Summary
Aerosol‐generating procedures such as tracheal intubation and extubation pose a potential risk to healthcare workers because of the possibility of airborne transmission of infection. Detailed characterisation of aerosol quantities, particle size and generating activities has been undertaken in a number of simulations but not in actual clinical practice. The aim of this study was to determine whether the processes of facemask ventilation, tracheal intubation and extubation generate aerosols in clinical practice, and to characterise any aerosols produced. In this observational study, patients scheduled to undergo elective endonasal pituitary surgery without symptoms of COVID‐19 were recruited. Airway management including tracheal intubation and extubation was performed in a standard positive pressure operating room with aerosols detected using laser‐based particle image velocimetry to detect larger particles, and spectrometry with continuous air sampling to detect smaller particles. A total of 482,960 data points were assessed for complete procedures in three patients. Facemask ventilation, tracheal tube insertion and cuff inflation generated small particles 30–300 times above background noise that remained suspended in airflows and spread from the patient’s facial region throughout the confines of the operating theatre. Safe clinical practice of these procedures should reflect these particle profiles. This adds to data that inform decisions regarding the appropriate precautions to take in a real‐world setting.
Keywords: aerosol‐generating procedures, extubation, intubation, occupational exposure
요약
기관내 삽관 및 발관과 같은 에어로졸 생성 시술은 공기매 개 전파의 가능성이 있으므로 의료 종사자에게 잠재적 위험 을 초래할 수 있다. 에어로졸의 양, 입자 크기 및 생성 활동에 대한 자세한 특성 분석이 다수의 모의상황에서 수행되었으나, 실제 임상에서는 수행되지 않았다. 본 연구의 목적은 마스크 환기, 기관내 삽관 및 발관 과정이 임상에서 에어로졸을 생성 하는지 여부를 파악하고 생성된 모든 에어로졸의 특성을 분석 하는 것이었다. 본 관찰 연구에서는 코로나‐19의 증상이 없으 며 경비강 접근법을 이용한 선택적 뇌하수체 수술을 받을 예 정이었던 환자를 모집하였다. 기관내 삽관 및 발관을 포함한 기도 관리는 에어로졸이 검출된 표준 양압 수술실에서 수행되 었다. 레이저 기반 입자영상유속계를 사용하여 크기가 큰 입 자를 검출하였으며, 연속식 공기 샘플링을 이용한 분광분석법 으로 작은 크기의 입자를 검출하였다. 3명의 환자를 대상으로 전체 시술에 대해 총 482,960개의 데이터 포인트를 평가하였 다. 마스크 환기, 기관내 튜브 삽입 및 커프 팽창은 기류에 부 유하는 배경 노이즈를 30‐300배 초과하는 작은 입자를 생성 하였으며, 환자의 안면부로부터 수술실 영역 전체로 확산되었 다. 이러한 시술의 안전한 임상 진료 지침에는 이와 같은 입자 프로파일을 반영해야 한다. 이는 실제 환경에 적합한 예방조 치와 관련된 의사결정 정보를 제공하는 데이터에 추가된다.
Aerosol‐generating procedures are a source of anxiety for healthcare workers because of the risk of transmission of infection by airborne particles [1], yet remain poorly understood [2, 3]. Mitigating the potential risk places strain on limited personal protective equipment resources and can change clinical practice standards of care [4, 5, 6]. Tracheal intubation is considered a high‐risk aerosol‐generating procedure [7], and 1 out of 10 proceduralists may develop symptoms or test positive for COVID‐19 within 14 days of intubating the tracheas of suspected or confirmed COVID‐19 patients [8]. Much of the data informing recommendations about aerosol‐generating procedures have been derived from laboratory simulations and cadaveric studies [9, 10, 11]. These have added useful information but do not replicate real‐world conditions. Live humans have different tissue, temperature, humidity and excretory characteristics compared with cadaveric and manikin models, which affect the aerosol produced [11]. In addition, operating theatres are positive pressure high airflow environments with multiple air exchanges per hour which affects the behaviour of any suspended small particles.
In order to help fill this knowledge gap, a collaboration was established between clinicians, fluid dynamicists and atmospheric scientists. We aimed to determine which stages of tracheal intubation and extubation generated aerosols, and to characterise the count, size, duration and direction of any aerosol produced.
Methods
Ethical approval was obtained from the St. Vincent’s Hospital Human Research Ethics Committee and informed consent was obtained from all participants. Before surgery, all patients were triaged for COVID‐19 symptoms as per the guidelines issued by the Neurosurgical Society of Australasia and the Victorian Department of Health and Human Services. This report details the aerosol particle characteristics during airway manipulation and instrumentation by the anaesthetist during induction and emergence from anaesthesia.
Patients undergoing elective endonasal pituitary surgery who provided informed consent were recruited. Exclusion criteria were patients who were < 18 years, unable to consent, pregnant or displayed symptoms of COVID‐19.
Surgery was performed in an operating theatre of size 6 × 7 × 3 m, volume 126 m3, under 20 Pa of positive pressure relative to the external areas, with four HEPA 530‐mm filters, air velocities of 0.97–1.04 ms, at a temperature of 20°C, and humidity 48.2%. There were 26 room volume air exchanges per hour. Air entered the room via four ceiling air diffusers and was extracted via four wall air intakes with an air exchange flow rate of 65 m3 per minute (online Supporting Information Appendix S1). All theatre entrants wore N95 masks to prevent leakage of staff‐generated aerosol into the field.
Once a patient was positioned on the operating table, and the imaging equipment aligned and arranged appropriately, 3 min of pre‐oxygenation with 100% oxygen and spontaneous ventilation was performed using the anaesthesia circuit. Anaesthesia was induced with propofol and remifentanil, followed neuromuscular blockade with rocuronium. A consultant anaesthetist or a directly supervised specialist registrar performed bag‐mask ventilation once the patient became apnoeic, followed by tracheal intubation and securing of the tracheal tube. At the end of surgery, neuromuscular block was reversed with neostigmine and glycopyrrolate, and the trachea was extubated once the patient was breathing adequately and responding to commands. No barriers or plastic sheets were used to cover the patient’s face during these procedures in order to replicate normal clinical practice.
Two non‐invasive methods that involved no modifications to standard of care were used to detect aerosol: particle image velocimetry, to detect relatively larger particles; and air sampling with spectrometry, to detect relatively smaller particles. In this paper, we define ‘aerosol’ as particles suspended in air, ‘small’ particles as ≤ 5 µm and ‘large’ particles as > 5 µm. Particle image velocimetry is an optical flow diagnostic technique for detecting moving particles in a fluid flow. The technique involves illuminating the target area with a 3‐mm thick light sheet generated by a class 3b 50 mW green laser with a 532‐nm wavelength (Fig. 1). The operating theatre was controlled with all entrants required to wear laser safety goggles and the anaesthetised patients’ eyes protected with laser safety tape. Disturbances in the light sheet were captured with a low‐speed Nikon D810 camera at 60 frames per second running continuously (Nikon Australia, Pty Ltd, Sydney, Australia), and a high‐speed PCO dimax HS4 camera (PCO AG, Kelheim, Germany) at 1000 frames per second for 10‐s bursts. The low speed system focused on a large field‐of‐view of 80 × 20 cm with a digital resolution of 110 µm/pixel, while the high‐speed system captured a smaller field‐of‐view of 15 × 15 cm with 75 µm/pixel.
Figure 1.
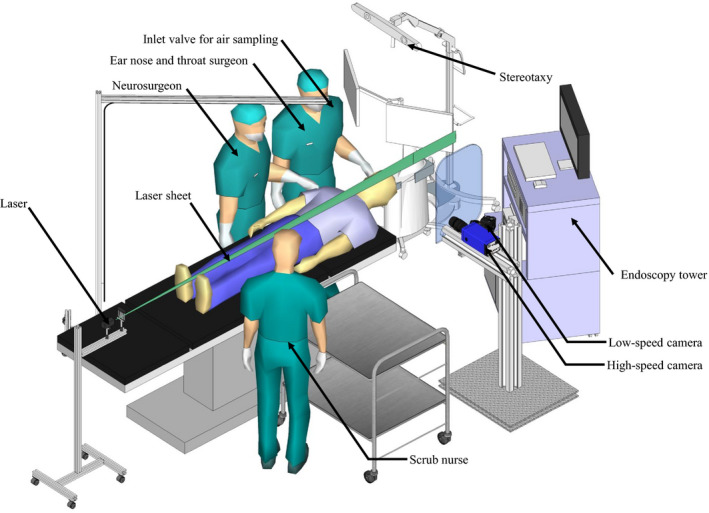
Theatre setup showing the location of aerosol measuring equipment.
For larger particles, an Aerodynamic Particle Sizer (APS model 3320, TSI Incorporated, Shoreview, MN, USA) continuously measured acceleration between two laser beams to determine the size distribution of aerosols sized 0.5–5 µm at 5‐s intervals using time‐of‐flight. For smaller particles, a Mini Wide Range Aerosol Spectrometer (MiniWRAS model 1371, GRIMM Aerosol Technik Ainring GmbH, Ainring, Germany) continuously measured the size distribution of aerosol at 1‐min intervals, using electrical mobility for particles between 0.01 and 0.2 µm and optical light scattering for particles between 0.25 and 35 µm.
Air was sampled at a rate of 6.3 l.min−1 through an inlet positioned 500 mm superior and 500 mm caudal to the patient’s nasal aperture (online Supporting Information Appendix S1), which has negligible effect on overall airflow in which the aerosol travelled given that the air exchange of theatre is 5460 l.min−1. The aerosol sample was transported to the instruments via a single 2500‐mm long, 12‐mm diameter conductive silicon tube. Other than transportation losses through the tube, the aerosol matrix was not altered.
The optical flow diagnostic technique measurement was carried out at three different locations – immediately superior to the patient’s nasal aperture, 300 mm caudal to the nasal aperture and 1100 mm caudal to the nasal aperture (online Supporting Information Appendix S1). Images were pre‐processed to increase the signal‐to‐noise ratio, and the high‐intensity peaks in each image used to calculate aerosol counts. For dispersion medium analysis, an in‐house particle tracking velocimetry algorithm detected particles with a specified intensity threshold in one frame, then searched for the pair of that particle in subsequent frames. Particles travelling at a faster speed than the recording rate of the low‐speed imaging system produced trajectory lines which were used to calculate the landing distance and time.
Before inducing general anaesthesia, background noise was evaluated with 12 healthcare workers, three researchers and the patient in the operating theatre, to establish the background noise created by normal theatre traffic (counts 0.09 cm−3 and 60 cm−3, respectively). Sets of low‐speed images were recorded and analysed for particle count, and the count was set as the noise level. Air sampling was performed in the empty operating theatre from 15.00 the previous day to 07.30 on the morning of surgery. The detection limit for each instrument was calculated as the mean plus three standard deviations during this overnight clean period (counts 0.04 cm−3 and 60 cm−3 for APS and MiniWRAS, respectively).
Independent t‐test samples were used to assess for differences between groups and a value of p < 0.05 was defined as significant. Analyses were performed using open source SciPy software (Scientific computing in Python©).
Results
Three cases were studied from patient arrival in the operating theatre until transfer to their bed from the operating table. A total of 423,000 frames were generated from semi‐continuous low speed camera detection, and 35,000 frames from intermittent high speed camera detection. A total of 23,400 samples were measured by the APS, and 1560 samples by the MiniWRAS.
Mean particle concentrations measured by APS during tracheal intubation were 12 times greater than baseline (p < 0.001). Passive oxygenation (spontaneous ventilation), laryngoscope introduction and throat pack insertion did not produce aerosols above background noise in particle counts from APS (Fig. 2) and MiniWRAS. Facemask ventilation in a patient who had received neuromuscular blockers using oxygen at 6–10 l.min−1, tracheal tube insertion and cuff inflation (to the point of gas leak elimination) produced mostly small particles < 5 µm in concentrations 30–300 times greater than background noise (p < 0.001) (Table 1). These counts were supported by the particle image velocimetry data.
Figure 2.
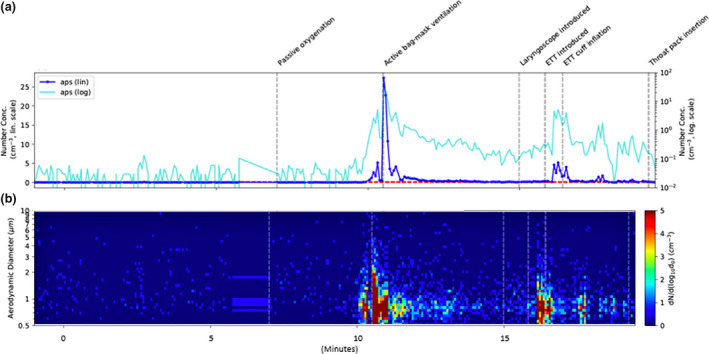
Particle count and diameter during intubation. (a) Time series of total number concentrations from the Aerodynamic Particle Sizer (APS) with linear and log scales shown in dark and light blue, respectively. Dashed lines represent the detection limit (mean + 3 standard deviations) during an empty theatre (green) and during theatre setup (red). (b) Measured aerosol size distributions over time from the APS, with size represented on the y‐axis and colours showing the number concentration in each size bin. The integrated size distributions correspond to total concentrations.
Table 1.
Disturbances above background levels caused by specific procedural steps from combined Aerodynamic Particle Sizer (0.09 cm−3) and Mini Wide Range Aerosol Spectrometer (60 cm−3) data.
| Procedure | Procedural step | Peak increase (multiples of background concentration) | Particle size (µm) |
|---|---|---|---|
| Intubation | Bag and mask ventilation | 200–300 | 0.05–2 |
| Intubation | Tracheal tube insertion | 30–50 | 0.15–2 |
| Intubation | Tracheal tube cuff inflation | 30–50 | 0.15–2 |
| Extubation | Bag and mask ventilation | 10–25 | 0.1–3 |
| Extubation | Throat pack removal | 5 | 0.75–3 |
| Extubation | Patient cough | 15–125 | 0.05–4 |
Mean particle concentrations measured by APS during tracheal extubation were 12 times greater than baseline (p < 0.001). At the end of the procedure, laryngoscope introduction, oropharyngeal suction, tracheal tube cuff deflation and tube removal, were not associated with increases in aerosol generation on air sampling methods (Fig. 3). However, facemask ventilation, throat pack removal and patient coughing, were associated with aerosol production of small particles < 4 µm on both air sampling measurements in concentrations that were significantly greater than baseline measurements (p < 0.001). The mean particle concentrations during intubation and extubation are summarised in Table 2.
Figure 3.
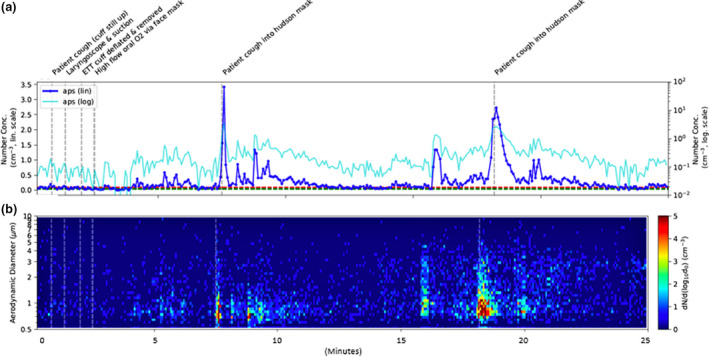
Particle count and diameter during extubation. (a) – (b) are as for Figure 2.
Table 2.
Mean particle concentrations during the epoch of specific procedural steps from Aerodynamic Particle Sizer data. Values are mean (SD).
| Procedure | Procedural step | Particles.cm−3 |
|---|---|---|
| Background level | 0.027 (0.022) | |
| Intubation | 0.324 (1.001) | |
| Bag and mask ventilation | 1.530 (4.577) | |
| Tracheal tube insertion | 2.221 (1.988) | |
| Tracheal tube cuff inflation | 1.125 (1.290) | |
| Extubation | 0.314 (0.693) | |
| Bag and mask ventilation | 0.319 (0.477) | |
| Throat pack removal | 0.105 (0.127) | |
| Patient cough | 1.897 (2.494) |
The dispersion medium of particles was obtained from the particle image velocimetry system, using two‐dimensional data and extrapolated to three dimensions. The procedural step that generated the largest particle count was used to calculate dispersion medium, which in the case of intubation and extubation was positive pressure bag and mask ventilation. Particles generated by facemask ventilation were relatively small and remained suspended in the air at 1100 mm from the patient’s nose. Based on their trajectories, these particles were calculated to travel distances limited by the confines of the theatre walls or air extraction points, with a tendency to travel craniocaudally from the patient’s nose towards air exhausts in the direction of the patient’s feet.
Discussion
Our data demonstrate that tracheal intubation and extubation are aerosol‐generating procedures. Importantly, there are key steps within each procedure that are particularly aerosol‐generating. Specifically, positive pressure bag and mask ventilation with high flow oxygen, and patient coughing into a Hudson mask, produced large signal spikes. A strength of this study is the use of experts across scientific disciplines to obtain, analyse and interpret the data.
Traditional models view aerosols as either small (≤ 5 µm) or large particles (> 5 µm) to understand behaviour [12, 13]. Larger particles travel shorter distances, do not remain airborne for long durations, and settle quickly resulting in surface contamination near the source [14, 15]. Smaller particles, or those that experience a low relative humidity, will shrink in size due to evaporation, resulting in a plume that moves with ambient air currents, remains airborne for longer durations and travels further [14, 16]. Newer aerosol models that add warm and moist micro‐environments within a plume sustain small particles for even longer durations and distances [17]. An example of smaller particles is those generated by facemask ventilation. Figure 4 demonstrates the general trend of smaller particles to travel from the patient’s nose in a caudal direction. While a portion of particle release via the nasal passages would be caudally directed, this would not explain oral particle release. We speculate that the trajectory is primarily dictated by the prevailing air currents in the operating theatre which suggests that, as a general principle, areas close to the procedural aperture and areas towards the closest airflow extraction point are best avoided.
Figure 4.
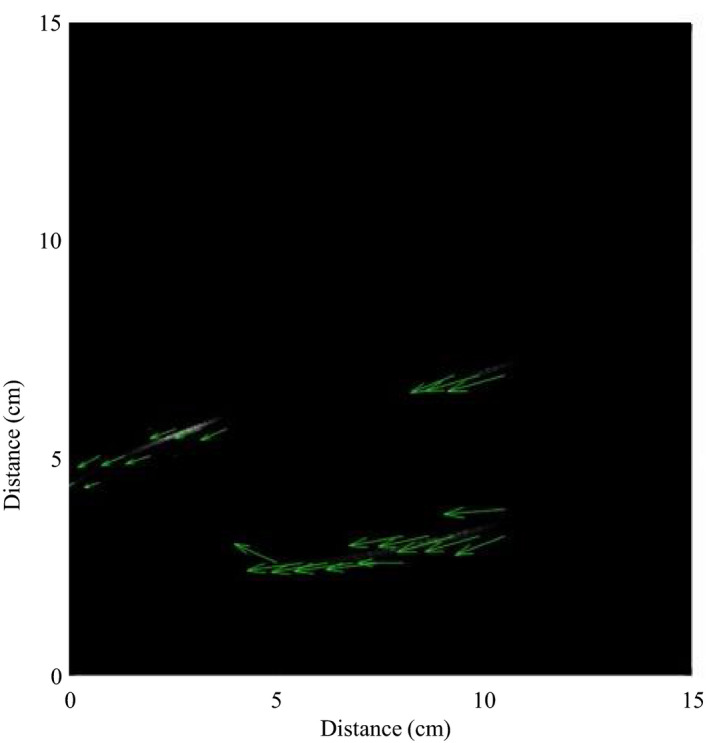
Particle image velocimetry high‐speed image taken during bag and mask ventilation. The image shows particles travelling from right (cranial) to left (caudal).
Calculating the total airborne duration of the suspended small particles is difficult. We estimate it to be at least the duration between volume air exchanges, or 138 s in a theatre with 26 volume air exchanges per hour. The US Centers for Disease Control and Prevention estimates that 99% removal of airborne contaminants in a room with 20 volume air exchanges per hour takes 14 min, although this is based on a 1973 paper citing physics principles rather than experimental data [18]. The truth probably lies somewhere in the middle, but further study is required to characterise aerosol clearance times.
There is a growing number of studies examining aerosol generation. Ten studies have examined healthcare workers retrospectively for exposure to infectious agents with serology and chest imaging to provide indirect evidence of aerosol production [2], two have looked at fluorescent dyes in manikins [19, 20], one looked at the protective effect of intubation shields with simulated aerosols [21], and another looked at viability of aerosolised SARS‐CoV‐2 on different surfaces [22]. These methods do not replicate the air temperature, humidity, viscosity, surface tension of mucosal surfaces, air‐mucus interface, liquid sheet fragmentation, flow induced particle dispersion or secretory/excretory characteristics of live humans [23] – variables affecting the characteristics of the aerosol produced [24]. Furthermore, previous studies do not replicate the clinical environment in terms of number and location of staff, turbulent flows from ducts, air exchange, positive or negative pressure ventilation, temperature or humidity – variables affecting the flow in which the aerosol resides [25].
Understanding background noise is essential in order to differentiate the often‐small signals from procedures above ambient changes in an aerosol population, which in an indoor setting can be substantial. Measures were taken to avoid false‐positive signals. We established background noise levels in an empty theatre by performing overnight recording, and in an occupied theatre with normal levels of healthcare worker traffic. In addition, staff wore N95 masks to prevent leakage of their own aerosols into the field.
Our results also demonstrate that the methods used were sufficiently sensitive to avoid false negatives. Both particle sizes down to 0.01 µm and very low concentrations of particles were detectable. This was shown by the fact that background noise quantifications were consistent with background counts performed as part of operating theatre accreditation which were measured over much longer time periods.
This study has several limitations. The sample size is small. This is tempered by the fact that the measurements were comprehensive, multiple technologies were used simultaneously, the anaesthetic procedures were standardised, and supported by consistent findings across all three cases. This study does not assess whether the aerosols generated were biologically active. However, the methods used detected unaltered particles sized 0.01 µm and above. COVID‐19 virions sizes range from 0.07 to 0.09 µm, and so we would expect that aerosolisation of virus directly, or aerosolisation of larger particles carrying smaller virions would be detected [13, 26]. There are data to suggest that the virus remains biologically active when aerosolised in a similar manner [23]. Thirdly, there are limitations with the aerosol measurement techniques used. Particle image velocimetry requires a clean line of sight for the laser light sheet which, at various times, was blocked by the proceduralist’s hands. Particle image velocimetry as used in this study provides information in two dimensions rather than three. This may overestimate the craniocaudal drift of smaller particles. The air sampling techniques also have limitations as the proximity of the aerosol source to the measurement point makes it possible that only the shoulder of an aerosol plume was being captured.
We believe that these findings inform clinical practice in several ways. Firstly, measures should be taken to limit unnecessary operating theatre traffic during aerosol‐generating steps, particularly facemask ventilation. Secondly, personal protective equipment needs to be appropriate for the size of particle generated. For example, standard surgical masks lack tight seals and would not prevent the entry of small particles travelling in flows through gaps between mask and face. COVID‐19 has changed the way we perform aerosol‐generating procedures. This study provides detailed data on aerosol generation from actual patients in an operating theatre setting. It demonstrates that positive pressure mask ventilation, tracheal intubation and procedures and events following extubation generate small particles in counts several hundred times over baseline, which remain suspended in air and spread throughout theatre. These findings should be used to inform safe anaesthetic practice, and lead to more rational personal protective equipment use.
Supporting information
Appendix S1. Layout of the operating theatre showing the location of ceiling air inlets (wavy lines) and wall air outlets (stripes) with direction of air outflow (arrows).
Acknowledgements
DS has received competitive research funding from the National Health and Medical Research Council, Medical Research Future Fund, Australia and New Zealand College of Anaesthetists Foundation and the Alzheimer’s Association. No other external funding or competing interests declared.
Appendix 1. The Clinical Aerosolisation Study Group
A. Yule, Y. C. Zhao, P. M. McNeill, N. Hutchins.
Contributor Information
R. S. Dhillon, Email: rana.dhillon@svha.org.au.
D. A. Scott, @scottdav44.
the Clinical Aerosolisation Study Group:
References
- 1. Morawska L, Milton D. It is time to address airborne transmission of COVID‐19. Clinical Infectious Diseases 2020. Epub 6 July. 10.1093/cid/ciaa939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tran K, Cimon K, Severn M, Pessoa‐Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One 2012; 7: e35797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheung JCH, Ho LT, Cheng JV, Cham EYK, Lam KN. Staff safety during emergency airway management for COVID‐19 in Hong Kong. Lancet Respiratory Medicine 2020; 8: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cook TM. Personal protective equipment during the coronavirus disease (COVID) 2019 pandemic – a narrative review. Anaesthesia 2020; 75: 920–7. [DOI] [PubMed] [Google Scholar]
- 5. Pandit JJ. Demand‐capacity modelling and COVID‐19 disease: identifying themes for future NHS planning. Anaesthesia 2020. 10.1111/anae.15144. Epub 21 May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019‐nCoV) patients. Canadian Journal of Anesthesia 2020; 67: 568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weissman DN, de Perio MA, Radonovich LJ Jr. COVID‐19 and risks posed to personnel during endotracheal intubation. Journal of the American Medical Association 2020; 323: 2027–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. El‐Boghdadly K, Wong DJN, Owen R, et al. Risks to healthcare workers following tracheal intubation of patients with COVID‐19: a prospective international multicentre cohort study. Anaesthesia 2020. Epub 9 July. 10.1111/anae.15170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sharma D, Rubel KE, Ye MJ, et al. Cadaveric simulation of endoscopic endonasal procedures: analysis of droplet splatter patterns during the COVID‐19 pandemic. Otolaryngology–Head and Neck Surgery 2020; 163: 145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ott M, Milazzo A, Liebau S, et al. Exploration of strategies to reduce aerosol‐spread during chest compressions: a simulation and cadaver model. Resuscitation 2020; 152: 192–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feldman O, Meir M, Shavit D, Idelman R, Shavit I. Exposure to a surrogate measure of contamination from simulated patients by emergency department personnel wearing personal protective equipment. Journal of the American Medical Association 2020; 323: 2091–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wells WF. On air‐borne infection: study II. Droplets and droplet nuclei. American Journal of Epidemiology 1934; 20: 611–8. [Google Scholar]
- 13. Gralton J, Tovey E, McLaws ML, Rawlinson WD. The role of particle size in aerosolised pathogen transmission: a review. Journal of Infection 2011; 62: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mittal R, Ni R, Seo JH. The flow physics of COVID‐19. Journal of Fluid Mechanics 2020; 894: 1–14. [Google Scholar]
- 15. Han ZY, Weng WG, Huang QY. Characterizations of particle size distribution of the droplets exhaled by sneeze. Journal of the Royal Society Interface 2013; 10: 20130560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xie X, Li Y, Chwang ATY, Ho PL, Seto WH. How far droplets can move in indoor environments – revisiting the Wells evaporation–falling curve. Indoor Air 2007; 17: 211–25. [DOI] [PubMed] [Google Scholar]
- 17. Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID‐19. Journal of the American Medical Association 2020; 323: 1837–8. [DOI] [PubMed] [Google Scholar]
- 18. Cook TM, Harrop‐Griffiths W. Aerosol clearance times to better communicate safety after aerosol‐generating procedures. Anaesthesia 2020; 75: 1122–3. [DOI] [PubMed] [Google Scholar]
- 19. Canelli R, Connor CW, Gonzalez M, Nozari A, Ortega R. Barrier enclosure during endotracheal intubation. New England Journal of Medicine 2020; 382: 1957–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Endersby RVW, Spencer AO, Ho ECY, Goldstein DH, Schubert E. Clear plastic drapes for aerosol‐generating medical procedures in COVID‐19 patients: questions still remain. Canadian Journal of Anesthesia 2020. Epub 11 May. 10.1007/s12630-020-01705-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Simpson J, Wong D, Verco L, Carter R, Dzidowski M, Chan P. Measurement of airborne particle exposure during simulated tracheal intubation using various proposed aerosol containment devices during the COVID‐19 pandemic. Anaesthesia 2020. Epub 19 June. 10.1111/anae.15188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Doremalen N, Bushmaker T, Morris DH. Aerosol and surface stability of SARS‐CoV‐2 as compared with SARS‐CoV‐1. New England Journal of Medicine 2020; 382: 1564–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wilson NM, Norton A, Young FP, Collins DW. Airborne transmission of severe acute respiratory syndrome coronavirus‐2 to healthcare workers: a narrative review. Anaesthesia 2020; 75: 1086–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cole EC, Cook CE. Characterization of infectious aerosols in health care facilities: an aid to effective engineering controls and preventive strategies. American Journal of Infection Control 1998; 26: 453–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tang JW, Li Y, Eames I, Chan PKS, Ridgway GL. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. Journal of Hospital Infection 2006; 64: 100–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim J‐M, Chung Y‐S, Jo HJ, et al. Identification of coronavirus isolated from a patient in Korea with COVID‐19 Osong Public Health and Research Perspectives. Osong Public Health and Research Perspectives 2020; 11: 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Layout of the operating theatre showing the location of ceiling air inlets (wavy lines) and wall air outlets (stripes) with direction of air outflow (arrows).


