biological and virological data.
The mortality rate at Day 60 was 17.1% [12.6%–22.4%], which is lower than in larger series, such as those published from New York City area (21%) 8 and Wuhan (28%). 10 Also, in the RECOVERY trial, overall 28‐day mortality was 25.7% in the usual care group. 11 This might be due, in part, to the enrollment of patients with mild COVID‐19 disease who were hospitalized at the very beginning of the epidemic in France. Indeed, again in the RECOVERY trial, 28‐day mortality was 17.8% and 14.0% for the dexamethasone group and the usual care group, respectively, in patients who did not require oxygen at the time of randomization. 11 Interestingly, the mortality rate was 10.2% [6.7–14.6] at Day 14 and rose up to 17.1% [12.6–22.4] at Day 60. Of note, no patient initially admitted to medical wards was transferred to ICU after Day 14 postadmission, suggesting delayed death in those admitted/transferred to ICU. In our study, 30% of patients had hypertension and 17% were obese, as compared with 57% and 42% in papers from the New York City area. 8 These numbers are close to estimates from the French general population, with prevalence rates of 30.6% and 15.7%, respectively. 12 , 13 Accordingly, the prevalence of diabetes (16%) was close to that in the general population, with a prevalence of 16% as compared with 20% in men aged between 75 and 85 years in the French general population. 14 The proportion of current smokers was equal to 8%, which is much lower than in the French general population, in the range 25%–30%. 15 This proportion is nonetheless highly age‐dependent and falls to 20% in people aged between 55 and 64 years, and to 10% in those aged between 65 and 75 years. 15
Age, male gender, chronic pulmonary disease (not asthma), obesity, and diabetes were associated with mortality, in line with previous reports. 8 , 10 Cardiovascular comorbidities, such as chronic cardiac disease, were associated with mortality in the univariate analysis but not in the multivariate analysis, possibly due to confounding factors, such as obesity and diabetes.
We compared the dynamics of clinical and biological variables over time in dead and alive patients. An increase in respiratory rate, in heart rate and/or biological parameters, in particular CRP, white blood count, and in blood urea nitrogen, and a drop in platelet count were early predictive markers of subsequent death and could be useful in the future to guide clinicians. 10
One of the strengths of this cohort was to collect nasopharyngeal viral load measurements at admission and prospectively. A striking observation was that viral load at admission decreased with the time since symptom onset, with an association with mortality adjusted on clinical risk factors.
Our study has certain limitations. The epidemic situation linked to COVID‐19 in France did not allow for an optimal data collection, leading to several missing data. Although patients from various geographical places in France were enrolled, most of the participating centers were University hospitals in which the proportion of most severe patients tends to be larger.
We systematically and prospectively collected data, including clinical, biological, and virological on 246 patients and found several risk factors of mortality. The results of this analysis could improve our knowledge of clinical and biological characteristics associated with disease evolution, thus improving medical care through better triage, earlier identification of patients with increased risk of mortality, and adapted management.
CONFLICT OF INTEREST
Pr. Yazdanpanah reports no conflict of interest.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
This study was supported by the REACTing (REsearch & ACtion emergING infectious diseases) consortium and by a grant of the French Ministry of Health (PHRC no. 20‐0424). The funders had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. The study included a scientific advisory board composed of Dominique COSTAGLIOLA, Astrid VABRET, Hervé RAOUL, and Laurence WEISS.
Contributors: Pr. Yazdanpanah had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Group Information: The members of the French COVID cohort investigator and study group include the following, the French COVID cohort study group (by alphabetical order): Alpha DIALLO, Christelle PAUL, Noémie MERCIER, Soizic LE MESTRE, Ventzislava PETROV‐SANCHEZ (ANRS, Paris, France), Claire ANDREJAK (CHU Amiens, France), Catherine CHIROUZE (CHU Jean Minjoz, Besançon, France), Denis MALVY (CHU Bordeaux, France), Dominique DEPLANQUE, François DUBOS (CHU Lille, France), Patrick ROSSIGNOL (CHU Nancy, France), Manuel ETIENNE (CHU Rouen, France), Bénédicte ROSSIGNOL, Claire LEVY‐MARCHAL, Morgane GILG, Tristan GIGANTE (FCRIN INI‐CRCT, Paris, France), BELUZE Marine (F‐CRIN Partners Platform, Paris, France), Jean‐Sebastien HULOT (Hôpital Européen Georges Pompidou, Paris, France), Anissa CHAIR, Antoine KHALIL, Benoit VISSEAUX, Camille COUFFIGNAL, Carine ROY, Cédric LAOUÉNAN, Charlene DA SILVEIRA, Coralie TARDIVON, Delphine BACHELET, Diane DESCAMPS, France MENTRÉ, François BOMPART, François‐Xavier LESCURE, Gilles PEYTAVIN, Isabelle GORENNE, Isabelle HOFFMANN, Jade GHOSN, Jean Christophe LUCET, Jean‐François TIMSIT, Jimmy MULLAERT, Krishna BHAVSAR, Lila BOUADMA, Lysa TAGHERSET, Marie‐Capucine TELLIER, Marie‐Pierre DEBRAY, Marina ESPOSITO‐FARESE, Marion SCHNEIDER, Minh LE, Nadia ETTALHAOUI, Nassima SI MOHAMMED, Nathalie GAULT, Nathan PEIFFER‐SMADJA, Ouifiya KEFIF, Philippine ELOY, Quentin LE HINGRAT, Sabrina KALI, Samira LARIBI, Sarah TUBIANA, Théo TRIOUX, Xavier DUVAL (Hôpital Bichat, Paris, France), Noémie VANEL (hôpital la timone, Marseille, France), Olivier PICONE, Romain BASMACI (Hôpital Louis Mourier, Colombes, France), François ANGOULVANT (Hôpital Necker, Paris, France), Florentia KAGUELIDOU, Justine PAGES (Hôpital Robert Debré, Paris, France), Aurélie VEISLINGER, Christelle TUAL (Inserm CIC‐1414, Rennes, France), Aurélie PAPADOPOULOS, Hélène ESPEROU, Salma JAAFOURA, Sandrine COUFFIN‐CADIERGUES (Inserm sponsor, Paris, France), Alexandra COELHO, Alexandre HOCTIN, Alphonsine DIOUF, Marina MAMBERT (Inserm UMR 1018, Paris, France), Alexandre GAYMARD, Bruno LINA, Manuel ROSA‐CALATRAVA, Maude BOUSCAMBERT, Olivier TERRIER (Inserm UMR 1111, Lyon, France), Amina MEZIANE, Céline DORIVAL, Dehbia BENKEROU, François TÉOULÉ (Inserm UMR 1136, Paris, France), Guillaume LINGAS, Hervé LE NAGARD, Jérémie GUEDJ, Nadège NEANT (Inserm UMR 1137, Paris, France), Laurent ABEL (Inserm UMR 1163, Paris, France), Coralie KHAN, Mathilde DESVALLÉE (Inserm UMR 1219, Bordeaux, France), Aurélie WIEDEMANN, Yves LEVY (Inserm UMR 955, Créteil, France), Hugo MOUQUET, Sylvie BEHILILL, Sylvie van der WERF, Vincent ENOUF (Pasteur Institute, Paris, France), Caroline SEMAILLE, Eric d'ORTENZIO, Minerva CERVANTES‐GONZALEZ, Oriane PUÉCHAL (REACTing, Paris, France), Marion NORET (RENARCI, Annecy, France); and the French COVID cohort investigators group (for inclusion of participants, follow‐up and local laboratory) (by alphabetical order): Mélanie RORIZ, Patrick RISPAL, Sarah REDL (CHG d'Agen), Laurent LEFEBVRE, Pascal GRANIER, Laurence MAULIN (CH du Pays d'Aix, Aix en Provence), Cédric JOSEPH, Julien MOYET, Sylvie LION‐DAOLIO (CHU Amiens Nord), Rafael MAHIEU, Alexandra DUCANCELLE, Vincent DUBEE (CHU d'Angers), Stéphane SALLABERRY, Aldric MANUEL, Gabriel MACHEDA, Mylène MAILLET, Bruno CHANZY (CH d'Annecy Genevois), Jean‐Charles GAGNARD (Hôpital privé d'Antony), Guillermo GIORDANO, Clara MOUTON PERROT, Vincent PESTRE (CH Henri Duffaut, Avignon), Cécile FICKO, Marie GOMINET, Aurore BOUSQUET (Hôpital d'instruction des armées Begin), Charline VAUCHY, Kévin BOUILLER, Maïder PAGADOY, Solène MARTY‐QUINTERNET, Quentin LEPILLER (CHU Jean Minjoz, Besançon), Cyril LE BRIS, Benoit THILL, Marie‐Laure CASANOVA, Georges LE FALHER, Eric OZIOL (CH de Béziers), Hugues CORDEL, Nathalie DOURNON, Olivier BOUCHAUD, Ségolène BRICHLER (Hôpital Avicenne, Bobigny), Duc NGUYEN, Pantxika BELLECAVE, Camille CICCONE, Marie‐Edith LAFON (CHU Pellegrin, Bordeaux), Ségolène GREFFE (CHU Ambroise Paré, Boulogne Billancourt), Camille BOUISSE, Nicholas SEDILLOT, Damien BOUHOUR (CH Fleyriat, Bourg en Bresse), Camille CHASSIN (CH Pierre Oudot, Bourgoin‐Jallieu), Erwan L'HER, Séverine ANSART, Cécile TROMEUR, Dewi GUELLEC (CHRU de Brest), Antoine MERCKX (CH de Cahors), Felix DJOSSOU (CH Andrée Rosemon, Cayenne), Vincent PEIGNE, Carola PIEROBON, Marie‐Christine CARRET, Florence JEGO, Margaux ISNARD (CHMS Chambéry NH), Johann AUCHABIE, Roxane COURTOIS (CH de Cholet), Olivier LESENS (CHU Gabriel Montpied, Clermont‐Ferrand), Martin MARTINOT (Hôpital Pasteur, Colmar), Olivier PICONE (Hôpital Louis Mourier, Colombes), Marie LACOSTE (CH Alpes Leman Contamine sur Arve), Brigitte ELHARRAR, Valérie GARRAIT, Isabelle DELACROIX, Thomas MAITRE, Jean Baptiste ASSIE (Centre Hospitalier Intercommunal de Créteil), Elsa NYAMANKOLLY (CH de Dax), François Xavier CATHERINE, Mathieu BLOT, Sophie MAHY, Marielle BUISSON, Lionel PIROTH, Florence GUILLOTIN, Alexis DE ROUGEMONT (CHU de Dijon Bourgogne), Valentine CAMPANA, Jérémie PASQUIER, André CABIE (CHU de Martinique, Fort de France), Simon BESSIS (Hôpital Raymond Poincaré, Garches), Olivier EPAULARD, Nicolas TERZI, Jean‐François PAYEN, Laurence BOUILLET, Rebecca HAMIDFAR, Marion Le MARECHAL (CHU de Grenoble), Moïse MACHADO, Audrey BARRELET, Alexandra BEDOSSA (Grand Hôpital de l'Est Francilien site Marne‐la‐Vallée, Jossigny), Gwenhaël COLIN, Romain DECOURS, Thomas GUIMARD (CHD Les Oudairies, La Roche Sur Yon), Cécile GOUJARD, Stéphane JAUREGUIBERRY, Antoine CHERET (Hôpital Universitaire Bicêtre, Le Kremlin‐Bicêtre), Anne Sophie RESSEGUIER (CH Emile Roux, Le Puy en Velay), Julien POISSY, Daniel MATHIEU, Saad NSEIR, Ilka ENGELMANN, Martine REMY (CHRU de Lille, Hôpital Salengro), Fanny VUOTTO, Benoit GACHET, Karine FAURE, Marielle BOYER‐BESSEYRE, Kazali Enagnon (CHRU de Lille, Hôpital Fourrier), Marc LAMBERT, Arnaud SCHERPEREEL, Dominique DEPLANQUE, Stéphanie FRY, Cécile YELNIK (CHRU de Lille, Hôpital Albert Calmette), Laurent BITKER, Mehdi MEZIDI, Hodane YONIS, Nicolas BENECH, Thomas PERPOINT, Anne CONRAD, Vinca ICARD, Martine VALETTE (Hôpital de la Croix Rousse, Lyon), Simon‐Djamel THIBERVILLE (Hôpital Louis Raffalli, Manosque), Stanislas REBAUDET (Hôpital Européen de Marseille), Bertrand DUSSOL (Hôpital Conception, Marseille), Sylvain DIAMANTIS, Catherine CHAKVEATZE, Clara FLATEAU (Groupe Hospitalier Sud Ile De France, hôpital de Melun), Vincent DINOT, Rostane GACI, Nadia OUAMARA (Hôpital de Mercy, CHR Metz‐Thionville, Ars laquenexy), Hajnal‐Gabriela ILLES, Louis GERBAUD MORLAES, Jérôme DIMET (CH de Mont Marsan), Vincent LE MOING, Nathalie PANSU, Clément LE BIHAN, Brigitte MONTES (CHU de Montpellier), Anne Sophie BOUREAU, Clotilde ALLAVENA, Sabelline BOUCHEZ, Romain GUERY, Paul LE TURNIER, Cécile MEAR‐PASSARD, Virginie FERRE (CHU de Nantes), Christophe RAPP (Hôpital Américain de Paris, Neuilly sur Seine), Elisa DEMONCHY, Céline MICHELANGELLI, Karine RISSO (CHU de Nice), Paul LOUBET, Jean‐Phillippe LAVIGNE (CHU Caremeau, Nîmes), Etienne DE MONTMOLLIN, Juliette PATRIER, Paul Henri WICKY, Lucie LE FEVRE, Pierre JACQUET, Raphael BORIE, Antoine DOSSIER, Dominique LUTON, Valentina ISERNIA, Sylvie LE GAC (Hôpital Bichat, Paris), Cécile AZOULAY, Nicolas CARLIER, Liem LUONG, Marie LACHATRE, Odile LAUNAY (Hôpital Cochin, Paris), Younes KERROUMI, Vanina MEYSSONNIER (Groupe Hospitalier Diaconesses Croix Saint‐Simon, Paris), Jean‐Luc DIEHL, Jean‐Sébastien HULOT, Bernard CHOLLEY, Jean‐Benoît ARLET, Olivier SANCHEZ (Hôpital Européen Georges Pompidou, Paris), Victoria MANDA, Laurène AZEMAR, Guylaine CASTOR‐ALEXANDRE (Hôpital Lariboisière, Paris), Karine LACOMBE, Thibault CHIARABINI, Bénédicte LEFEBVRE, Laurence MORAND‐JOUBERT, Djeneba FOFANA, Aurélie SCHNURIGER (Hôpital Saint Antoine, Paris), Nathalie DE CASTRO, Constance DELAUGERRE, Marie‐Laure CHAIX (Hôpital Saint Louis, Paris), Julie CHAS (Hôpital Tenon, Paris), Valérie GABORIEAU, Eve LE COUSTUMIER, Walter PICARD (CH de Pau), Jean‐Benoît ZABBE, Florent PEELMAN, Edouard SOUM (CH de Périgueux), Hugues AUMAÎTRE (CH de Perpignan), Elodie CURLIER, Rachida OUISSA, Isabelle FABRE (CHU de Pointe à Pitre, Guadeloupe), Blandine RAMMAERT (CHU de Poitiers), Nadia SAIDANI (CH Quimper), Firouzé BANI‐SADR, Maxime HENTZIEN, Yohan N'GUYEN, Juliette ROMARU, Kévin DIDIER (CHU Reims), Isabelle ENDERLE, Vincent THIBAULT, Fabrice LAINE, Matthieu LESOUHAITIER, Matthieu REVEST, Pierre TATTEVIN (CHU Rennes), Jean‐Christophe PLANTIER, Manuel ETIENNE, Véronique LEMEE, Eglantine FERRAND DEVOUGE, Kévin ALEXANDRE, Elise ARTAUD‐MACCARI (CHU Rouen), Nathalie ALLOU, Marie LAGRANGE, Julien JABOT (CH Saint Denis et Saint Pierre, La réunion), Benoît ROZE, Delphine BREGEAUD, Younes AIT TAMLIHAT (CH Saintes), Ali HACHEMI (CH Soisson), Hélène SALVATOR, Erwan FOURN, David ZUCMAN (Hôpital Foch, Suresnes), Eric DELAVEUVE, Coline JAUD‐FISCHER, Paul DUNAND (Hôpital Bel Air, Thionville), François BISSUEL (CH Thonon les Bains), Karen DELAVIGNE, Marie PIEL‐JULIAN, Pierre DELOBEL, Benjamine SARTON, Marie RAFIQ, Guillaume MARTIN‐BLONDEL, Laurent GUILLEMINAULT, Marlène MURRIS, Agnès SOMMET (CHU de Toulouse), Eric SENNEVILLE, Olivier ROBINEAU, Agnès MEYBECK (CH Tourcoing), Denis GAROT, Laurent PLANTIER, Valérie GISSOT, Julien MARLET, Karl STEFIC, Catherine GAUDY‐GRAFFIN, Francis BARIN, Adrien LEMAIGNEN (CHU Bretonneau, Tours), Grégory CORVAISIER, Delphine LARIVIERE, Marie LANGELOT‐RICHARD (CH Vannes), Elisabeth BOTELHO‐NEVERS, Amandine GAGNEUX‐BRUNON, Tiffany TROUILLON, Thomas BOURLET, Thomas BOURLET, Sylvie PILLET, Bruno POZZETTO (CHU de St Etienne), Antoine KIMMOUM, Bruno LEVY, Mathieu MATTEI, François GOEHRINGER, Christian RABAUD, Sibylle BEVILACQUA, Benjamin LEFEVRE, Anne GUILLAUMOT, Véronique VENARD, Hélène JEULIN, Evelyne SCHVOERER, Cédric HARTARD (CHU de Nancy, Vandoeuvre les Nancy), Pauline CARAUX PAZ, Laurent RICHIER, Mohamed EL SANHARAWI (CH Villeneuve St Georges). None of these individuals received compensation for their role in the study.
Yazdanpanah Y. Impact on disease mortality of clinical, biological, and virological characteristics at hospital admission and overtime in COVID‐19 patients. J Med Virol. 2021;93:2149–2159. 10.1002/jmv.26601
Membership of the French COVID cohort investigators and study group is provided in Acknowledgments.
Contributor Information
Yazdan Yazdanpanah, Email: yazdan.yazdanpanah@aphp.fr.
French COVID cohort investigators and study group:
Mélanie RORIZ, Patrick RISPAL, Sarah REDL, Laurent LEFEBVRE, Pascal GRANIER, Laurence MAULIN, Cédric JOSEPH, Julien MOYET, Sylvie LION‐DAOLIO, Rafael MAHIEU, Alexandra DUCANCELLE, Vincent DUBEE, Stéphane SALLABERRY, Aldric MANUEL, Gabriel MACHEDA, Mylène MAILLET, Bruno CHANZY, Jean‐Charles GAGNARD, Guillermo GIORDANO, Clara MOUTON PERROT, Vincent PESTRE, Cécile FICKO, Marie GOMINET, Aurore BOUSQUET, Charline VAUCHY, Kévin BOUILLER, Maïder PAGADOY, Solène MARTY‐QUINTERNET, Quentin LEPILLER, Cyril LE BRIS, Benoit THILL, Marie‐Laure CASANOVA, Georges LE FALHER, Eric OZIOL, Hugues CORDEL, Nathalie DOURNON, Olivier BOUCHAUD, Ségolène BRICHLER, Duc NGUYEN, Pantxika BELLECAVE, Camille CICCONE, Marie‐Edith LAFON, Ségolène GREFFE, Camille BOUISSE, Nicholas SEDILLOT, Damien BOUHOUR, Camille CHASSIN, Erwan L'HER, Séverine ANSART, Cécile TROMEUR, Dewi GUELLEC, Antoine MERCKX, Felix DJOSSOU, Vincent PEIGNE, Carola PIEROBON, Marie‐Christine CARRET, Florence JEGO, Margaux ISNARD, Johann AUCHABIE, Roxane COURTOIS, Olivier LESENS, Martin MARTINOT, Olivier PICONE, Marie LACOSTE, Brigitte ELHARRAR, Valérie GARRAIT, Isabelle DELACROIX, Thomas MAITRE, Jean Baptiste ASSIE, Elsa NYAMANKOLLY, François Xavier CATHERINE, Mathieu BLOT, Sophie MAHY, Marielle BUISSON, Lionel PIROTH, Florence GUILLOTIN, Alexis DE ROUGEMONT, Valentine CAMPANA, Jérémie PASQUIER, André CABIE, Simon BESSIS, Olivier EPAULARD, Nicolas TERZI, Jean‐François PAYEN, Laurence BOUILLET, Rebecca HAMIDFAR, Marion Le MARECHAL, Moïse MACHADO, Audrey BARRELET, Alexandra BEDOSSA, Gwenhaël COLIN, Romain DECOURS, Thomas GUIMARD, Cécile GOUJARD, Stéphane JAUREGUIBERRY, Antoine CHERET, Anne Sophie RESSEGUIER, Julien POISSY, Daniel MATHIEU, Saad NSEIR, Ilka ENGELMANN, Martine REMY, Fanny VUOTTO, Benoit GACHET, Karine FAURE, Marielle BOYER‐BESSEYRE, Kazali Enagnon, Marc LAMBERT, Arnaud SCHERPEREEL, Dominique DEPLANQUE, Stéphanie FRY, Cécile YELNIK, Laurent BITKER, Mehdi MEZIDI, Hodane YONIS, Nicolas BENECH, Thomas PERPOINT, Anne CONRAD, Vinca ICARD, Martine VALETTE, Simon‐Djamel THIBERVILLE, Stanislas REBAUDET, Bertrand DUSSOL, Sylvain DIAMANTIS, Catherine CHAKVEATZE, Clara FLATEAU, Vincent DINOT, Rostane GACI, Nadia OUAMARA, Hajnal‐Gabriela ILLES, Louis GERBAUD MORLAES, Jérôme DIMET, Vincent LE MOING, Nathalie PANSU, Clément LE BIHAN, Brigitte MONTES, Anne Sophie BOUREAU, Clotilde ALLAVENA, Sabelline BOUCHEZ, Romain GUERY, Paul LE TURNIER, Cécile MEAR‐PASSARD, Virginie FERRE, Christophe RAPP, Elisa DEMONCHY, Céline MICHELANGELLI, Karine RISSO, Paul LOUBET, Jean‐Phillippe LAVIGNE, Etienne DE MONTMOLLIN, Juliette PATRIER, Paul Henri WICKY, Lucie LE FEVRE, Pierre JACQUET, Raphael BORIE, Antoine DOSSIER, Dominique LUTON, Valentina ISERNIA, Sylvie LE GAC, Cécile AZOULAY, Nicolas CARLIER, Liem LUONG, Marie LACHATRE, Odile LAUNAY, Younes KERROUMI, Vanina MEYSSONNIER, Jean‐Luc DIEHL, Jean‐Sébastien HULOT, Bernard CHOLLEY, Jean‐Benoît ARLET, Olivier SANCHEZ, Victoria MANDA, Laurène AZEMAR, Guylaine CASTOR‐ALEXANDRE, Karine LACOMBE, Thibault CHIARABINI, Bénédicte LEFEBVRE, Laurence MORAND‐JOUBERT, Djeneba FOFANA, Aurélie SCHNURIGER, Nathalie DE CASTRO, Constance DELAUGERRE, Marie‐Laure CHAIX, Julie CHAS, Valérie GABORIEAU, Eve LE COUSTUMIER, Walter PICARD, Jean‐Benoît ZABBE, Florent PEELMAN, Edouard SOUM, Hugues AUMAÎTRE, Elodie CURLIER, Rachida OUISSA, Isabelle FABRE, Blandine RAMMAERT, Nadia SAIDANI, Firouzé BANI‐SADR, Maxime HENTZIEN, Yohan N'GUYEN, Juliette ROMARU, Kévin DIDIER, Isabelle ENDERLE, Vincent THIBAULT, Fabrice LAINE, Matthieu LESOUHAITIER, Matthieu REVEST, Pierre TATTEVIN, Jean‐Christophe PLANTIER, Manuel ETIENNE, Véronique LEMEE, Eglantine FERRAND DEVOUGE, Kévin ALEXANDRE, Elise ARTAUD‐MACCARI, Nathalie ALLOU, Marie LAGRANGE, Julien JABOT, Benoît ROZE, Delphine BREGEAUD, Younes AIT TAMLIHAT, Ali HACHEMI, Hélène SALVATOR, Erwan FOURN, David ZUCMAN, Eric DELAVEUVE, Coline JAUD‐FISCHER, Paul DUNAND, François BISSUEL, Karen DELAVIGNE, Marie PIEL‐JULIAN, Pierre DELOBEL, Benjamine SARTON, Marie RAFIQ, Guillaume MARTIN‐BLONDEL, Laurent GUILLEMINAULT, Marlène MURRIS, Agnès SOMMET, Eric SENNEVILLE, Olivier ROBINEAU, Agnès MEYBECK, Denis GAROT, Laurent PLANTIER, Valérie GISSOT, Julien MARLET, Karl STEFIC, Catherine GAUDY‐GRAFFIN, Francis BARIN, Adrien LEMAIGNEN, Grégory CORVAISIER, Delphine LARIVIERE, Marie LANGELOT‐RICHARD, Elisabeth BOTELHO‐NEVERS, Amandine GAGNEUX‐BRUNON, Tiffany TROUILLON, Thomas BOURLET, Thomas BOURLET, Sylvie PILLET, Bruno POZZETTO, Antoine KIMMOUM, Bruno LEVY, Mathieu MATTEI, François GOEHRINGER, Christian RABAUD, Sibylle BEVILACQUA, Benjamin LEFEVRE, Anne GUILLAUMOT, Véronique VENARD, Hélène JEULIN, Evelyne SCHVOERER, Cédric HARTARD, Pauline CARAUX PAZ, Laurent RICHIER, and Mohamed EL SANHARAWI
REFERENCES
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lescure F‐X, Bouadma L, Nguyen D, et al. Clinical and virological data of the first cases of COVID‐19 in Europe: a case series. Lancet Infect Dis. 2020;20:697–706. 10.1016/S1473-3099(20)30200-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO . Coronavirus disease (COVID‐19) technical guidance: laboratory testing for 2019‐nCoV in humans. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/laboratory-guidance. Accessed April 30, 2020.
- 4. Institut Pasteur, Paris . Protocol: real‐time RT‐PCR assays for the detection of SARS‐CoV‐2. https://www.who.int/docs/default-source/coronaviruse/real-time-rt-pcr-assays-for-the-detection-of-sars-cov-2-institut-pasteur-paris.pdf?sfvrsn=3662fcb6_2. Accessed April 24, 2020.
- 5. Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019‐nCoV) by real‐time RT‐PCR. Euro Surveill. 2020;25. 10.2807/1560-7917.ES.2020.25.3.2000045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dunning JW, Merson L, Rohde GGU, et al. Open source clinical science for emerging infections. Lancet Infect Dis. 2014;14:8–9. 10.1016/S1473-3099(13)70327-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 8. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA. 2020;323:2052. 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS‐CoV‐2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574. 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Horby P, Lim WS, Emberson JR, et al, RECOVERY Collaborative Group . Dexamethasone in hospitalized patients with Covid‐19: preliminary report [published online ahead of print July 17, 2020]. N Engl J Med. 2020. 10.1056/NEJMoa2021436 [DOI] [Google Scholar]
- 12. Matta J, Zins M, Feral‐Pierssens AL, et al. Prévalence du surpoids, de l'obésité et des facteurs de risque cardio‐métaboliques dans la cohorte Constances. Bull Epidémiol Hebd. 2016;(35‐36):640–646. [Google Scholar]
- 13. Perrine AL, Lecoffre C, Blacher J, Olié V. L'hypertension artérielle en France: prévalence, traitement et contrôle en 2015 et évolutions depuis 2006. Bull Epidémiol Hebd. 2018;(10):170–179. [Google Scholar]
- 14. Mandereau‐Bruno L, Fosse‐Edorh S. Prévalence du diabète traité pharmacologiquement (tous types) en France en 2015. Disparités territoriales et socio‐économiques. Bull Epidémiol Hebd. 2017;(27‐28):586–591. [Google Scholar]
- 15. Santé Publique France . Baisse de la prévalence du tabagisme quotidien parmi les adultes: résultats du Baromètre de Santé publique France 2018; 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.


