Abstract
The national importance of telemedicine for safe and effective patient care has been highlighted by the current COVID‐19 pandemic. Prior to the 2020 pandemic the Division of Genetics and Metabolism piloted a telemedicine program focused on initial and follow‐up visits in the patients' home. The goals were to increase access to care, decrease missed work, improve scheduling, and avoid the transport and exposure of medically fragile patients. Visits were conducted by physician medical geneticists, genetic counselors, and biochemical dietitians, together and separately. This allowed the program to develop detailed standard operating procedures. At the onset of the COVID‐19 pandemic, this pilot‐program was deployed by the full team of 22 providers in one business day. Two physicians remained on‐site for patients requiring in‐person evaluations. This model optimized patient safety and workforce preservation while providing full access to patients during a pandemic. We provide initial data on visit numbers, types of diagnoses, and no‐show rates. Experience in this implementation before and during the pandemic has confirmed the effectiveness and value of telemedicine for a highly complex medical population. This program is a model that can and will be continued well‐beyond the current crisis.
Keywords: COVID‐19, genetics, pediatrics, telemedicine
1. INTRODUCTION
“The best way to predict the future is to create it yourself.” Peter Diamandis
Prior to the COVID‐19 pandemic, the Rare Disease Institute (RDI) at Children's National Hospital identified a novel need in the rare disease community. New treatments for rare disease are emerging rapidly (Pogue et al., 2018), including enzyme replacement, gene, RNA, and new small molecule therapies, but major delays remain in the time from presentation of clinical symptoms to diagnosis with caregiver access being a primary driver. Barriers to accessing genetics care include distance to hospitals and clinics, small size of the genetics workforce, and insurance coverage gaps (Penon‐Portmann, Chang, Cheng, & Shieh, 2020). Telemedicine focused on telegenetics offered an innovative solution that may improve access and has been tried with success in genetic counseling (Zierhut, MacFarlane, Ahmed, & Davies, 2018), cancer counseling (Solomons, Lamb, Lucas, McDonald, & Miesfeldt, 2018), presymptomatic testing of patients at risk for genetic conditions (Gattas, MacMillan, Meinecke, Loane, & Wootton, 2001) and patient evaluation of genetic conditions at the regional (Otten, Birnie, Lucassen, Ranchor, & Van Langen, 2016), national (Vrecar, Hristovski, & Peterlin, 2017), and international levels (Hilgart, Hayward, Coles, & Iredale, 2012; Otten, Birnie, Ranchor, & van Langen, 2017). In pediatrics telemedicine visits are associated with improvements in efficiency and equal or greater patient satisfaction than in‐person visits (Waller, Taylor, & Portnoy, 2019). This work expands on previous care models and provides the framework of rapidly transitioning a pilot telemedicine program to telemedicine‐first model for a complete genetics and metabolism division.
In this article, we describe the program overview and implementation, delivery of clinical services, rapid training and education, and the challenges and limitations faced and addressed within the context of such urgent deployment. We provide initial metrics data pre‐COVID‐19 and post‐COVID‐19, including number of visits, types of patients seen, and no‐show rates in comparison between in‐person and telemedicine visits. In conclusion, we provide our provider perspective on the implementation, expansion, and sustainability of a telemedicine‐first care model in the context of an international pandemic response effort.
2. METHODS
2.1. Team demographics
The Genetics and Metabolism team consists of 13 geneticists, 7 genetic counselors, 2 dietitians, and a strong support team of office personnel. The full team is located at Children's National Hospital, Washington, District of Columbia.
2.2. Pilot development
We piloted a telemedicine clinic for genetics and metabolic patients in which patients were seen at home. This pilot clinic was part of our organizations broader telemedicine program, initiated in 2016. The mission of the program was to increase patient access to care by easing the burdens of transportation, missed work, school disruption, and nosocomial exposure. It also was designed to improve clinical efficiency and ensure rapid turn‐around for new and follow‐up appointments. Our model flipped the current care paradigm in our patient population (only follow‐up visits by telemedicine) by using a telemedicine‐first model for initial visits for intakes, assessments, and plans. Beyond our local patient population, this fits our broader scope of work with national and international organizations to provide better access for patients in underserved areas. When creating the telemedicine program for genetics and metabolism, the vision was simple: optimize the patient and provider experience. Our hope was to make medical care easy, practical, and accessible. The patient experience was designed around reducing the burden of care for families. By utilizing the home environment, patients are more comfortable and more relaxed than in a busy clinic setting. To reinforce and facilitate patient education we have deployed freely accessible educational videos on a variety of genetics and clinical topics as well as the telemedicine program in English and Spanish (BearGenes: https://childrensnational.org/departments/rare-disease-institute/beargenes).
In terms of the provider experience, we used time‐saving solutions including digital intake forms and templates with auto‐text phrases to facilitate documentation.
The pilot telemedicine program started in December 2018 and continued until our pandemic response began on March 16, 2020. Based on patient and provider feedback and technology assessment, we developed standard operating procedures (SOP) for our telemedicine practice and care delivery model (Appendix S1, Standard Operating Procedure). The SOP was based on institutional experience with 22 pediatric specialties represented in the telemedicine program pre‐COVID‐19, and 44 pediatric specialties post‐COVID‐19.
Patients were scheduled by the hospital call center and received either an English or Spanish consent form and automated telephone reminders. The technology platform used was HIPAA compliant. Interpreters for all languages were accessible and could be directly incorporated into the visit via the telehealth platform. Each new patient intake included a history of present illness, medication list, allergy list, review of systems, developmental history, school history including therapies and services, family history with three‐generation pedigree, review of growth curves and previous records when available, and telemedicine appropriate physical examination. In cases where the video quality for physical features was inadequate, patients/families provided still images of sufficient quality via secure email. The technology platform allowed secure recording of visits (with consent) that could be useful for review or trainee education.
2.3. Post‐COVID‐19 rapid deployment
The post‐COVID‐19 deployment was done in close collaboration with the Children's National telemedicine program which provided access to secure laptop computers, scheduling systems, and billing integration. During the weekend before the division wide telemedicine conversion, we developed and used a training presentation which detailed the following: how to initiate a visit, schedule a patient, use the equipment, utilize documentation templates, place orders, bill a telemedicine visit, and understand particulars of individual state regulations. In addition, instructions were given to providers on how to access the specific telehealth software platform, in this case end‐to‐end encrypted secure Zoom (Zoom Video Communications, Inc., San Jose California) system. On Monday, March 16, 2020, telemedicine clinics were running concurrently with on‐site clinics. Complete conversion was achieved for the division by the next day. The goal was to maintain seamless access to care for our patients. A redundant system was implemented for patient contact to ensure all individuals were aware of the telemedicine transition and prepared for their in‐home visit.
Recognizing that some patients required in‐person evaluation, monitoring, or sample collection, one nonrotating physician was designated to cover the physical outpatient clinic. This physician also handled patients who did not get the conversion message due to out of date contact information or communication gaps. Surprisingly few patients fell into this group. To care for inpatient cases, another nonrotating physician was designated to conduct exams, round, and provide bedside care, while the previously scheduled on‐call physician handled all pages and outside calls remotely. This on‐call physician also provided telemedicine care at other hospitals in consultation. This model (currently ongoing) provided a manageable workload for all team members over the 6 weeks since inception and exposed only two physicians to potential hospital‐acquired infection. As of the date of submission of this manuscript, none of the caregivers in the program have shown symptoms of or had positive testing for COVID‐19, nor have their families.
A necessary component of our telemedicine effort included program management at the level of the Division of Genetics and Metabolism. The program manager, lead geneticist, and genetic counselor from our pilot program comprised a core genetics telemedicine leadership team, which coordinated the other teams and provided real‐time troubleshooting and training as needed. The program manager streamlined all administrative processes, coordinated training implementation and volunteer inclusion, managed and responded quickly to provider concerns, and served as point person to interface with hospital‐wide telemedicine initiatives, technology support services, and public relations teams. A virtual “all hands‐on deck” approach was taken; our entire staff, including providers, scheduling staff, and administrative assistants were heavily involved. An organized daily video‐conference meeting structure optimized provider participation, maintained team connectivity, and created an efficient and effective mechanism to identify and overcome challenges. This resulted in continuous iteration for quality improvement and development of SOPs which were subsequently used by other hospital teams.
2.4. Medical education interventions
Medical education has also suffered in the pandemic. We implemented a rapid technical training program and developed a formalized remote‐education curriculum to minimize gaps for medical students, residents, and fellows. Our medical genetics trainees were integrated in real‐time with the telemedicine program, and they maintained their education by participating in telemedicine and home‐call consultations. This educational program included telemedicine participation and evaluation of skill‐set development; providers also received continuing medical education via accredited virtual case‐conferences and presentations.
We incorporated medical students, residents, and fellows into our program. Our education program director designed a formalized program which leveraged remote clinical activities and provided training on telemedicine, including how to take a remote history and physical, evaluate a patient, generate a differential diagnosis, develop management recommendations, and adapt care plans to the current pandemic restrictions and shortages. A CME approved virtual case‐based learning and dysmorphology weekly conference was a requirement for virtual learners and continues to draw an average of 30 staff and trainees per week.
3. RESULTS
Billing charges were used to assess the pilot and post‐COVID‐19 deployment. From the launch of the pilot program in December 21, 2018 to March 16, 2020, medical geneticists, genetic counselors and dietitians participated; however, only medical geneticist billing was allowed by the hospital compliance team. Thus, the 136 billed encounters represent the minimum number of patients reached via telemedicine. The pilot program included many providers but focused primarily on one geneticist and genetic counselor. From the start of the post‐COVID‐19 telemedicine conversion for the entire Division of Genetics and Metabolism in March 17, 2020 to April 15, 2020, 150 medical geneticist encounters were captured. There were 116 additional charges by genetic counselors and dietitians. These do not include all charges from multispecialty clinics which also involved genetics providers. These charges were submitted after new guidance was provided by Centers for Medicare and Medicaid Services and review by our compliance team. By week three, the program was at 80% of normal clinical productivity.
Patient demographics are described in Table 1. Figure 1 shows the top 10 diagnostic billing codes for pre‐COVID and post‐COVID patients. Autism was a targeted visit type in our pilot program which may account for the higher pre‐COVID‐19 numbers.
TABLE 1.
Patient demographic characteristics pre‐COVID‐19 and post‐COVID‐19
| Pre‐COVID‐19 | Post‐COVID‐19 | ||
|---|---|---|---|
| Gender [N (%)] | Male | 62 (45%) | 65 (43%) |
| Female | 76 (55%) | 85 (57%) | |
| Age range | Min. | 1 week | 1 week |
| Max. | 40+ years | 40+ years | |
| Visit type [N (%)] | New | 95 (69%) | 65 (43%) |
| Follow‐up | 43 (31%) | 85 (57%) | |
Notes: Pre‐COVID and Post‐COVID demographics of patients evaluated in the telehealth program. Gender was self‐identified by patients or parents. New visits were defined as not having been seen in the Genetics and Metabolism Clinic within the last 5 years.
FIGURE 1.
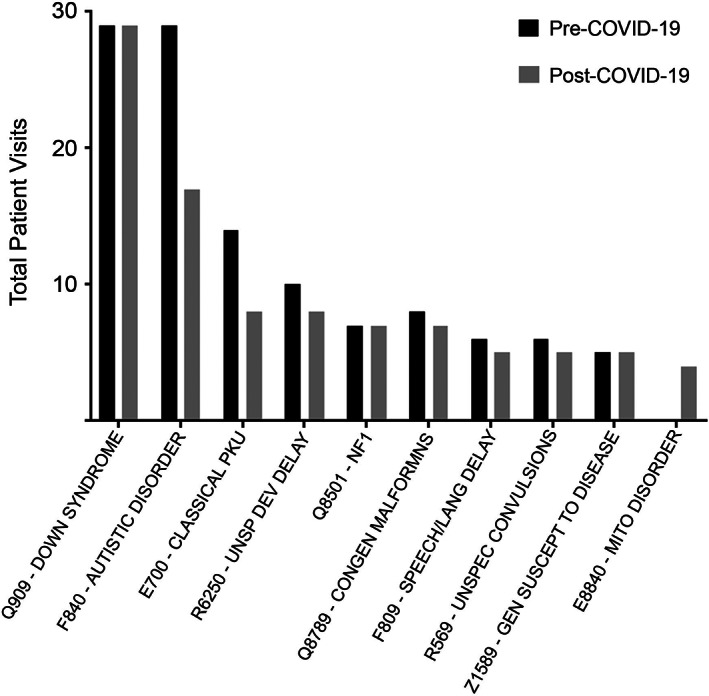
Top 10 diagnoses by patient number based on billing codes in the post‐COVID telemedicine program. The black bars indicate pre‐COVID visits and the gray bars represent post‐COVID visits
Patient no‐show rates from comparable time periods (2018, n = 860 and 2019, n = 1,079) for in‐person visits were 13.6 and 14.4% respectively. For pre‐COVID telemedicine visits (n = 136) the no‐show rate averaged 9.1% and post‐COVID (n = 474) was 8.9%.
Providers expressed satisfaction with this model and internal polling showed that all providers wanted some form of telemedicine in their practice with the majority targeting around 50% (data not shown).
4. DISCUSSION
4.1. Lessons learned
The importance of creating and implementing quick educational tools and working documents for the whole team cannot be understated. A mechanism to identify pitfalls and technology gaps along with capturing and distributing workarounds was vital to maintaining momentum. As an example, our pilot program experience taught us that patients commonly forgot to turn on the audio. Our workaround was a phone call to the patient at the time of the visit to remind them and help with connecting. Having a tech‐savvy teenager in the patient's home was often a successful tech solution.
A simple SODOTO (see one, do one, and teach one) approach was necessary to execute the plan. The core team provided one‐on‐one training with practice mock clinical visits. Once the newly trained clinician acquired the skill‐set, that clinician in turn shifted to training the next provider while also seeing patients. A buddy system enabled providers with real‐time visit challenges to reach out and receive immediate support in order to reduce workflow interruptions. Hospital wide support for IT and telemedicine was also available, including a physician peer‐support system.
Based on our review of the data, pre‐COVID‐19 and post‐COVID‐19, the demographics of our patients, and types of diagnoses seen in the genetics telemedicine program were very similar. Post‐COVID‐19, our genetics telemedicine volumes rapidly increased. The majority of encounters both pre‐COVID‐19 and post‐COVID‐19 were new visits, which should positively impact access to care.
Challenges included scheduling issues, technical problems, billing questions, and, most significantly, state licensure regulations. Technical challenges peaked in the first week. Of note, the technical platform in use has continued to update the software to address identified needs and security issues. Access to language interpreters was initially limited; however our hospital system rapidly improved access through our virtual interpreter service. We created an online folder, accessible to the entire division, with information, SOPs, workarounds, the division‐wide contacts list, and documented rapid solutions. A daily division‐wide virtual meeting identified additional problems to the team.
Licensure barriers continue to be an ongoing issue for access to care. This is particularly true for pediatric specialties with a limited number of providers, including genetics. Providers could only participate in care for new patients in states where they already held licenses. Given our location, we regularly see patients from Virginia, Maryland, West Virginia, and the District of Columbia. In addition, we provide metabolic newborn screening support for Delaware. Several states waived license restrictions for established patients. Our call center had the extra burden of checking individual provider licenses prior to scheduling each patient, which sometimes created confusion and incorrect appointment assignments. Requirements changed daily and by state which required a nimble response on the part of all team members.
A limitation of this work is a lack of standardized surveys of either patients or providers. A nonvalidated survey of our providers requested a significant amount of telemedicine in their practice going forward. Patients consistently provided verbal and written positive feedback. In the future, patient feedback surveys will be incorporated for quality improvement. Another limitation of this report is the accuracy of diagnostic decisions from telemedicine visits compared with in‐person visits. Future studies will compare positive results from diagnostic sequencing, biochemical monitoring levels, and hospitalization/ER visit rates.
5. CONCLUSIONS
Provider access across state jurisdictions is key issue for the future of telemedicine. As the demand for telemedicine grows from patients, this issue will need to be addressed either through state compacts or federal licensure. Provider wellness can be enhanced with a flexible telemedicine model. Both patients and providers can benefit from the ability to schedule visits outside regular clinic business hours. Medically fragile patients can be safely seen in their home environment and avoid unnecessary exposure to nosocomial infections. Telemedicine also can help address geographic disparities in access to care.
In conclusion, our rapid implementation of a telemedicine‐first model during COVID‐19 and rapid‐deployment approach demonstrates a sustainable and effective approach for delivery of services that can be carried forward into a post‐pandemic future. From a provider perspective, when we consider the impact of this work, we summarize the core of our experience over the last month with the following viewpoint: In the middle of this pandemic and international crisis, we have lost much including everyday freedoms, a general feeling of security, and most tragically human lives. As care providers, we refuse to lose a fundamental part of ourselves—the connection with our patients and families. While we cannot hold every patient's hand at the bedside, carry a toddler around clinic, and give our friends and colleagues a hug, we have not forgotten the value of human touch. Our goal was to leave no child without care. Therefore, we reached out, crossed barriers, and overcame obstacles until we found new ways of coming together with patients, families, and each other.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
All of the authors participated in conception or design of the work. Data collection was primarily the work of the following authors: Shur, Kisling, Tabarani, Williams,Atabaki. All authors participated in data analysis and interpretation. Shur drafted the original article. Regier and Fraser drafted and revised figures and tables. All authors participated in critical revision of the article and final acceptance of the version to be published.
Supporting information
Appendix S1: Supporting information
ACKNOWLEDGMENTS
Special thanks to dietitians Danielle Starin, RD and Erin McLeod, RD, PhD who worked on weekly patient newsletters and always remain on call for patients and their families. Thanks to our administrative staff who moved mountains of paperwork into digital bits. Special thanks to: Children's National Hospital institution‐wide telemedicine team, including Dr. Alejandro Lopez‐Magallon, Dr. Ricardo Munez, Dr. Marc Difazio, and Dr. Rachel Selekman; Arial McDade, Benjamin Parrish and Ryan Lee Pearman; to our call center team: John Schultz, Conklin Blain Gentry III, and Zenobia Burns who worked around the clock to provide people with access and us data; to our telemedicine trainees and students led by Dr. Andrea Cohen and Maxwell Summerlin. Thank you for our patients and families who provided incredible support and serve as our source of inspiration and motivation. We are privileged to be part of their lives.
Shur N, Atabaki SM, Kisling MS, et al. Rapid deployment of a telemedicine care model for genetics and metabolism during COVID‐19. Am J Med Genet Part A. 2021;185A:68–72. 10.1002/ajmg.a.61911
Members of Rare Disease Institute: Marshall Summar, MD; Nicholas Ah Mew, MD; Seth Berger, MD, PhD; Kimberly Chapman, MD, PhD; Jamie Fraser, MD, PhD; Christina Grant MD, PhD; Kim Keppler‐Noreuil, MD; Eyby Leon, MD; Amy Feldman Lewanda, MD; Debra Regier, MD, PhD; Kenneth Rosenbaum, MD; Tamanna Roshan Lal, MBChB; Natasha Shur, MD; Hallie Andrew, MS, CGC; Holly Babcock, MS,CGC; Kathleen Crosby, MS, CGC; Jullianne Diaz, MS, CGC; Haley Nisson, MS, CGC; Hillary Porter, MS, CGC; Monisha Kisling, MS, CGC; Rhonda Schonberg, MS, CGC; Kara Simpson, MS, CGC; Joyce Turner, MS, CGC; Erin MacLeod, PhD, RD, LD; Danielle Starin, MS,RD,LD; Kelly McNulty; Emily Louise Able; Nancy Cheng; Charlie Billington, MD, PhD; Angela Grochowsky, MD; Teodoro Jerves, MD; Abir Tabarani, Amelia Roberts; Tiffany Swaringer; Melissa Vivas; Jin Zhang; and Diana Julca, MD.
Funding information BlueCross BlueShield CareFirst Foundation; Health Resources and Services Administration (HRSA), Grant/Award Number: Cooperative Agreement #UH7MC30773 (6/2017‐5/2020); Unrestricted philanthropic grant from BioMarin (San Rafael, California) and Retrophin Inc
DATA AVAILABILITY STATEMENT
N/A.
REFERENCES
- Gattas, M. R. , MacMillan, J. C. , Meinecke, I. , Loane, M. , & Wootton, R. (2001). Telemedicine and clinical genetics: Establishing a successful service. Journal of Telemedicine and Telecare, 7(Suppl 2), 68–70. 10.1258/1357633011937191 [DOI] [PubMed] [Google Scholar]
- Hilgart, J. S. , Hayward, J. A. , Coles, B. , & Iredale, R. (2012). Telegenetics: A systematic review of telemedicine in genetics services. Genetics in Medicine, 14(9), 765–776. 10.1038/gim.2012.40 [DOI] [PubMed] [Google Scholar]
- Otten, E. , Birnie, E. , Lucassen, A. M. , Ranchor, A. V. , & Van Langen, I. M. (2016). Telemedicine uptake among genetics professionals in Europe: Room for expansion. European Journal of Human Genetics, 24(2), 157–163. 10.1038/ejhg.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten, E. , Birnie, E. , Ranchor, A. V. , & van Langen, I. M. (2017). Telegenetics use in presymptomatic genetic counselling: Patient evaluations on satisfaction and quality of care. European Journal of Human Genetics, 24(4), 513–520. 10.1038/ejhg.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penon‐Portmann, M. , Chang, J. , Cheng, M. , & Shieh, J. T. (2020). Genetics workforce: Distribution of genetics services and challenges to health care in California. Genetics in Medicine, 22(1), 227–231. 10.1038/s41436-019-0628-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogue, R. E. , Cavalcanti, D. P. , Shanker, S. , Andrade, R. V. , Aguiar, L. R. , de Carvalho, J. L. , & Costa, F. F. (2018). Rare genetic diseases: Update on diagnosis, treatment and online resources. Drug Discovery Today, 23(1), 187–195. 10.1016/j.drudis.2017.11.002 [DOI] [PubMed] [Google Scholar]
- Solomons, N. M. , Lamb, A. E. , Lucas, F. L. , McDonald, E. F. , & Miesfeldt, S. (2018). Examination of the patient‐focused impact of cancer telegenetics among a rural population: Comparison with traditional in‐person services. Telemedicine Journal and E‐Health, 24(2), 130–138. 10.1089/tmj.2017.0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrecar, I. , Hristovski, D. , & Peterlin, B. (2017). Telegenetics: An update on availability and use of telemedicine in clinical genetics service. Journal of Medical Systems, 41(2), 21. 10.1007/s10916-016-0666-3 [DOI] [PubMed] [Google Scholar]
- Waller, M. , Taylor, L. , & Portnoy, J. (2019). The medical virtualist: Is pediatric patient care using telemedicine, a new specialty? Pediatric Annals, 48(6), e243–e248. 10.3928/19382359-20190520-01 [DOI] [PubMed] [Google Scholar]
- Zierhut, H. A. , MacFarlane, I. M. , Ahmed, Z. , & Davies, J. (2018). Genetic Counselors' experiences and interest in telegenetics and remote counseling. Journal of Genetic Counseling, 27(2), 329–338. 10.1007/s10897-017-0200 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information
Data Availability Statement
N/A.


