Abstract
Background
Many laboratory parameters have been associated with morbidity and mortality in SARS‐CoV‐2 (COVID‐19), which emerged in an animal market in Wuhan, China in December 2019 and has infected over 20 million people. This study investigated the relationship between serum interleukin (IL)‐18, IL‐1 receptor antagonist (IL‐1Ra), and alpha defensin levels and the clinical course and prognosis of COVID‐19.
Materials and Methods
This study included 100 patients who were admitted to the chest diseases department and intensive care unit of our hospital and diagnosed with COVID‐19 by real‐time polymerase chain reaction (PCR) of nasopharyngeal swab samples between March 24 and May 31, 2020. The control group consisted of 50 nonsymptomatic health workers with negative real‐time PCR results in routine COVID‐19 screening in our hospital.
Results
Serum alpha defensin, IL‐1Ra, and IL‐18 levels were significantly higher in patients who developed macrophage activation syndrome (MAS) and acute respiratory distress syndrome (ARDS) compared to patients who did not (p < .001 for all). Alpha defensin, IL‐1Ra, and IL‐18 levels were significantly higher in COVID‐19 patients with and without MAS or ARDS when compared to the control group (p < .001 for all). When the 9 patients who died were compared with the 91 surviving patients, IL‐1Ra and IL‐18 levels were found to be significantly higher in the nonsurvivors (p < .001).
Conclusion
Our findings of correlations between alpha defensin and levels of IL‐1Ra and IL‐18, which were previously shown to be useful in COVID‐19 treatment and follow‐up, indicates that it may also be promising in treatment.
Keywords: alpha defensin, COVID‐19, IL‐18, IL‐1Ra
1. INTRODUCTION
To date, COVID‐19 has infected over 20 million people worldwide since it appeared in December 2019, and case numbers continue to rise daily. Infected individuals may be asymptomatic or may exhibit mild symptoms such as fatigue, muscle, and joint pain, decreased appetite, and loss of smell and taste. However, the clinical presentation can be severe in certain individuals, especially older adults, people with hypertension, diabetes mellitus, or chronic kidney failure, patients receiving immunosuppressive therapy, and pregnant women. 1 , 2
The main severe clinical manifestations of COVID‐19 are acute respiratory failure and macrophage activation syndrome (MAS). In both presentations, excessive production of proinflammatory cytokines can cause endothelial dysfunction in many vital organs, especially the lungs. This state of intense cytokine discharge is known as a cytokine storm, and helper T cells are instrumental in its formation. Th‐1 cells synthesize many of the major proinflammatory cytokines involved in the cytokine storm, particularly interleukin (IL)‐1, IL‐6, IL‐12, and IL‐17. Failure to maintain the pro‐/anti‐inflammatory balance is the most important cause of morbidity and mortality in patients with COVID‐19. 3 , 4
The proinflammatory side of this equilibrium also includes IL‐18, which is a member of the IL‐1 family and similar to IL‐12 in its cytotoxic effect. In conjunction with IL‐12, IL‐18 stimulates interferon‐gamma synthesis from natural killer cells and T cells, thereby activating the macrophages that play an important role in cellular immunity. 5 IL‐18 dysregulation has been associated with several autoimmune and inflammatory diseases. 6 , 7 IL‐1 receptor antagonist (IL‐1Ra) is involved in the antagonization of IL‐1, which plays an important role in the cytokine storm syndrome. An attenuated form of this protein is now able for use in patients with COVID‐19 associated cytokine storm syndrome. 8
Alpha defensin is another cytokine that helps prevent the excessive cytokine response from macrophages that contributes to the cytokine storm. Alpha defensin is a protein mainly synthesized by apoptotic and necrotic neutrophils as a part of the innate immune defense against viral and bacterial infections. 9 Studies have shown that alpha defensin has an important role in preventing virus entry into cells and in the transmission of viral infection between cells. This antiviral effect has been interpreted as evidence that it may be used as a part of treatment to prevent and control viral infections. 9 , 10
The aim of this study was to investigate the relationships between clinical course, prognosis, and mortality in COVID‐19 patients and their levels of IL‐18, IL‐1Ra, and alpha defensin, which are important players in the inflammatory/anti‐inflammatory balance in viral infections.
2. MATERIALS AND METHODS
The study included patients who presented to the emergency department of Atatürk University with suspicious symptoms such as fever, cough, dyspnea, fatigue, and sudden loss of smell and taste and had a history of travel abroad or contact with a suspected COVID‐19 patient within the past 14 days.
High‐resolution computed tomography (HRCT) was performed routinely in all patients at high risk for COVID‐19. Patients whose HRCT results suggested COVID‐19 and those whose radiologic findings were atypical but had consistent clinical symptoms were admitted. COVID‐19 diagnosis was established by real‐time polymerase chain reaction (PCR) testing of nasopharyngeal swab samples. The study group comprised 121 patients who were followed up in the chest diseases department between March 24, 2020 (the date of admission of the first COVID‐19‐positive patient to Atatürk University) and May 31, 2020. The control group consisted of 50 nonsymptomatic health workers in our hospital who tested negative in routine real‐time PCR screening for COVID‐19. As inflammatory dysregulation is more common in COVID‐19 patients with comorbidities, for our study focusing on inflammatory parameters, we excluded 10 patients presenting with uncontrolled diabetes mellitus, two patients who presented with COVID‐19 and concomitant acute coronary syndrome, one patient with COVID‐19 and concomitant infarction in the temporoparietal region, and eight patients who were active smokers.
Following admission, the patients' hematological parameters, biochemical parameters including liver and kidney function tests, coagulation parameters, ferritin, d‐dimer, troponin‐I, C‐reactive protein (CRP), and arterial blood gas parameters were examined.
2.1. Definitions and treatment
Fever was defined as an axillary temperature of 37.3°C or higher. Secondary bacterial infection was defined as the presence of signs and symptoms of bacteremia or pneumonia with endotracheal aspirate or lower respiratory tract sputum culture positive for a new pathogen. Patients diagnosed as having ventilator‐associated or hospital‐acquired pneumonia were treated according to current guidelines. Acute respiratory distress was diagnosed and graded using the Berlin 2015 diagnostic criteria. Cardiac‐specific troponin was checked daily and patients whose values were above the normal range were evaluated by echocardiography for emerging cardiac pathologies. Coagulopathy was defined as prothrombin and partial thromboplastin times prolonged by 3 s and 5 s, respectively. Based on disease severity, the treatment was planned according to the COVID‐19 adult diagnosis and treatment guidelines published by the Turkish Ministry of Health. Patients exhibiting findings such as persistent fever, CRP, and ferritin levels that remained high or continued to increase, d‐dimer elevation, lymphopenia or thrombocytopenia, abnormal liver function tests, hypofibrinogenemia, or elevated triglyceride levels despite treatment were evaluated as having MAS. If these parameters deteriorated in repeated measures and could not be explained by a secondary bacterial infection, the patients were treated with 400 mg tocilizumab for MAS if not contraindicated. Patients who did not show significant improvement in laboratory parameters and vital signs after 24 h were given another dose of 400 mg tocilizumab.
2.2. Measurement of biochemical markers
After 15 min of semi‐supine rest, blood samples were collected from the antecubital vein into tubes containing ethylenediaminetetraacetic acid to prevent coagulation. Troponin‐I concentrations were measured by a chemiluminescent immunoassay using an Immulite 2500 (Siemens Medical Solutions). Alpha defensin, IL‐1Ra, and IL‐18 levels were measured by enzyme‐linked immunosorbent assay (Elabscience Human ELISA Kit).
2.3. Statistical analysis
The data were analyzed using IBM SPSS Statistics for Windows version 24.0 (IBM Corp.). Pearson's χ 2 test and Mann–Whitney U test were used for intergroup comparisons of parametric data and nonnormally distributed numerical data, respectively. An independent‐samples t‐test was used to compare demographic data and laboratory parameters between groups. Analysis of variation was used to compare levels of alpha defensin, IL‐1Ra, and IL‐18 between patient groups with and without MAS and ARDS and the control group. Pearson's correlation analysis was used to evaluate relationships between laboratory parameters. A p‐value of less than .05 was considered statistically significant.
3. RESULTS
Of the 100 patients with COVID‐19 included in the study, 55 were women and 45 were men. The control group comprised of 25 men and 25 women. The mean age of the female patients with COVID‐19 was 46.2 ± 19.1 years and that of the male patients was 51.9 ± 21.5 years. There was no statistically significant difference in mean age according to sex (p = .171). The mean age of the participants in the control group was 49.2 ± 17.6 years. There was no statistically significant difference in age between the patient and control groups (p = .09).
In the COVID‐19 group, 33 patients had hypertension, 16 had diabetes mellitus, 5 had coronary artery disease, and 20 patients were ex‐smokers. In the subgroup of patients who developed MAS, 15 of the patients had hypertension (HT), eight had diabetes mellitus (DM), and one had coronary artery disease (CAD); in the ARDS subgroup, 30 patients had hypertension, 10 had diabetes mellitus, and three had coronary artery disease. There were 15 nonsmokers in each subgroup. Participants in the control group had no comorbidities and 10 were ex‐smokers.
Comparisons of laboratory data and age between patients with and without MAS are shown in Table 1. The patients who developed MAS showed a significantly higher mean age, white blood cell (WBC) count, neutrophil count, neutrophil/lymphocyte ratio (NLR), aspartate transaminase (AST), lactate dehydrogenase (LDH), gamma‐glutamyltransferase (GGT), creatine, prothrombin time, CRP, Troponin‐I, PaO2/FiO2, d‐dimer, and ferritin levels and a significantly lower lymphocyte count compared with those who did not develop MAS (p = .01 for AST, p < .001 for all other parameters). Comparisons of laboratory data and age between patients with and without ARDS are shown in Table 2. The patients who developed ARDS showed a significantly higher mean age, WBC count, neutrophil count, neutrophil/lymphocyte ratio, AST, LDH, GGT, creatine, prothrombin time, CRP, Troponin‐I, PaO2/FiO2, d‐dimer, and ferritin levels and a significantly lower lymphocyte count compared with those who did not develop ARDS (p = .05 for WBC, p = .02 for creatine, p = .008 for ferritin, and p < .001 for all other parameters).
Table 1.
Comparison of laboratory parameters at admission in patients with COVID‐19 who did and did not develop macrophage activation syndrome (MAS)
| MAS patients admission (mean ± SD; n = 21) | Non‐MAS patients admission (mean ± SD; n = 79) | p | |
|---|---|---|---|
| Age (year) | 69.6 ± 12.6 | 43.4 ± 18.2 | .001 |
| WBC (/µl) | 8770 ± 7007.7 | 6641.5 ± 2244.3 | .02 |
| Lymphocytes (/µl) | 831.4 ± 388.6 | 1779.4 ± 870.4 | .001 |
| Neutrophils (/µl) | 7159.1 ± 6189.3 | 4201.5 ± 1883.2 | .001 |
| NLR | 12.8 ± 14.8 | 3.1 ± 2.7 | .001 |
| AST (U/L) | 41.5 ± 18.8 | 29.9 ± 19.1 | .01 |
| ALT (U/L) | 35.1 ± 27.2 | 28.6 ± 23.5 | .33 |
| LDH (U/L) | 439.2 ± 205.4 | 268.1 ± 111.9 | .001 |
| GGT (U/L) | 61.2 ± 51.7 | 33.2 ± 22.6 | .001 |
| ALP (U/L) | 84.8 ± 36.9 | 78.5 ± 41.9 | .54 |
| Sodium (mmol/L) | 137.9 ± 6 | 139.1 ± 3.1 | .22 |
| Potassium (mmol/L) | 4.1 ± 0.7 | 4.2 ± 0.4 | .72 |
| Creatine (mg/dL) | 1.6 ± 1.7 | 0.9 ± 0.5 | .001 |
| Prothrombin time (s) | 20.1 ± 12.1 | 14.7 ± 3.3 | .001 |
| CRP (mg/dL) | 176.4 ± 87.6 | 24.7 ± 36.6 | .001 |
| Troponin‐I (ng/dL) | 253 ± 580.7 | 10.6 ± 26.2 | .001 |
| PaO2/FiO2 | 209.4 ± 64.8 | 325.4 ± 47.2 | .001 |
| d‐Dimer (ng/ml) | 2947.1 ± 3642.8 | 876.2 ± 1706.9 | .001 |
| Ferritin (ng/ml) | 1093.8 ± 1485.5 | 322.4 ± 151.9 | .001 |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; COVID‐19, coronavirus disease 2019; GGT, gamma‐glutamyltransferase; LDH, lactate dehydrogenase; MAS, macrophage activation syndrome; NLR, neutrophil/lymphocyte ratio; SD, standard deviation; WBC, white blood cells.
Table 2.
Comparison of laboratory parameters at admission in patients with COVID‐19 who did and did not develop acute respiratory distress syndrome (ARDS)
| ARDS patients (n = 35; mean ± SD) | Non‐ARDS patients (n = 65; mean ± SD) | p | |
|---|---|---|---|
| Age (year) | 67.9 ± 12.2 | 38.3 ± 15.6 | .001 |
| WBC (/µl) | 8109.1 ± 5749.5 | 6535.3 ± 2070.6 | .05 |
| Lymphocytes (/µl) | 960 ± 467.3 | 1927.6 ± 874.1 | .001 |
| Neutrophils (/µl) | 6411.4 ± 5117.7 | 3951.4 ± 1595.8 | .001 |
| NLR | 9.8 ± 11.9 | 2.4 ± 1.6 | .001 |
| AST (U/L) | 42.9 ± 25.1 | 26.6 ± 12.8 | .001 |
| ALT (U/L) | 34.1 ± 30.6 | 27.9 ± 20.3 | .23 |
| LDH (U/L) | 427.7 ± 186.9 | 234.6 ± 64.5 | .001 |
| GGT (U/L) | 55.2 ± 44.6 | 30.4 ± 19.8 | .001 |
| ALP (U/L) | 90.1 ± 55.1 | 74.5 ± 29.9 | .1 |
| Sodium (mmol/L) | 137.3 ± 4.9 | 139.7 ± 2.9 | .04 |
| Potassium (mmol/L) | 4.1 ± 0.6 | 4.2 ± 0.4 | .4 |
| Creatine (mg/dL) | 1.3 ± 1.4 | 0.9 ± 0.5 | .02 |
| Prothrombin time (s) | 19.1 ± 9.8 | 13.9 ± 2 | .001 |
| CRP (mg/dL) | 132.1 ± 92.4 | 15.9 ± 26.1 | .001 |
| Troponin‐I (ng/dL) | 160.5 ± 460.3 | 8.1 ± 25.7 | .009 |
| PaO2/FiO2 | 228.5 ± 58.8 | 342.6 ± 29.2 | .001 |
| d‐Dimer (ng/ml) | 2401.9 ± 3097.1 | 707.3 ± 1655.2 | .001 |
| Ferritin (ng/ml) | 742.4 ± 1204.9A | 327.5 ± 154.6 | .008 |
Abbreviations: ARDS, acute respiratory distress syndrome; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; COVID‐19, coronavirus disease 2019; GGT, gamma‐glutamyl transferase; LDH, lactate dehydrogenase; NLR, neutrophil/lymphocyte ratio; SD, standard deviation; WBC, white blood cells.
The alpha defensin, IL‐1Ra, and IL‐18 levels of patients with and without MAS and ARDS are shown in Table 3. All parameters were significantly higher in patients who developed MAS and ARDS compared with patients who did not (p < .001 for all). Alpha defensin, IL‐1Ra, and IL‐18 levels were significantly higher in COVID‐19 patients with and without MAS or ARDS when compared with the control group (p < .001 for all). When the nine patients who died were compared with the 91 surviving patients, IL‐1Ra and IL‐18 levels were found to be significantly higher in the nonsurvivors (p < .001), but there was no significant difference in alpha defensin levels (p = .08).
Table 3.
Comparison of alpha defensin, IL‐1Ra, and IL‐18 levels at admission between COVID‐19 patients with and without MAS and ARDS and the control group
| MAS | ARDS | |||||
|---|---|---|---|---|---|---|
| + | − | + | − | Control | ||
| (n = 21) | (n = 79) | (n = 35) | (n = 65) | (n = 50) | ||
| (mean ± SD) | (mean ± SD) | (mean ± SD) | (mean ± SD) | (mean ± SD) | p * | |
| Alpha defensin (pg/ml) | 1021.1 ± 260.5 | 674.5 ± 347.6 | 984.8 ± 254.7 | 613.7 ± 342.6 | 348.4 ± 120.1 | .001 |
| IL‐1Ra (pg/ml) | 704.7 ± 488.1 | 110.2 ± 87.9 | 493.9 ± 460.6 | 87.6 ± 45.9 | 65.4 ± 48.8 | .001 |
| IL‐18 (pg/ml) | 239.7 ± 153.7 | 145.5 ± 45.5 | 204.4 ± 119.3 | 139.4 ± 43.3 | 90.4 ± 25.6 | .001 |
Abbreviations: ARDS, acute respiratory distress syndrome; COVID‐19, coronavirus disease 2019; IL, interleukin; IL‐1Ra, IL‐1 receptor antagonist; MAS, macrophage activation syndrome.
p: Comparisons of all parameters within each disease subgroup and with the control group were significant at the p < .001 level.
When analyzed by sex, alpha defensin levels were 863.8 ± 326.5 pg/ml in men and 649.8 ± 360.5 pg/ml in women, while IL‐1Ra levels were 318.1 ± 401.4 pg/ml in men and 161 ± 256.6 pg/ml in women. Both parameters were significantly higher among male patients (p = .03, p = .02). Twelve of the 21 patients who developed MAS and 21 of the 45 patients who developed ARDS were male. Although the difference in MAS development between the sexes was not statistically significant (p = .3), the prevalence of ARDS was significantly higher among male patients (p = .03).
Comparisons of alpha defensin, IL‐1Ra, and IL‐18 levels based on comorbidities revealed no significant differences between the 15 patients with HT and the six patients without HT who developed MAS (p = .08, p = .09, p = .2, respectively). Likewise, no statistically significant differences were observed when comparing the eight patients with DM and the 13 patients without DM in the MAS group, which was a more homogeneous grouping (p = .2, p = .12, p = .07). This analysis could not be done for CAD due to the small number of patients.
Correlation analysis showed that alpha defensin, IL‐1Ra, and IL‐18 levels were negatively correlated with PaO2/FiO2 (r = −.437, p = .01; r = −.629, p = .01; r = −.475, p = .01, respectively) and positively correlated with CRP (r = .445, p = .01; r = .676, p = .01; r = .344, p = .01, respectively; Figures 1 and 2). Alpha defensin, IL‐1Ra, and IL‐18 levels were positively correlated with both LDH (r = .576, p = .01; r = .499, p = .01; r = .547, p = .01) and ferritin levels (r = .2, p = .05, r = .519, p = .01, r = .751, p = .01). Positive correlations were also detected between alpha defensin level and IL‐1Ra (r = .552, p = .01) and IL‐18 (r = .285 p = .01) and between IL‐1Ra and IL‐18 (r = .552, p = .01; Figure 3).
Figure 1.
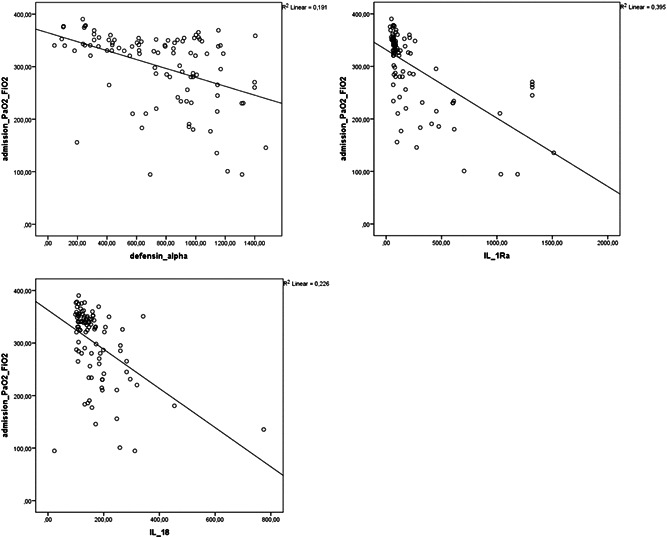
Correlation analysis of PaO2/FiO2 ratios and alpha defensin, IL‐1Ra, and IL‐18 levels in patients with COVID‐19 at admission. COVID‐19, coronavirus disease 2019; IL‐18, interleukin‐18; IL‐1Ra, IL‐1 receptor antagonist
Figure 2.
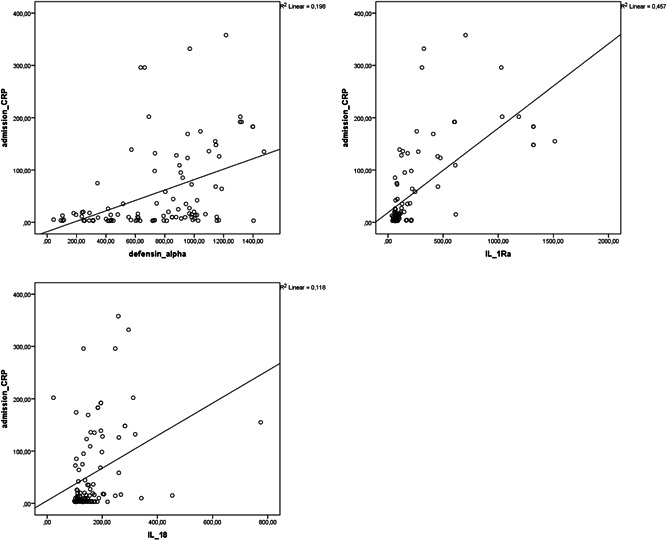
Correlation analysis between CRP level and alpha defensin, IL‐1Ra, and IL‐18 levels in patients with COVID‐19 at admission. COVID‐19, coronavirus disease 2019; CRP, C‐reactive protein; IL‐18, interleukin‐18; IL‐1Ra, IL‐1 receptor antagonist
Figure 3.
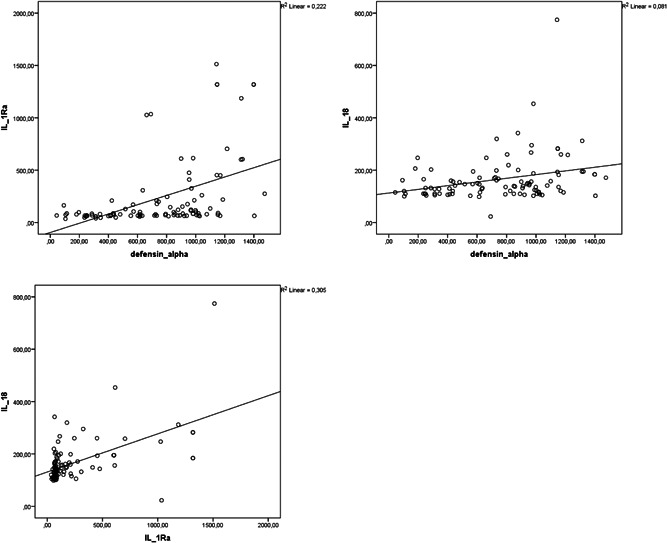
Correlation analysis of alpha defensin, IL‐1Ra, and IL‐18 levels of patients with COVID‐19. COVID‐19, coronavirus disease 2019; IL‐18, interleukin‐18; IL‐1Ra, IL‐1 receptor antagonist
NLR was found to be positively correlated with CRP (r = .527, p = .01) and LDH levels (r = .281, p = .01) and negatively correlated with PaO2/FiO2 ratio (r = −.468, p = .01). Positive correlations were detected between ferritin level and CRP (r = .547, p = .01), d‐Dimer (r = .54, p = 0.01), and Troponin‐I (r = .453, p = .01), whereas a negative correlation was observed between ferritin and PaO2/FiO2 ratio (r = −.418, p = .01).
4. DISCUSSION
In this study, we determined that alpha defensin, IL‐1Ra, and IL‐18 levels were elevated in patients with COVID‐19. We also observed that this elevation was more pronounced in patients with MAS and ARDS compared with those without. In addition, IL‐1Ra and IL‐18 levels were higher in nonsurvivors compared to surviving patients. Alpha defensin, IL‐1Ra, and IL‐18 levels were positively correlated with CRP and negatively correlated with the PaO2/FiO2 ratio.
SARS‐CoV‐2 was discovered in the Wuhan province of China in December 2019. This virus was found to be closely related to SARS‐CoV and Middle East respiratory syndrome‐related coronavirus, viruses that previously caused outbreaks with high rates of morbidity and mortality. 11 The rapid spread of this viral disease, named COVID‐19 by the World Health Organization, resulted in a global pandemic that has infected over 20 million people and claimed over 700,000 lives to date.
SARS‐CoV‐2 infects the human respiratory tract and vascular and immune cells and can cause severe damage to the respiratory tract, especially in the lungs. 12 Disease severity is linked to immune system activity. The currently available data suggest that viral infection can lead to an exaggerated immune reaction in the host in some cases that is severe enough to be labeled as MAS, which is a hyperinflammatory condition. 13
Numerous proinflammatory cytokines are released in MAS, particularly tumor necrosis factor‐α (TNF‐α), IL‐1, IL‐2, IL‐6, IL‐18, and nitric oxide. These cytokines can increase vascular permeability, leading to impaired tissue perfusion, as well as endothelial damage and microthrombus formation. 14 This increase in vascular permeability causes fluid to accumulate in the lung tissue and interstitial spaces, which manifests clinically with acute respiratory failure. Suppression of these pro‐inflammatory cytokines has been therapeutically beneficial in many inflammatory conditions, including viral infections. 15 MAS has a proven role in severe COVID‐19, and early identification of hyperinflammation and the use of currently available and approved therapies such as steroids, intravenous immunoglobulins, and selective inhibitors of cytokines such as IL‐1 and IL‐6 can prevent morbidity and mortality in COVID‐19 patients. 14
Alpha defensin, a component of innate immunity that plays a role in the anti‐inflammatory balance and acts as a brake in MAS, is released by apoptotic and necrotic neutrophils at the site of inflammation. 10 , 16 Defensins have antimicrobial activity against bacteria, fungi, and enveloped viruses such as influenza A, SARS‐CoV, and HIV. During inflammation, neutrophil granules release large amounts of alpha‐defensins, which are called human neutrophil peptides 1, 2, and 3. 16 , 17 Defensins released from the site of inflammation have a chemotactic effect on monocytes, T cells, and dendritic cells. According to studies on the action of alpha defensin, levels of this peptide rise in response to influenza A infection and it increases neutrophil uptake of the virus. 18 In addition, it has also been found to minimize oxidative damage while increasing viral uptake. 19 In a study conducted on animals infected with Pseudomonas aeruginosa, it was found that alpha defensin synthesized by apoptotic and necrotic neutrophils led to a decrease in levels of TNF‐α, IL‐1 beta, IL‐6, and IL‐8, which are important proinflammatory cytokines. 20
Consistent with previous SARS‐CoV‐2 studies, we observed in the present study that prothrombin time, PaO2/FiO2, and levels of CRP, Troponin‐I, d‐dimer, and ferritin, which are known to be associated with morbidity and mortality, were relatively higher during hospitalization in patients who developed MAS and ARDS than those who did not. Furthermore, the older age of these patients again demonstrates the importance of age as a risk factor in the COVID‐19 pandemic. The present study focused on alpha defensin, IL‐1Ra, and IL‐18 levels and showed that these are also higher in patients who develop MAS and ARDS. This may be interpreted as a sign of a more intense inflammatory/anti‐inflammatory battle in these patients. Given the data indicating that IL‐1 antagonists may yield positive treatment outcomes, alpha defensin increasing with IL‐1Ra in the anti‐inflammatory balance suggests that alpha defensin may also be utilized as a part of treatment. Elevated CRP levels and increased oxygen demand during follow‐up are important in the administration of IL‐1 antagonist therapy in SARS‐CoV‐2. As with IL‐1Ra, the positive correlation between alpha defensin and CRP and its negative correlation with the PaO2/FiO2 ratio suggests that it might be a useful parameter in the diagnosis and follow‐up of COVID‐19 and MAS. NLR was found to be positively correlated with CRP (r = .527, p = .01) and LDH levels (r = .281, p = .01) and negatively correlated with the PaO2/FiO2 ratio (r = −.468, p = .01). Positive correlations were detected between ferritin level and CRP (r = .547, p = .01), d‐Dimer (r = .54, p = .01), and Troponin‐I (r = .453, p = .01), whereas a negative correlation was observed between ferritin and PaO2/FiO2 ratio (r = −.418, p = .01).
The most important limitation of the present study was that the patients who developed MAS and ARDS were older and had more comorbidities. However, the fact that SARS‐CoV‐2 causes higher morbidity and mortality especially in older adults and those with chronic diseases may complicate attempts to form study groups that are homogeneous in terms of age and comorbidities.
In conclusion, with a steady stream of new information about its diagnosis and treatment and no sign of it losing virulence, the treatment of COVID‐19 remains an important issue today. Alpha defensin has been shown to have anti‐inflammatory activity in viral infections such as influenza A and SARS‐CoV, and its elevation in correlation with agents used in the diagnosis and treatment of patients infected with SARS‐CoV‐2 may be a new beacon of hope for treatment.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Conceptualization, methodology, software, validation, and formal analysis: Buğra Kerget and Ferhan Kerget. Investigation, resources, and data curation: Alperen Aksakal and Seda Aşkın. Writing—original draft, writing—review and editing: Buğra Kerget. Visualization, supervision, and project administration: Leyla Sağlam and Metin Akgün.
Kerget B, Kerget F, Aksakal A, Aşkın S, Sağlam L, Akgün M. Evaluation of alpha defensin, IL‐1 receptor antagonist, and IL‐18 levels in COVID‐19 patients with macrophage activation syndrome and acute respiratory distress syndrome. J Med Virol. 2021;93:2090–2098. 10.1002/jmv.26589
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID‐19): a review. JAMA. 2020;323(18):1824–1836. [DOI] [PubMed] [Google Scholar]
- 2. Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID‐19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cao X. COVID‐19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20(5):269–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arend WP, Palmer G, Gabay C. IL‐1, IL‐18, and IL‐33 families of cytokines. Immunol Rev. 2008;223(1):20–38. [DOI] [PubMed] [Google Scholar]
- 6. Boraschi D, Dinarello CA. IL‐18 in autoimmunity. Eur Cytokine Netw. 2006;17(4):224–252. [PubMed] [Google Scholar]
- 7. Akgun M, Saglam L, Kaynar H, et al. Serum IL‐18 levels in tuberculosis: comparison with pneumonia, lung cancer and healthy controls. Respirology. 2005;10(3):295–299. [DOI] [PubMed] [Google Scholar]
- 8. Vaninov N. In the eye of the COVID‐19 cytokine storm [published online ahead of print April 06, 2020]. Nat Rev Immunol. 2020. https://dx.doi.org/10.1038%2Fs41577-020-0305-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Holly MK, Diaz K, Smith JG. Defensins in viral infection and pathogenesis. Ann Rev Virol. 2017;4:369–391. [DOI] [PubMed] [Google Scholar]
- 10. Wilson SS, Bromme BA, Holly MK, et al. Alpha‐defensin‐dependent enhancement of enteric viral infection. PLoS Pathog. 2017;13(6):e1006446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Singhal T. A review of coronavirus disease‐2019 (COVID‐19). Indian J Pediatr. 2020;87:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chua RL, Lukassen S, Trump S, et al. COVID‐19 severity correlates with airway epithelium–immune cell interactions identified by single‐cell analysis. Nature Biotechnol. 2020:1–10. [DOI] [PubMed] [Google Scholar]
- 13. Henderson LA, Canna SW, Schulert GS, et al. On the alert for cytokine storm: immunopathology in COVID‐19. Arthritis Rheumatol. 2020;72:1059–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McGonagle D, Sharif K, O'Regan A, Bridgewood C. Interleukin‐6 use in COVID‐19 pneumonia related macrophage activation syndrome. Autoimmun Rev. 2020;19:102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ingraham NE, Lotfi‐Emran S, Thielen BK, et al. Immunomodulation in COVID‐19. Lancet Respir Med. 2020;8:544–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilson SS, Wiens ME, Smith JG. Antiviral mechanisms of human defensins. J Mol Biol. 2013;425(24):4965–4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Trabattoni D, Caputo SL, Maffeis G, et al. Human α defensin in HIV‐exposed but uninfected individuals. J Acquir Immune Defic Syndr. 2004;35(5):455–463. [DOI] [PubMed] [Google Scholar]
- 18. Salvatore M, García‐Sastre A, Ruchala P, Lehrer RI, Chang T, Klotman ME. β‐defensin inhibits influenza virus replication by cell‐mediated mechanism (s). J Infect Dis. 2007;196(6):835–843. [DOI] [PubMed] [Google Scholar]
- 19. Tecle T, White MR, Gantz D, Crouch EC, Hartshorn KL. Human neutrophil defensins increase neutrophil uptake of influenza A virus and bacteria and modify virus‐induced respiratory burst responses. J Immunol. 2007;178(12):8046–8052. [DOI] [PubMed] [Google Scholar]
- 20. Schneider JJ, Unholzer A, Schaller M, Schäfer‐Korting M, Korting HC. Human defensins. J Mol Med. 2005;83(8):587–595. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


