Abstract
The factors predicting the progression of coronavirus disease‐2019 (COVID‐19) from mild to moderate to critical are unclear. We retrospectively evaluated risk factors for disease progression in Japanese patients with COVID‐19. Seventy‐four patients with laboratory‐confirmed COVID‐19 were hospitalized in our hospital between February 20, 2020, and June 10, 2020. We excluded asymptomatic, non‐Japanese, and pediatric patients. We divided patients into the stable group and the progression group (PG; requiring mechanical ventilation). We compared the clinical factors. We established the cutoff values (COVs) for significantly different factors via receiver operating characteristic curve analysis and identified risk factors by univariate regression. We enrolled 57 patients with COVID‐19 (median age 52 years, 56.1% male). The median time from symptom onset to admission was 8 days. Seven patients developed critical disease (PG: 12.2%), two (3.5%) of whom died; 50 had stable disease. Univariate logistic analysis identified an elevated lactate dehydrogenase (LDH) level (COV: 309 U/l), a decreased estimated glomerular filtration rate (eGFR; COV: 68 ml/min), lymphocytopenia (COV: 980/μl), and statin use as significantly associated with disease progression. However, in the Cox proportional hazards analysis, lymphocytopenia at admission was not significant. We identified three candidate risk factors for progression to critical COVID‐19 in adult Japanese patients: statin use, elevated LDH level, and decreased eGFR.
Keywords: comorbidity, COVID‐19, critical disease, disease progression, Japanese patients, laboratory finding, risk factors
Highlights
To evaluate risk factors for disease progression in Japanese patients with COVID‐19, we examined seventy‐four patients with laboratory‐confirmed COVID‐19.
Seven patients (12.2%) developed critical disease requiring mechanical ventilation, two (3.5%) of whom died.
We identified three candidate risk factors for progression to critical COVID‐19 in adult Japanese patients: statin use elevated LDH level and decreased eGFR.
1. INTRODUCTION
In December 2019, an outbreak of infections with a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), occurred in Wuhan, China. In February 2020, the World Health Organization (WHO) named this disease coronavirus disease‐2019 (COVID‐19). It has rapidly spread and resulted in a pandemic. The clinical presentation of patients with COVID‐19 ranges from asymptomatic to mild to critical, and the disease can lead to death. The WHO classified COVID‐19 into four severity levels: mild, moderate, severe, and critical. 1 The proportion of patients with severe or critical disease may vary with location, but most patients have mild to moderate disease. 2 , 3 However, some patients rapidly progress to critical illness. 4 The mortality risk dramatically increases once a patient develops critical disease. 5 Therefore, the early prediction of progression to critical COVID‐19 is required to enable the timely initiation of interventions to improve the prognosis. In addition, ethnic differences may affect the risk factors because of the different extent of the pandemic in various regions. However, there is no established evidence of predictive factors for the development of critical COVID‐19 in the Japanese population. Our hospital is a medium‐volume hospital with 613 beds, including 14 in the Infectious Disease Unit, in an urban area of Osaka Prefecture, Japan. It is also one of 351 designated medical institutions for Type II infectious diseases in Japan, with a total of 1758 beds. We have accepted patients with COVID‐19 since February 2020 and have expanded the number of beds for infectious disease patients from 14 to 45 to make more room for COVID‐19 patients. In the present study, we retrospectively examined the risk factors for progression to critical disease in Japanese patients with COVID‐19 using clinical characteristics and laboratory findings in a single hospital.
2. PATIENTS AND METHODS
This study was a retrospective single‐center cohort study that examined a total of 75 consecutive patients with laboratory‐confirmed COVID‐19 from February 20, 2020 to June 10, 2020. We followed the patients until July 7, 2020. In the present study, various reverse transcription‐polymerase chain reaction (RT‐PCR) assays were used, but all findings were based on the results of tests by public health centers in the Osaka area in Japan. Osaka Prefecture established the Osaka Prefecture Inpatient Follow‐up Center to coordinate broad‐based hospitalization based on patients’ symptoms on March 30, 2020. Patients with confirmed or suspected COVID‐19 were triaged in four stages depending on the disease severity: (1) patients with critical illness were hospitalized at designated medical institutions for infectious diseases, university hospitals, or the National Hospital Organization; (2) patients with moderate disease were hospitalized in general hospitals (negative pressure room or special ward); (3) patients with mild disease were isolated in a closed or decommissioned ward; and (4) asymptomatic pathogen carriers were quarantined in private accommodations or at home. Our hospital was a designated general hospital for the treatment of patients with moderate COVID‐19.
We reviewed the medical records and examined the clinical factors that predicted progression to critical disease among patients with mild to moderate and severe COVID‐19. According to the clinical course, we divided the patients into two groups: the stable group (SG) and the progression group (PG).
The present study was conducted in accordance with the Declaration of Helsinki, and approval was obtained from the Institutional Review Board of Toyonaka Municipal Hospital (No. 2020‐07‐01). The requirement for informed consent was waived via the opt‐out method on our hospital website.
2.1. Medical treatment
We provided basic supportive care, including supplemental oxygen, and treatment with acetaminophen, antibiotics, and mechanical ventilatory support when indicated.
Regarding specific medications, we used ciclesonide for patients with lung infiltrates on imaging, hydroxychloroquine for patients with a high fever (higher than 38℃) or diarrhea, and favipiravir for patients with moderate pneumonia, according to the advice of respiratory disease specialists.
2.2. Definition
A diagnosis of laboratory‐confirmed COVID‐19 was made in cases of the positive direct detection of SARS‐CoV‐2 nucleic acid by RT‐PCR. 6 The classification of the severity of COVID‐19 was as follows: (1) mild to moderate disease with or without pneumonia; (2) severe disease with dyspnea, a respiratory rate more than or equal to 30 breaths/min, a blood oxygen saturation level less than or equal to 93%, a PaO2/FiO2 ratio less than 300, and/or lung infiltrates in more than 50% of the lung field within 24–48 h; and (3) critical disease with respiratory failure, septic shock, or multiple organ dysfunction/failure according to the criteria in the Report of the WHO‐China Joint Mission on COVID‐19. 1 In the present study, progression was defined when a patient required mechanical ventilatory support.
2.3. Statistical analysis
The medians and interquartile ranges (IQRs) are reported for continuous variables. Categorical variables are summarized as frequencies (percentages). We used the values of the potential risk factors affecting disease progression measured at admission, and there was consequently some missing data. Missing continuous variables were imputed using a matrix imputation method, and missing categorical variables were imputed using random proportional methods. Differences in variables between the SG and PG were evaluated using Fisher's exact test for categorical data and the Mann–Whitney U test for continuous data. For the factors that differed significantly between the two groups and could be used to differentiate between the SG and PG, we performed receiver operating characteristic (ROC) curve analysis to determine the cutoff values (COVs) for predicting disease progression. Then, we used a univariate logistic regression model to obtain the odds ratios (ORs) with 95% confidence intervals (CIs) between the two groups according to the COVs for the significant factors. We defined the observation time as the interval from the onset of symptoms to the development of critical disease or discharge. The cumulative rates of progression to critical disease were calculated using the Kaplan–Meier method and compared using the log‐rank test. Similarly, we used univariate Cox proportional hazards models to obtain the hazard ratios (HRs) with 95% CIs.
All reported p values were two‐sided, and p < .05 was considered significant. Statistical analyses were performed with JMP statistical software (ver. 14.3, SAS Institute, Inc.).
3. RESULTS
3.1. Baseline characteristics and clinical course
All consecutive patients with COVID‐19 hospitalized at Toyonaka Municipal Hospital between February 20 and June 10, 2020, were enrolled. There were a total of 74 patients with laboratory‐confirmed COVID‐19. Among these patients, we excluded seven asymptomatic patients, five readmitted patients, one patient transferred from another hospital, seven non‐Japanese patients, and three patients younger than 16 years of age (some patients were included in more than one of these categories). Figure 1 shows the flow chart of patient enrollment. By the end of the follow‐up period, all patients had been discharged, and we ultimately enrolled a total of 57 patients with newly diagnosed laboratory‐confirmed mild to moderate or severe COVID‐19.
Figure 1.
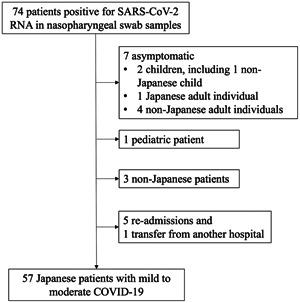
Flow chart of patient enrollment. COVID‐19, coronavirus disease‐2019; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
Table 1 shows the patients' comorbidities, medication use, treatments and clinical courses during hospitalization. Of the 57 patients with mild to moderate COVID‐19, 32 (56.1%) were male, and the median (IQR age was 52 (35–69.5) years. Sixteen patients (28.1%) had a history of close contact with individuals with confirmed COVID‐19 cases. At admission, the median disease duration from symptom onset was 8 (5–12) days.
Table 1.
Characteristics and clinical course of patients with mild to moderate or severe confirmed COVID‐19
| Characteristics | Patients with mild to moderate or severe COVID‐19 N = 57 |
|---|---|
| Age, median (IQR) | 52 (35–69.5) |
| Male sex, n (%) | 32 (56.1) |
| Body mass index, median (IQR) | 23.8 (21.0–26.5) |
| Pneumonia, n (%) | 37 (64.9) |
| Smoking history (none/past/current) | 33/17/7 |
| History of close contact with individuals with confirmed cases | 16 (28.1) |
| Days from onset of symptoms to admission, median (IQR) | 8 (5–12) |
| Comorbidities | |
| Hypertension, n (%) | 16 (28.1) |
| Cardiovascular diseases, n (%) | 5 (8.8) |
| Chronic obstructive pulmonary disease, n (%) | 4 (7.0) |
| Asthma, n (%) | 8 (14.0) |
| Diabetes mellitus, n (%) | 13 (22.8) |
| Hyperlipidemia, n (%) | 20 (35.1) |
| Chronic kidney disease, n (%) | 5 (8.8) |
| Hemodialysis, n (%) | 3 (5.3) |
| Solid tumor, n (%) | 1 (1.8) |
| Pregnancy, n (%) | 2 (3.5) |
| Use of medication for comorbidities | |
| ARB, n (%) | 8 (14.0) |
| Calcium blocker, n (%) | 9 (15.8) |
| Statin, n (%) | 12 (21.1) |
Abbreviations: ARB, angiotensin receptor blocker; COVID‐19, coronavirus disease‐2019; IQR, interquartile range.
Most patients (89.9%) had a fever at disease onset; the second most common symptom was diarrhea (25.9%). In total, 37 (64.9%) and 20 (35.1%) patients had mild to moderate disease and severe disease, respectively. Regarding the medications used to treat COVID‐19, we treated 29 patients with ciclesonide, 14 with hydroxychloroquine, and 12 with favipiravir (Table 2).
Table 2.
Initial presentation, treatment, and clinical course
| Initial presentation | |
|---|---|
| Fever, n (%) | 51 (89.9) |
| Fatigue, n (%) | 12 (21.1) |
| Cough, n (%) | 5 (8.8) |
| Dyspnea, n (%) | 13 (22.8) |
| Sputum production, n (%) | 5 (8.8) |
| Anorexia, n (%) | 7 (12.3) |
| Headache, n (%) | 5 (8.8) |
| Diarrhea, n (%) | 14 (25.9) |
| New loss of taste or smell, n (%) | 9 (15.8) |
| Erythema, n (%) | 3 (5.3) |
| Severity of COVID‐19 | |
| Mild to moderate/severe, n (%) | 37 (64.9)/20 (35.1) |
| Treatment | |
| Required oxygen, n (%) | 20 (35.1) |
| Medication for COVID‐19 | |
| Ciclesonide, n (%) | 29 (50.9) |
| Hydroxychloroquine, n (%) | 14 (24.6) |
| Favipiravir, n (%) | 12 (21.1) |
| Clinical course | |
| Length of hospital stay, median (IQR) (days) | 12 (8–20) |
| Required mechanical ventilatory support, n (%) | 7 (12.3) |
| Mortality, n (%) | 2 (3.5) |
Abbreviation: COVID‐19, coronavirus disease‐2019; IQR, interquartile range.
The median length of hospital stay was 12 (8–20) days. During the hospital stay, seven patients developed critical COVID‐19 (PG: 12.2%), and 50 patients did not experience progression to critical disease (SG: 78.8%). The median time from symptom onset to disease progression was nine days, and the time from admission to progression was one day. Most patients who experienced progression rapidly developed critical disease after admission, but one 61‐year‐old woman developed critical disease 20 days after the onset of symptoms and 9 days after admission. She had been infected with SARS‐CoV‐2 in a previous hospital while she was being treated with high‐dose steroid therapy for nephrotic syndrome. In the PG group, two patients (3.5%) died of COVID‐19. Both were men aged 70 years or older (71 and 75 years old) with diabetes.
3.2. Risk factors affecting disease progression
Compared with the SG, the PG had a significantly higher proportion of patients using statins, a lower lymphocyte count, a higher lactate dehydrogenase (LDH) level, a higher C‐reactive protein (CRP) level, a higher blood urea nitrogen (BUN) level, and a lower estimated glomerular filtration rate (eGFR; Table 3). We evaluated the COVs of these laboratory parameters for predicting disease progression via ROC curve analysis. Our analysis demonstrated that the COVs for the lymphocyte count, LDH level, CRP level, and eGFR were 980 (area under the ROC curve [AUC]: 0.85, sensitivity = 1.00 and specificity = 0.62), 309 (AUC: 0.81, sensitivity = 0.857, and specificity = 0.70), 2.92 mg/dl (AUC: 0.76, sensitivity = 1.00, and specificity = 0.56), and 68 ml/min (AUC: 0.81, sensitivity = 1.00, and specificity = 0.68), respectively. We excluded BUN (COV: 17 mg/dl, AUC: 0.74, sensitivity = 0.71, and specificity = 0.76) because it had lower sensitivity and was a confounding factor for the eGFR. We performed univariate logistic analysis with statin use and the six laboratory factors. This analysis revealed that an elevated LDH level (OR: 14, p = .0188), decreased eGFR (OR: 12.8, p = .0233), decreased lymphocyte count (OR: 9.8, p = .0414), and statin use (OR: 7.0, p = .0230) were significantly associated with disease progression (Table 4).
Table 3.
Comparison of the disease progression group and stable group
| Characteristics | Progression group N = 7 | Stable group N = 50 | p |
|---|---|---|---|
| Age, median (IQR) | 61 (59–71) | 48 (31–69.3) | .1224 |
| Sex, male (%) | 5 (71.4) | 27 (54.0) | .4498 |
| Body mass index, median (IQR) | 24.9 (21.1–25.6) | 24.6 (21.0–22.9) | .6794 |
| Smoking (none/past/current) | 2/3/2 | 31/14/5 | .1860 |
| Days from onset of symptoms to disease progression, median (IQR) | 9 (7–9) | NA | |
| Days from admission to disease progression, median (IQR) | 1 (0–3) | NA | |
| Severity of COVID‐19 mild to moderate/severe | 0/7 | 37/13 | .0003 |
| Mortality | 2 (28.6) | 0 (0) | .0132 |
| Comorbidities | |||
| Any comorbidity, n (%) | 6 (85.7) | 29 (58) | .2303 |
| Hypertension, n (%) | 3 (42.9) | 13 (26.0) | .3878 |
| Cardiovascular diseases, n (%) | 0 (0) | 5 (10.2) | 1.000 |
| Chronic obstructive pulmonary disease, n (%) | 0 (0) | 4 (8.0) | 1.000 |
| Asthma, n (%) | 0 (0) | 8 (16.3) | .5767 |
| Diabetes mellitus, n (%) | 2 (28.6) | 11 (22) | .6592 |
| Hyperlipidemia, n (%) | 4 (57.1) | 16 (32.0) | .2261 |
| Chronic kidney disease, n (%) | 1 (14.3) | 4 (8) | .4940 |
| Hemodialysis, n (%) | 0 (0) | 3 (6.0) | 1.000 |
| Solid tumor, n (%) | 0 (0) | 1 (2.0) | 1.000 |
| Pregnancy, n (%) | 0 (0) | 2 (4.0) | 1.000 |
| Medication | |||
| ARB, n (%) | 1 (14.3) | 7 (14.0) | 1.000 |
| Calcium blocker, n (%) | 3 (42.9) | 6 (12) | .0706 |
| Statin, n (%) | 4 (57.1) | 8 (16) | .0297 |
| Initial assessment | |||
| Days from the onset of symptoms to admission, median (IQR) | 8 (5–9) | 8 (5, 13.3) | .6087 |
| WBC, median (IQR) count/μl | 4600 (3800–7000) | 5950 (4675–7500) | .3187 |
| Lymphocyte, median (IQR) (count/μl) | 617 (374–864) | 1231 (840–1553) | .0033 |
| Neutrophil, median (IQR) (count/μl) | 3795 (2690–5859) | 4049 (3001–5508) | .8458 |
| Hemoglobin, median (IQR) (g/dl) | 14.2 (12.4–14.5) | 14.3 (12.0–15.2) | .8553 |
| Platelet count, median (IQR) (109/L) | 18.7 (14.1–23.2) | 21.1 (16.4–29.3) | .1851 |
| LDH, median (IQR) (IU/L) | 450 (309–562) | 263 (205–323) | .0075 |
| CRP, median (IQR) (mg/dl) | 5.69 (3.73–10.9) | 2.11 (0.2–6.51) | .0261 |
| AST, median (IQR) (IU/L) | 51 (31–85) | 39 (21–55) | .0663 |
| ALT, median (IQR) (IU/L) | 41 (29–63) | 35 (18–58) | .6180 |
| Total bilirubin, median (IQR) (mg/dl) | 0.51 (0.4–1.29) | 0.61 (0.49–0.72) | .6267 |
| HbA1c, median (IQR) (%) | 8.2 (7.9–9.5) | 7.5 (6.8–8.4) | .0843 |
| Cr, median (IQR) (mg/dl) | 0.99 (0.84–1.3) | 0.8 (0.66–1.1) | .0842 |
| BUN, median (IQR) (mg/dl) | 17 (14–25) | 13 (10–17) | .0396 |
| eGFR, median (IQR) | 56.8 (46.8–65.6) | 76.8 (63.5–91.7) | .0093 |
Abbreviations: ARB, angiotensin receptor blocker; AST, aspartate aminotransferase; BUN, blood urea nitrogen; COVID‐19, coronavirus disease‐2019; Cr, creatinine; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; IQR, interquartile range; LDH, lactate dehydrogenase.
Table 4.
Univariate logistic analysis and univariate Cox proportional hazards analysis of risk factors for progression to critical COVID‐19
| Univariate logistic analysis | Univariate Cox proportional hazards analysis | |||||
|---|---|---|---|---|---|---|
| Characteristics | Odds ratio | 95% CI | p | Hazard ratio | 95% CI | p |
| Statin use | ||||||
| No | 1 | 1 | ||||
| Yes | 7.0 | 1.31–37.4 | .0230 | 6.3 | 1.40–28.3 | .0167 |
| Lymphocyte count | ||||||
| ≥980 | 1 | 1 | ||||
| <980 | 9.8 | 1.09–87.7 | .0414 | 7.7 | 0.93–64.1 | .0584 |
| LDH | ||||||
| <309 | 1 | 1 | ||||
| ≥309 | 14.0 | 1.55–123 | .0188 | 11.0 | 1.32–91.4 | .0265 |
| CRP | ||||||
| <2.92 | 1 | 1 | ||||
| ≥2.92 | NC | NC | .9960 | N.C | NC | .9989 |
| eGFR | ||||||
| ≥68 | 1 | 1 | ||||
| <68 | 12.8 | 1.41–115 | .0233 | 10.2 | 1.23–84.8 | .0315 |
Abbreviations: CI, confidence interval; COVID‐19, coronavirus disease‐2019; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; LDH, lactate dehydrogenase; NC, not calculated.
A plot of the progression‐free intervals of the 57 patients is shown in Figure 2A. Univariate Cox regression analysis showed that statin use (HR: 6.28, p = .0167), an elevated LDH level (HR: 11.0, p = .0265), and a decreased eGFR (OR: 10.2 p = .0315) but not lymphocytopenia were significantly associated with disease progression (Figure 2).
Figure 2.
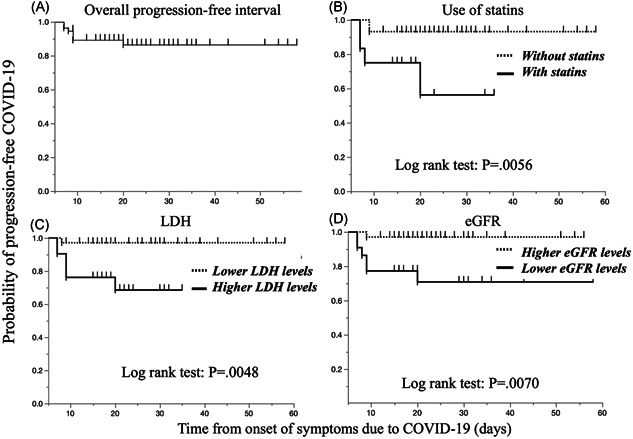
Overall progression‐free interval in patients with mild to moderate COVID‐19. Significant differences were found in the progression‐free interval between patients stratified by statin use, LDH levels and eGFRs at admission. COVID‐19, coronavirus disease‐2019; eGFR, estimated glomerular filtration rate; LDH, lactate dehydrogenase
4. DISCUSSION
This study aimed to identify early clinical factors that are predictive of the progression of mild to moderate COVID‐19 to critical COVID‐19 in a Japanese patient population. To date, several laboratory findings have been reported to be associated with worse outcomes: lower lymphocyte counts, elevated liver enzyme levels, elevated LDH levels, high levels of inflammatory markers (e.g., CRP and ferritin), elevated d‐dimer levels, prolonged prothrombin times, and acute kidney injury. 7 , 8 Our results were consistent with those in previous reports. We identified four candidate risk factors for disease progression in patients with mild to moderate COVID‐19—an elevated LDH level, statin use, a decreased eGFR, and a decreased lymphocyte count—by univariate logistic analysis, and three of these factors (excluding lymphocytopenia) significantly differed when the time‐to‐event was considered. Unlike statin use, the LDH level and eGFR are considered organ damage markers but not inflammatory factors. Recent studies have reported that severe COVID‐19 is commonly complicated with coagulopathy and that disseminated intravascular coagulation may be involved in most deaths. 9 An elevated LDH level and a decreased eGFR can indicate liver and kidney dysfunction. In addition, the lymphocyte count and the CRP level, an inflammatory marker, had high sensitivities but lower specificities (lymphocyte 0.62 and CRP 0.56) than those of those markers (LDH: 0.70 and eGFR 0.68). Therefore, we believe that an elevated LDH level and a decreased eGFR are superior risk factors for the prediction of progression to critical disease or death compared with inflammatory markers.
Regarding clinical factors, advanced age and male sex were reported to be significant risk factors for disease progression, 10 , 11 , 12 but we did not identify these clinical factors as risk factors. Advanced age and comorbid conditions such as diabetes have been reported as risk factors for mortality, 1 , 7 , 13 although severe disease can occur in healthy individuals of any age. 13 , 14 In the present study, we did not identify clinical background parameters as risk factors; however, both of our patients who died were men with diabetes aged 70 years or older, which supports the above previous reports.
In the present study, statin use was identified as a significant risk factor for the development of critical disease. Statins have anti‐inflammatory, antithrombotic, and immunomodulatory effects. 15 , 16 Two observational studies of hospitalized patients with influenza during the 2009 H1N1 pandemic revealed reductions of 41% 17 and 59% 18 in 30‐day mortality rates associated with the use of statins. Those studies showed that statin use could decrease the disease severity in hospitalized patients with influenza. However, a later investigation by Laidler et al. 18 concluded that statins should not be used as an adjunct treatment to prevent death because of unmeasured confounding. We agree with Laidler's conclusion because our cohort showed negative results with statin use, and our patients who used statins were of an advanced age (median, 62 years) and had comorbidities.
This report is the first to evaluate risk factors for disease progression in Japanese patients with COVID‐19. Risk factors may be related to ethnic differences. In the symptomatic Japanese cohort in this study, 65% had mild to moderate disease, and 35% had severe disease; the condition of 12.2% of the patients deteriorated, and two died (3.5%). In a large cohort of Chinese patients, including approximately 44,500 patients with confirmed COVID‐19, 81% of the cases were classified as mild, 14% as severe, and 5% as critical, and the mortality rate was 2.3%. 13 The outcomes in our Japanese cohort were similar to or worse than those in that Chinese population, and there seemed to be no ethnic differences between that Chinese population and the Japanese patients in our cohort.
This study has several limitations due to its retrospective nature. First, we enrolled only a small number of COVID‐19 patients. Consequently, we did not perform multivariate analysis. Second, our data set had missing data because we were not accustomed to treating COVID‐19 patients and avoided unnecessary or nonurgent contact with these patients to reduce the risk of infection. Therefore, we statistically handled missing data using imputation methods in statistical software.
In conclusion, we report for the first time three candidate risk factors for disease progression in Japanese adult patients with mild to moderate COVID‐19: statin use, an elevated LDH level, and a decreased eGFR.
CONFLICT OF INTERESTS
The authors report that they have no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
AUTHOR CONTRIBUTIONS
Takatoshi Higuchi and Tsutomu Nishida contributed equally to this study. Takatoshi Higuchi and Tsutomu Nishida analyzed the data and wrote the manuscript. Takatoshi Higuchi, Tsutomu Nishida, and Yukiyoshi Okauchi collected the data. Takatoshi Higuchi, Tsutomu Nishida, Osamu Morimura, Yasushi Otani, Yukiyoshi Okauchi, and Masaru Yokoe provided medical care for the patients enrolled in this study. Hiromi Iwahashi, Norihiro Suzuki, Masami Inada, and Kinya Abe critically reviewed the manuscript.
ACKNOWLEDGMENTS
We thank all medical staff members and doctors at Toyonaka Municipal Hospital. The collaborators involved in the study are as follows: Sanae Fukuda, Kazumi Ohkubo (Nursing Department), Dr. Masashi Yamamoto, Dr. Kengo Matsumoto, Dr. Kaori Mukai, Dr. Dai Nakamatsu, Dr. Aya Sugimoto, Dr. Naoto Osugi, Dr. Sho Yamaoka, Dr. Tatsuya Sakamoto, Dr. Akino Okamoto, Dr. Yuri Tsujii, Dr. Ryo Sugio, Dr. Kazumasa Souma (Department of Gastroenterology), Dr. Masayuki Moriya, Dr. Katsuya Araki, Dr. Yuri Sugiura (Department of Neurology), Dr. Masanobu Takeji, Dr. Satoko Yamamoto, Dr. Yasuo Kusunoki, Dr. Natsuko Ikeda, Dr. Kumie Teramoto, Dr. Momoko Okawara, Dr. Yuki Iwahashi, Dr. Masashi Yokoyama, Dr. Toru Kida, Dr. Chihiro Hasegawa, Dr. Shunsuke Shiode, Dr. Tomoko Isaka, Dr. Naohiko Ito, Dr. Kanae Matsuno (Department of Internal Medicine), Dr. Yukinori Okazaki, Dr. Yukika Mizukami, Dr. Takuma Iida, Dr. Naoki Fukushima, Dr. Ai Miyaoka, and Dr. Takamori Yamamoto (Department of Cardiology).
Higuchi T, Nishida T, Iwahashi H, et al. Early clinical factors predicting the development of critical disease in Japanese patients with COVID‐19: A single‐center, retrospective, observational study. J Med Virol. 2021;93:0–21412148 10.1002/jmv.26599
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Report of the WHO‐China Joint Mission on Coronavirus Disease 2019. (COVID‐19). 2020. https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf. Accessed June 16, 2020.
- 2. Chan JFW, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Feng Z, Yu Q, Yao S, et al. Early prediction of disease progression in 2019 novel coronavirus pneumonia patients outside Wuhan with CT and clinical characteristics [published online ahead of print February 23, 2020]. medRxiv. 2020. 10.1101/2020.02.19.20025296 [DOI] [Google Scholar]
- 5. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patel A, Jernigan DB. Initial public health response and interim clinical guidance for the 2019 novel coronavirus outbreak ‐ United States, December 31, 2019‐February 4, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(5):140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu Y, Sun W, Li J, et al. Clinical features and progression of acute respiratory distress syndrome in coronavirus disease 2019 [published online ahead of print February 27, 2020]. medRxiv. 2020. 10.1101/2020.02.17.20024166 [DOI] [Google Scholar]
- 12. Cen Y, Chen X, Shen Y, et al. Risk factors for disease progression in patients with mild to moderate COVID‐19‐ a multi‐center observational study. Clin Microbiol Infect. 2020;26(9):1242–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239. [DOI] [PubMed] [Google Scholar]
- 14. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blanco‐Colio LM, Tunon J, Martin‐Ventura JL, Egido J. Anti‐inflammatory and immunomodulatory effects of statins. Kidney Int. 2003;63(1):12–23. [DOI] [PubMed] [Google Scholar]
- 16. Violi F, Calvieri C, Ferro D, Pignatelli P. Statins as antithrombotic drugs. Circulation. 2013;127(2):251–257. [DOI] [PubMed] [Google Scholar]
- 17. Vandermeer ML, Thomas AR, Kamimoto L, et al. Association between use of statins and mortality among patients hospitalized with laboratory‐confirmed influenza virus infections: a multistate study. J Infect Dis. 2012;205(1):13–19. [DOI] [PubMed] [Google Scholar]
- 18. Laidler MR, Thomas A, Baumbach J, et al. Statin treatment and mortality: propensity score‐matched analyses of 2007‐2008 and 2009‐2010 laboratory‐confirmed influenza hospitalizations. Open Forum Infect Dis. 2015;2:ofv028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


