Dear Editor,
The clinical features of coronavirus disease 2019 (COVID‐19) are diverse, causing multiple organ failure, cytokine storm, coagulopathy, and still more to be fully characterized. A cluster of methemoglobinemia cases was identified among COVID‐19 patients. 1 Methemoglobin cannot bind and release oxygen reversibly and results in decreased oxygen delivery leading to hypoxia. 2 Severe methemoglobinemia can have life‐threatening potential and will be further deleterious when compounded by the coexistent cardiopulmonary dysfunction in COVID‐19 patients. In this report, red cell exchange (REX) transfusion by apheresis is shown to be an effective treatment of dangerously rising methemoglobinemia that was refractory to other conventional treatments in a patient with severe COVID‐19.
A 52‐year‐old African American male with hypertension, type 2 diabetes mellitus, and morbid obesity (148 kg) was admitted with acute hypoxic respiratory failure. Nasopharyngeal swabs were positive for SARS‐CoV‐2 by real‐time reverse transcription‐polymerase chain reaction assay. On hospital day 2 (D2), he was placed on mechanical ventilation and norepinephrine was started. On D3, uncontrolled hyperglycemia required insulin drip, and hemodialysis was initiated for acute kidney injury. He completed a trial of hydroxychloroquine and azithromycin treatment over D3 to D7.
Methemoglobinemia was rising rapidly from his baseline of <1% (normal range 0%‐1.5%) to 16.8% on D6, and continued to rise to 25.3% on D7 (Figure 1). Treatments of methylene blue (MB) and ascorbic acid were given on D7 and D8. MB was administered safely without causing hemolysis after confirming that the patient did not have glucose‐6‐phosphate dehydrogenase deficiency. Methemoglobinemia, however, was refractory and reached greater than 30% (above the measurable range of the assay) on D9. A venous blood specimen drawn on D9 showed red cells of dark brown color and plasma of dark greenish color, consistent with severe methemoglobinemia and plasma free methemoglobin released from intravascular hemolysis. There was growing concern for the detrimental effects of sharply rising methemoglobinemia in this anemic (Hb 8.3 g/dL) patient with comorbidities of respiratory failure, shock, and renal failure. To mitigate the adverse effects from worsening methemoglobinemia, a REX by apheresis with replacement of 12 red cell units was performed emergently. The patient was on vasopressors at the time, but hemodynamic status remained stable during REX. Post‐REX methemoglobin was reduced to 8.7%. During the following 4 days, methemoglobinemia had gradually resolved completely and there was no rebound (Figure 1), suggesting that the oxidizing events had been discontinued. Intravascular hemolysis was also resolved completely after REX.
FIGURE 1.
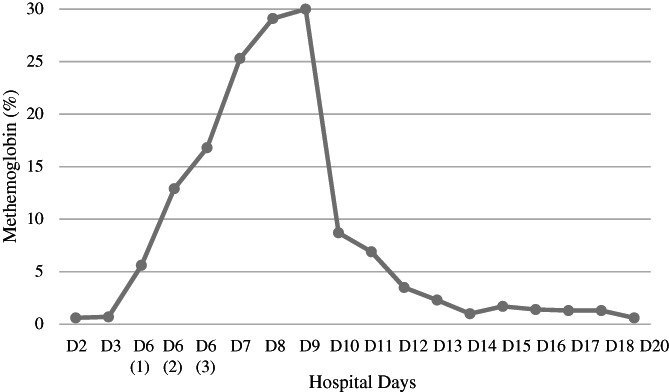
The course of methemoglobinemia as measured by blood gas analysis with co‐oxymeter. The result of >30% (beyond the measurable range of the assay) on hospital day 9 (D9) was obtained prior to red cell exchange transfusion (REX). The D10 result (8.7%) was obtained after REX. (methemoglobin normal range 0%‐1.5%)
Acquired methemoglobinemia is usually caused by exogenous oxidizing chemicals or drugs, 2 and hydroxychloroquine could have precipitated methemoglobinemia in this case. Chloroquine was reported to provoke methemoglobinemia when it was used as malarial prophylaxis. 3 Hydroxychloroquine was administered on D3 to D7, and methemoglobinemia rose rapidly over D6 to D9. Hydroxychloroquine has been used for the patients with rheumatoid arthritis and systemic lupus erythematosus, but methemoglobinemia has not been reported among them. It is probable that certain COVID‐19‐associated conditions and hydroxychloroquine together may confer increased risks for developing methemoglobinemia.
Various modalities of apheresis have been tried for treatment of methemoglobinemia of different etiologies. 4 This case is the first report demonstrating that emergent REX could be a potentially life‐saving treatment of refractory severe methemoglobinemia in COVID‐19 patients with coexistent compounding comorbidities.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
The author thanks all the healthcare workers who dedicated themselves to caring COVID‐19 patients.
REFERENCES
- 1. Naymagon L, Berwick S, Kessler A, Lancman G, Gidwani U, Troy K. The emergence of methemoglobinemia amidst the COVID‐19 pandemic. Am J Hematol. 2020;95(8):E196–E197. 10.1002/ajh.25868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mansouri A, Lurie AA. Methemoglobinemia. Am J Hematol. 1993;42(1):7–12. 10.1002/ajh.2830420104. [DOI] [PubMed] [Google Scholar]
- 3. Cohen RJ, Sachs JR, Wicker DJ, Conrad ME. Methemoglobinemia provoked by malarial chemoprophylaxis in Vietnam. N Engl J Med. 1968;279(21):1127–1131. 10.1056/nejm196811212792102. [DOI] [PubMed] [Google Scholar]
- 4. Singh P, Rakesh K, Agarwal R, et al. Therapeutic whole blood exchange in the management of methaemoglobinemia: Case series and systematic review of literature. Transfus Med. 2020;30:231–239. 10.1111/tme.12666. [DOI] [PubMed] [Google Scholar]


