Abstract
Aims
Coronavirus disease 2019 (COVID‐19) is a widespread pandemic with an increased morbidity and mortality, especially for patients with cardiovascular diseases. Angiotensin‐converting enzyme 2 (ACE2) has been identified as necessary cell entry point for SARS‐CoV‐2. Previous animal studies have demonstrated an increased ACE2 expression following treatment with either angiotensin‐converting enzyme inhibitors (ACEi) or angiotensin II receptor blockers (ARB) that have led to a massive precariousness regarding the optimal cardiovascular therapy during this pandemic.
Methods and results
We have measured ACE2 mRNA expression using real‐time quantitative polymerase chain reaction in atrial biopsies of 81 patients undergoing coronary artery bypass grafting and we compared 62 patients that received ACEi/ARB vs. 19 patients that were not ACEi/ARB‐treated. We found atrial ACE2 mRNA expression to be significantly increased in patients treated with an ACEi or an ARB, independent of potential confounding comorbidities. Interestingly, the cardiac ACE2 mRNA expression correlated significantly with the expression in white blood cells of 22 patients encouraging further evaluation if the latter may be used as a surrogate for the former. Similarly, analysis of 18 ventricular biopsies revealed a significant and independent increase in ACE2 mRNA expression in patients with end‐stage heart failure that were treated with ACEi/ARB. On the other hand, cardiac unloading with a left ventricular assist device significantly reduced ventricular ACE2 mRNA expression.
Conclusion
Treatment with ACEi/ARB is independently associated with an increased myocardial ACE2 mRNA expression in patients with coronary artery disease and in patients with end‐stage heart failure. Further trials are needed to test whether this association is deleterious for patients with COVID‐19, or possibly protective. Nevertheless, haemodynamic factors seem to be equally important for regulation of cardiac ACE2 mRNA expression.
Keywords: COVID‐19 pandemic, SARS‐CoV‐2, ACE2, ACE inhibitor, Heart failure, Left ventricular assist device
Treatment with angiotensin‐converting enzyme inhibitors or angiotensin II receptor blockers increases independently myocardial mRNA expression of the SARS‐CoV‐2 cell entry point ACE2 in high‐risk patients.

Introduction
Coronavirus disease 2019 (COVID‐19) is a widespread pandemic with an extensive morbidity and a mortality with up to 10% in certain countries. 1 , 2 , 3 , 4 , 5 Besides typical symptoms like fever, cough or dyspnoea, COVID‐19 is also associated with severe cardiovascular complications, like acute myocardial injury, arrhythmias, or thrombosis. 1 , 2 , 3 , 4 , 5 Importantly, patients with cardiovascular diseases are at increased risk for COVID‐19 infection and exhibit a five‐fold increased COVID‐19‐associated mortality. 1 , 2 , 3 , 4 , 5 , 6
COVID‐19 is caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) that is known to use cell‐bound angiotensin‐converting enzyme 2 (ACE2) for cell entry. 7 Intriguingly, recent investigations have shown that both treatment with angiotensin‐converting enzyme inhibitor (ACEi) or angiotensin II receptor blocker (ARB) leads to an increase in cardiac ACE2 mRNA expression in rats. 8 Moreover, treatment with the ARB olmesartan seems to upregulate urinary ACE2 secretion in hypertensive patients, proposing that upregulation of ACE2 may also be present in humans. 9 Recently, Nicin et al. 10 have demonstrated an increased myocardial ACE2 mRNA expression in patients treated with an ACEi, but the number of investigated patients was very low and important confounding comorbidities have not been investigated. On the other hand, in a large scale cross‐sectional study of heart failure (HF) patients, plasma ACE2 protein levels did not correlate with ACEi/ARB treatment. 11 Although the latter is more functionally relevant, the mechanisms of ACE2 protein plasma shedding are complex and may be differentially affected by HF, angiotensin II signalling and drug treatment. 12 Moreover, it is unclear if non‐membrane bound plasma ACE2 would be relevant for SARS‐CoV‐2 cell entry and damage. The binding of soluble ACE2 protein to SARS‐CoV‐2 in plasma might result in some level of reduction of viral particles and even block early stages of SARS‐CoV‐2 infections, at least in engineered human tissue. 13 On the other hand, soluble ACE2‐SARS‐CoV‐2 fusion particles may also be dangerous since they can interact with endothelial membrane‐bound ACE2 leading to endothelial damage. For instance, epidemiological studies suggest that COVID‐19 patients with cardiovascular diseases and high plasma ACE2 levels have a more than five times increased mortality rate possibly due to endothelial morbidities with thrombotic sequelae. 1 , 3 , 4 , 5 , 14
Despite overwhelming evidence that ACEi/ARB treatment reduces morbidity and mortality in cardiovascular patients, these recent concerns about a potential increase in the susceptibility for SARS‐CoV‐2 infection with potentially deleterious outcome have already encouraged physicians to withhold ACEi/ARB treatment for patients with cardiovascular disease, despite a complete lack of evidence. 6 , 15
The present study adds novel data about the impact of ACEi/ARB treatment on cardiac and white blood cellular ACE2 mRNA expression in high‐risk patients undergoing heart surgery.
Methods
All methods are described in detail in the online supplementary material. This cross‐sectional experimental study is a retrospective analysis of patients enrolled in the observational trial CONSIDER‐AF between May 2016 and August 2018 (online supplementary Figure S1 ). We measured ACE2 mRNA expression using real‐time quantitative polymerase chain reaction in right atrial appendage biopsies of 81 patients and in white blood cells (WBCs) of 22 patients undergoing elective coronary artery bypass grafting. Additionally, we measured ventricular ACE2 mRNA expression of 18 patients with end‐stage HF before and after cardiac unloading with a left ventricular assist device (LVAD). Differences between groups were compared using t‐test for normally distributed continuous variables, the Wilcoxon–Mann–Whitney test for non‐normally distributed continuous variables and the Chi‐square test of independence for categorical variables. If the expected frequencies during contingency analysis were low, Fisher's exact test was used. Univariable regression analyses were conducted with predisposing risk factors as independent variables and with myocardial ACE2 mRNA expression as a dependent variable. To test for interactions, multivariable regression models, including selected independent variables, were calculated. A two‐sided P‐value ≤0.05 was considered statistically significant for all analyses.
Results
Baseline characteristics of patients that donated atrial myocardium
Eighty‐one patients undergoing elective coronary artery bypass grafting donated their right atrial appendage biopsy and were included in the study (Table 1 ). This cohort was stratified according to their medical treatment into patients treated with either ACEi or ARB (n = 62: 46 ACEi, 16 ARB), and patients without ACEi/ARB treatment (n = 19). The patient cohorts did not differ in age, showed equal gender distribution, body mass index (BMI), New York Heart Association (NYHA) status and heart function. However, arterial hypertension and hyperlipidaemia were significantly more common in patients treated with ACEi/ARB (Table 1 ). Consistently, ACEi/ARB‐treated patients were more frequently treated with thiazide diuretics and statins (Table 1 ).
Table 1.
Baseline characteristics of patients undergoing elective coronary artery bypass grafting
| Total cohort (n = 81) | No ACEi/ARB (n = 19) | ACEi/ARB (n = 62) | P‐value | |
|---|---|---|---|---|
| Age, years, mean ± SD | 67.0 ± 8.6 | 68.9 ± 7.5 | 66.5 ± 8.9 | 0.27T |
| Male sex, n (%) | 72 (89) | 16 (84) | 56 (90) | 0.46Chi |
| BMI, kg/m2, mean ± SD | 28.1 ± 4.3 | 28.4 ± 3.8 | 28.0 ± 4.5 | 0.72T |
| Cardiovascular risk factors | ||||
| Arterial hypertension, n (%) | 69 (85) | 13 (68) | 56 (90) | 0.02 Chi |
| Systolic blood pressure, mmHg, mean ± SD | 137.9 ± 20.6 | 137.6 ± 21.7 | 138.0 ± 20.4 | 0.94T |
| Diastolic blood pressure, mmHg, mean ± SD | 77.7 ± 11.1 | 78.4 ± 11.1 | 77.5 ± 11.2 | 0.74T |
| Smoker, n (%) | 52 (64) | 15 (79) | 37 (60) | 0.13Chi |
| Previous smoker | 38 (47) | 12 (63) | 26 (42) | 0.10Chi |
| Current smoker | 14 (17) | 3 (16) | 11 (18) | 0.84Chi |
| Diabetes mellitus, n (%) | 21 (26) | 7 (37) | 14 (23) | 0.21Chi |
| Hyperlipidaemia, n (%) | 52 (64) | 7 (37) | 45 (73) | 0.004 Chi |
| Atrial fibrillation, n (%) | 9 (11) | 3 (16) | 6 (10) | 0.46Chi |
| History of TIA or stroke, n (%) | 8 (10) | 3 (16) | 5 (8) | 0.32Chi |
| Heart function | ||||
| Heart failure a , n (%) | 30 (37) | 7 (37) | 23 (37) | 0.98Chi |
| NYHA functional class b , n (%) | ||||
| I | 14 (17) | 4 (21) | 10 (16) | 0.62Chi |
| II | 16 (20) | 3 (16) | 13 (21) | 0.62Chi |
| III–IV | 0 (0) | 0 (0) | 0 (0) | 1.00Chi |
| NT‐proBNP, pg/mL, median (IQR) | 259 (87–705) | 166 (73–881) | 259 (99–705) | 0.85W |
| LVEF, %, mean ± SD | 56.5 ± 8.6 | 57.2 ± 7.0 | 56.4 ± 9.1 | 0.72T |
| LVEF <50%, n (%) | 11 (14) | 2 (11) | 9 (15) | 0.66Chi |
| LAVI, mL/m2, mean ± SD | 42.4 ± 16.3 | 45.3 ± 5.3 | 41.6 ± 18.6 | 0.74T |
| LVMI, g/m2, mean ± SD | 83.5 ± 26.4 | 92.8 ± 40.4 | 81.0 ± 22.0 | 0.34T |
| Diastolic dysfunction, n (%) | 38 (47) | 8 (42) | 30 (48) | 0.77Chi |
| GFR, mL/min, mean ± SD | 75.6 ± 21.8 | 75.8 ± 17.7 | 75.6 ± 23.0 | 0.98T |
| Medical treatment, n (%) | ||||
| ACEi | 46 (57) | 0 (0) | 46 (74) | <0.001 Chi |
| ARB | 16 (20) | 0 (0) | 16 (26) | 0.01 Chi |
| CCB | 26 (32) | 4 (21) | 22 (35) | 0.24Chi |
| MRA | 3 (4) | 0 (0) | 3 (5) | 0.33Chi |
| Beta‐blockers | 56 (69) | 12 (63) | 44 (71) | 0.52Chi |
| Loop diuretics | 14 (17) | 3 (16) | 11 (18) | 0.84Chi |
| Thiazide diuretics | 21 (26) | 1 (5) | 20 (32) | 0.02 Chi |
| Statins | 61 (75) | 11 (58) | 50 (81) | 0.04 Chi |
Note: Bold value shown comparison is statistically significant. ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; CCB, calcium channel blocker; Chi, Chi‐square test; GFR, glomerular filtration rate; IQR, interquartile range; LAVI, left atrial volume index; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro brain natriuretic peptide; NYHA, New York Heart Association; SD, standard deviation; T, Student's t‐test; TIA, transient ischaemic attack; W, Wilcoxon rank‐sum test.
Requirement of symptoms and LVEF <40% or elevated NT‐proBNP levels and either left ventricular hypertrophy (left ventricular mass index ≥115 g/m2 for male, ≥95 g/m2 for female patients), left atrial enlargement (LAVI >34 mL/m2), or diastolic dysfunction.
If heart failure was diagnosed.
Atrial angiotensin‐converting enzyme 2 mRNA expression is increased in patients treated with angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker
Figure 1 shows the frequency distribution of atrial ACE2 mRNA expression (normalized to β‐actin) for the total patient cohort (Figure 1A ) and for the patient subgroups stratified according to ACEi/ARB treatment (Figure 1B ). In addition, scatter plots stratified according to treatment with ACEi/ARB are presented (Figure 1B ). Importantly, compared to untreated patients, there was a significant increase in atrial ACE2 mRNA expression about 1.41‐fold from (in % of β‐actin) 0.37 ± 0.16 to 0.52 ± 0.32 in patients treated with ACEi/ARB (P = 0.009; Figure 1B ). This corresponds to an effect size (Cohen's d) of 0.58, which represents a medium effect size with potential biological significance. This increase was consistent across both patients treated with either ACEi or ARB although the latter did not reach statistical significance (online supplementary Figure S3A ).
Figure 1.
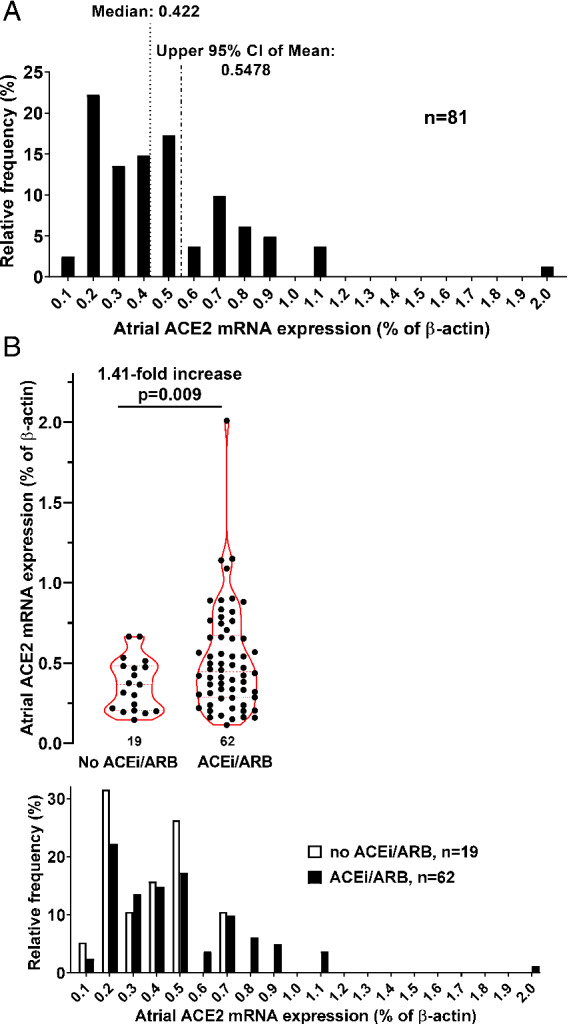
Atrial angiotensin‐converting enzyme 2 (ACE2) mRNA expression in patients treated with angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker (ACEi/ARB). (A) Frequency distribution of ACE2 expression. Vertical lines indicate median and upper 95% confidence interval (CI). (B) Scatter and violin plots of atrial ACE2 mRNA expression stratified by patients treated with either ACEi/ARB or not and the corresponding frequency distribution underneath.
Since ACE2 can also be found in WBCs and may be used as a biomarker for clinical routine, we also measured ACE2 mRNA expression in WBCs of a subgroup of 22 patients. The frequency distribution plot (online supplementary Figure S2A ) indicates a much lower absolute expression level compared to the atrium. On the other hand, there was a significant correlation in the ACE2 mRNA expression level between atrium and the corresponding blood (R2 = 0.26, P = 0.02; online supplementary Figure S2B ) with higher ACE2 mRNA levels in patients treated with ACEi/ARB (online supplementary Figure S2C ).
The increase in atrial angiotensin‐converting enzyme 2 mRNA expression was independent of clinical covariates
The lack of randomization implies that heterogeneities in the patient cohorts may substantially confound our results. Therefore, we performed multiple univariate logistic and linear regression analysis of atrial ACE2 mRNA expression. We defined an ACE2 mRNA expression >0.5478 as increased, which corresponds to the upper 95% confidence interval derived from the frequency distribution of the total cohort (Figure 1A ). In both the logistic and linear models, cardiac ACE2 mRNA expression was increased in patients treated with ACEi/ARB, but only in the linear model this increase reached statistical significance (P = 0.052 vs. P = 0.04; Figure 2 and online supplementary Table S 3 ). Intriguingly, presence of HF and elevated N‐terminal pro brain natriuretic peptide (NT‐proBNP) levels were associated with increased ACE2 mRNA expression levels in both logistic and linear regression models (Figure 2 and online supplementary Table S 3 ). On the other hand, there was a significant negative association of BMI with increased atrial ACE2 mRNA expression (Figure 2 and online supplementary Table S 3 ). Importantly, neither the presence of arterial hypertension nor hyperlipidaemia or pharmacological treatment with mineralocorticoid receptor antagonists (MRAs), thiazides or statins affected atrial ACE2 mRNA expression (Figure 2 and online supplementary Table S 3 ).
Figure 2.
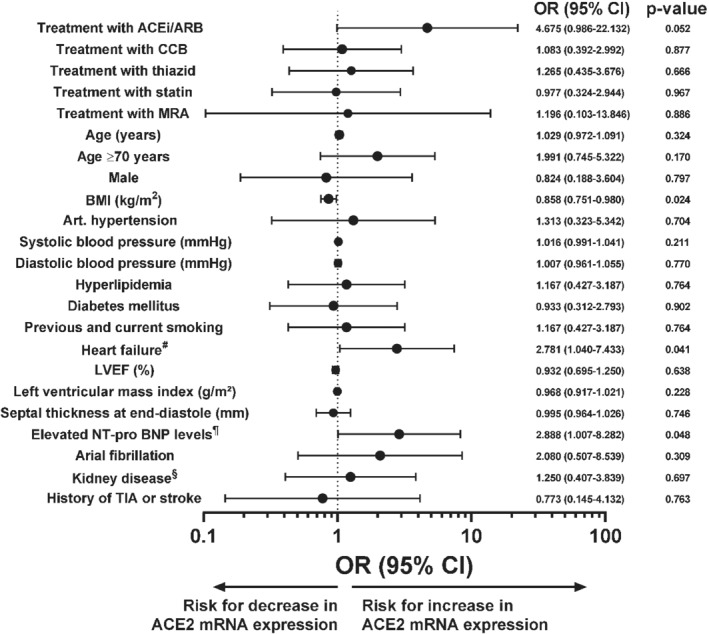
Univariate logistic regression analysis for atrial angiotensin‐converting enzyme 2 (ACE2) mRNA expression. Forest plot showing odds ratio (OR) and 95% confidence interval (CI) derived from univariate logistic regression analysing the risk of increased atrial ACE2 mRNA expression (>0.5478) for multiple clinical variables. ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; CCB, calcium channel blocker; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro brain natriuretic peptide; TIA, transient ischaemic attack. #Requirement of symptoms and LVEF <40% or elevated NT‐proBNP levels and either left ventricular hypertrophy (left ventricular mass index ≥115 g/m2 for male, ≥95 g/m2 for female patients), left atrial enlargement (left atrial volume index >34 mL/m2), or diastolic dysfunction. ¶>300 pg/mL for patients without atrial fibrillation, >900 pg/mL for patients with atrial fibrillation. §Glomerular filtration rate <60 mL/min.
To further analyse the impact of potential confounders, a multivariate logistic and linear regression analysis was performed including advanced age (age ≥70 years), BMI, presence of HF, atrial fibrillation and history of transient ischaemic attack or stroke into the model (Table 2 and online supplementary Table S 3 ). Importantly, the association of increased ACE2 mRNA expression with ACEi/ARB treatment was independent of all analysed comorbidities suggesting that there may be a mechanistic link. To further explore the impact of ACEi/ARB treatment on ACE2 expression, subgroups of patients were analysed for the mean difference in atrial ACE2 mRNA expression between patients treated with ACEi/ARB and untreated patients by linear regression and weighting by sample size. Figure 3 indicates that the impact of ACEi/ARB treatment on ACE2 mRNA expression levels was maintained across all investigated subgroups including older patients (age ≥70 years), patients with increased BMI, patients with arterial hypertension, hyperlipidaemia, diabetes mellitus, HF, or kidney disease.
Table 2.
Multivariate logistic regression analysis for factors that influence increased atrial angiotensin‐converting enzyme 2 mRNA expression (n = 81)
| OR (95% CI) | P‐value | |
|---|---|---|
| Multivariate model for increased ACE2 mRNA expression | 0.026 | |
| Treatment with ACEi/ARB | 5.745 (1.047–31.533) | 0.044 |
| Age ≥70 years | 1.964 (0.640–6.024) | 0.238 |
| Male sex | 0.844 (0.149–4.749) | 0.847 |
| BMI (kg/m2) | 0.892 (0.769–1.034) | 0.130 |
| Heart failure a | 2.327 (0.747–7.253) | 0.145 |
| Atrial fibrillation | 2.647 (0.520–13.484) | 0.241 |
| History of TIA or stroke | 0.794 (0.105–5.997) | 0.823 |
Note: Bold value shown comparison is statistically significant. Increased ACE2 mRNA expression was defined as >0.5478.
ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; CI, confidence interval; OR, odds ratio; TIA, transient ischaemic attack.
Requirement of symptoms and left ventricular ejection fraction <40% or elevated N‐terminal pro brain natriuretic peptide levels and either left ventricular hypertrophy (left ventricular mass index ≥115 g/m2 for male, ≥95 g/m2 for female patients), left atrial enlargement (left atrial volume index >34 mL/m2), or diastolic dysfunction.
Figure 3.
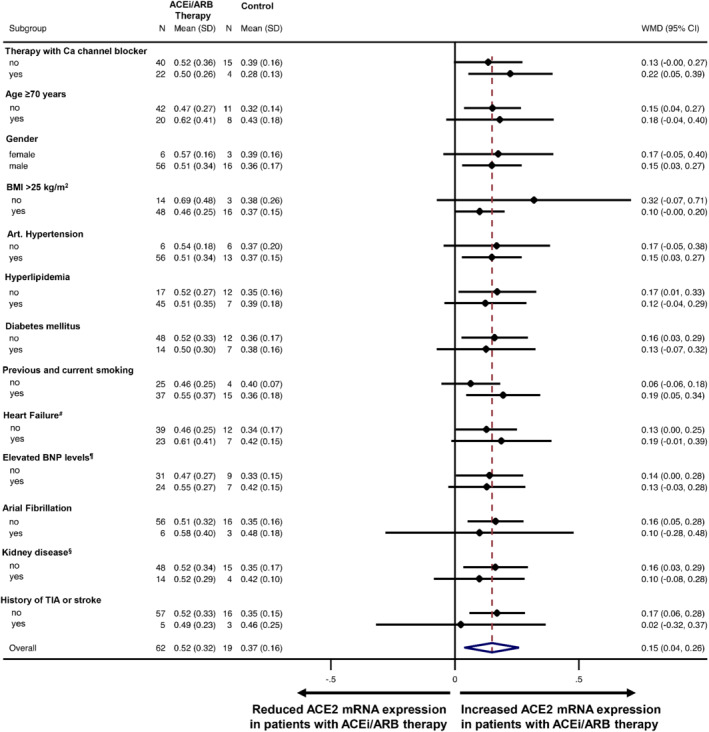
The effect of angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker (ACEi/ARB) therapy on angiotensin‐converting enzyme 2 (ACE2) mRNA expression. The mean difference in ACE2 mRNA expression (and 95% confidence interval, CI) between patients treated with ACEi/ARB vs. patients not treated is shown as forest plot. The mean difference was weighted by sample size (weighted mean difference, WMD). BMI, body mass index; BNP, brain natriuretic peptide; Ca, calcium; TIA, transient ischaemic attack. #Requirement of symptoms and left ventricular ejection fraction <40% or elevated N‐terminal proBNP levels and either left ventricular hypertrophy (left ventricular mass index ≥115 g/m2 for male, ≥95 g/m2 for female patients), left atrial enlargement (left atrial volume index >34 mL/m2), or diastolic dysfunction. ¶>300 pg/mL for patients without atrial fibrillation, >900 pg/mL for patients with atrial fibrillation. §Glomerular filtration rate <60 mL/min.
Baseline characteristics of patients with end‐stage heart failure
Heart failure has been shown to be associated with an increased ACE2 expression. 16 , 17 Moreover, we have shown here a significant association of HF and elevated NT‐proBNP levels with atrial ACE2 mRNA expression (Figure 2 ). On the other hand, only 37% of coronary artery bypass graft patients presented with HF (Table 1 ) and only 5% of patients had HF with reduced ejection fraction. Therefore, we additionally analysed left ventricular ACE2 mRNA expression in 18 patients with end‐stage HF that were scheduled for implantation of an LVAD and were later subjected to heart transplantation. The baseline characteristics of these patients stratified according to treatment with ACEi/ARB (n = 12) vs. patients without ACEi/ARB (n = 6) are shown in online supplementary Table S 1 . The latter six HF patients were not treated with an ACEi/ARB since they were either haemodynamically unstable (acute decompensated HF or cardiogenic shock), or they suffered from severe symptomatic hypotension. The mean duration on LVAD (about 300 days) was not different between groups. All patients were middle‐aged and both groups showed a similar gender distribution. While there was no difference in the prevalence of arterial hypertension, diabetes mellitus, or atrial fibrillation, ACEi/ARB‐treated patients were more frequently treated with MRAs (10/12 vs. 2/6; online supplementary Table S 1 ) and beta‐blockers (11/12 vs. 2/6; online supplementary Table S 1 ).
Ventricular angiotensin‐converting enzyme 2 mRNA expression was increased in heart failure patients treated with angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker
Figure 4 shows the frequency distribution of ventricular ACE2 mRNA expression (normalized to hypoxanthine ribosyltransferase) for patients subjected to LVAD implantation (Figure 4A ). Importantly, at LVAD implantation, ventricular ACE2 mRNA expression was approximately quadrupled in patients that were treated with an ACEi/ARB inhibitor (to 198.5 ± 75.0%) compared to patients without ACEi/ARB inhibition (44.7 ± 43.1%, P = 0.0004; Figure 4B ). This increase was consistent across both patient groups treated with either ACEi or ARB (online supplementary Figure S3B ). Interestingly, we also observed a strong trend towards an increased ACE2 mRNA expression in patients treated with MRAs (P = 0.10) or beta‐blockers (P = 0.08; online supplementary Table S2 ).
Figure 4.
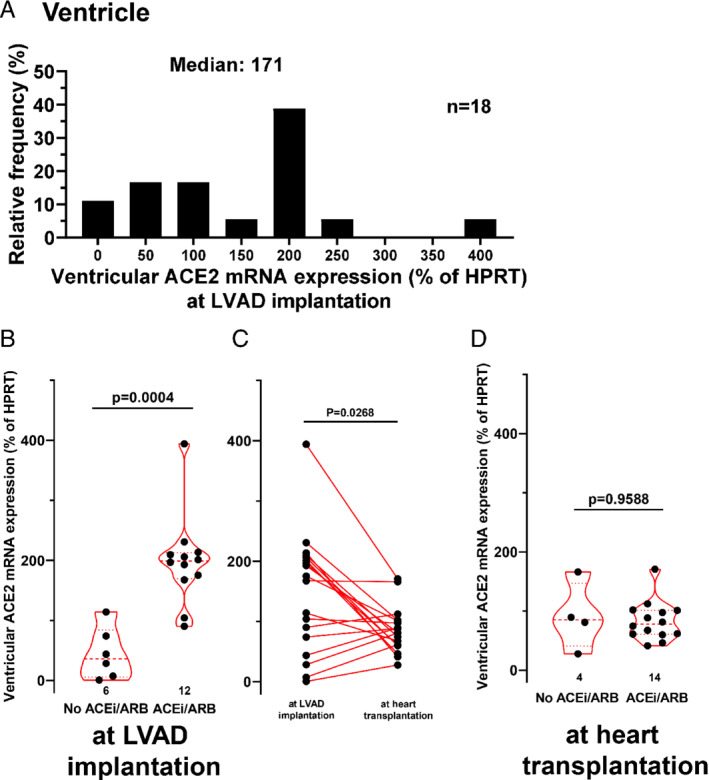
Ventricular angiotensin‐converting enzyme 2 (ACE2) mRNA expression in failing hearts. (A) Frequency distribution of ventricular ACE2 mRNA expression at left ventricular assist device (LVAD) implantation. (B) Scatter and violin plots of ventricular ACE2 mRNA expression stratified by patients treated with either angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker (ACEi/ARB) or not. (C) Spaghetti plot of ventricular ACE2 mRNA expression at LVAD implantation and at heart transplantation (about 300 days later). (D) Scatter and violin plots of ACE2 mRNA expression at heart transplantation. HPRT, hypoxanthine ribosyltransferase.
To further analyse the clinical covariates that may influence ventricular ACE2 expression at LVAD implantation we conducted a multivariate linear regression analysis accounting for the treatment with ACEi/ARB, age, BMI, atrial fibrillation, treatment with beta‐blockers, treatment with MRAs, diabetes mellitus, and the glomerular filtration rate (online supplementary Table S 2 ). Pre‐existing treatment of patients with ACEi/ARB was independently associated with increased ventricular ACE2 mRNA expression at LVAD implantation (P = 0.01 for ACEi/ARB treatment, R2 = 0.72 and P = 0.04 for the whole multivariate model; online supplementary Table S 2 ).
Cardiac unloading by LVAD is known to induce structural, molecular and genomic alterations in the heart that may also affect ACE2 expression. 18 Therefore, we measured ventricular ACE2 mRNA expression of the same patients after about 300 days of LVAD therapy at heart transplantation (Figure 4C and 4D). Interestingly, there was a significant reduction in ventricular ACE2 mRNA expression upon LVAD treatment (Figure 4C ) indicating that ventricular ACE2 expression may be reduced by haemodynamic improvement. Moreover, at heart transplantation, the ventricular ACE2 mRNA expression was reduced by LVAD therapy to such an extent that there was no detectable difference between patients treated with ACEi/ARB and untreated patients (Figure 4D ).
Discussion
In this study we investigated the effect of ACEi/ARB treatment on cardiac mRNA expression of ACE2, the cell entry mechanism of SARS‐CoV‐2, in a larger scale cohort of patients undergoing heart surgery and in patients with end‐stage HF. Interestingly, this is the first study demonstrating that treatment with ACEi/ARB was significantly and independently associated with increased cardiac ACE2 mRNA expression in patients at high risk of severe COVID‐19. In addition, we found no difference between ACEi or ARB treatment on ACE2 mRNA expression. On the other hand, we showed that in patients with end‐stage HF, cardiac unloading by LVAD therapy led to a significant reduction of ventricular ACE2 mRNA expression abolishing the impact of ACEi/ARB treatment. This suggests that – in addition to ACEi/ARB treatment – haemodynamic factors or cardiovascular diseases may also be important for the regulation of cardiac ACE2 expression. This is in accordance with observational and retrospective clinical trials showing no harm by continuing ACEi/ARB treatment in patients with COVID‐19 and cardiovascular disease. 19 , 20 , 21
Potential regulation of SARS‐CoV‐2 cell entry mechanism angiotensin‐converting enzyme 2 by angiotensin‐converting enzyme/angiotensin II receptor blocker treatment
Since December 2019, COVID‐19 is spreading from Wuhan, China, all over the world with dramatic infection, morbidity and mortality rates. 1 , 2 , 3 , 4 , 5 It is induced by SARS‐CoV‐2, which uses ACE2 for cell entry. 7 COVID‐19 is frequently associated with severe cardiovascular complications and cardiovascular patients suffering from COVID‐19 have a dramatically increased mortality rate with an increased risk of life‐threatening arrhythmias and myocardial injury. 1 , 2 , 3 , 4 , 5 , 6 A recent study has demonstrated that 56% of COVID‐19 patients with initial chest pain and ST‐segment elevation had non‐coronary myocardial injury, indicating that direct effects of SARS‐CoV‐2 (e.g. potentially via ACE2) on the myocardium may be important. 22
Angiotensin‐converting enzyme 2 is a key enzyme that counteracts the renin–angiotensin system by degrading angiotensin I to angiotensin‐(1–9) and angiotensin II to angiotensin‐(1–7), thereby alleviating the detrimental angiotensin signalling. 14 , 17 In fact, a protective effect of ACE2 in the context of severe inflammation‐dependent lung injury has been shown by multiple studies. 23 , 24 , 25 Interestingly, the beneficial effect may be not limited to membrane‐bound ACE2 since administration of recombinant human ACE2 has been proven beneficial in improving lung pathologies. 23 , 24 , 25 Therefore, the fact that ACEi/ARB treatment leads to a significant and independent increase in myocardial ACE2 mRNA expression may even be beneficial for patients with COVID‐19 infection. In accordance, there are already a few studies showing that ACEi/ARB treatment does not increase the risk for SARS‐CoV‐2 infection or even improve the outcome if COVID‐19 develops. 19 , 26 , 27
Recently, several animal studies have suggested that ACE2 expression may be upregulated by ACEi/ARB inhibition. Ferrario et al. 8 have shown that treatment of rats with lisinopril or losartan (only eight rats each) resulted in increased cardiac ACE2 mRNA expression. Other animal studies have suggested an upregulation of ACE2 by ACEi/ARB treatment also in murine kidney arterioles 28 and in the aorta of hypertensive rats. 29 On the other hand, Burchill and colleagues did not detect a change in cardiac ACE2 mRNA expression following ACEi or ARB treatment in rats. 30
In contrast to animal data, very few studies have addressed ACE2 expression in humans. In a Japanese study comparing 101 healthy controls with 100 hypertensive patients under antihypertensive therapy, only olmesartan treatment (13 patients) was associated with increased urinary ACE2 secretion, while neither enalapril, losartan, candesartan, valsartan nor telmisartan showed that effect. 9 In this context, a large scale observational study has recently shown that plasma ACE2 protein levels do not correlate with existing ACEi/ARB therapy in HF patients. 11 The relevance of these finding, however, is unclear, since urinary or plasma ACE2 level do not necessarily correlate with membranous ACE2 expression – and only the latter is relevant for SARS‐CoV‐2 cell entry. 7 , 17 Most recently, Nicin et al. 10 demonstrated an increased ACE2 mRNA expression in patients treated with ACEi compared to patients with an ARB. Unfortunately, this study was dramatically limited due to a very small sample size of four vs. two patients, respectively. 10 In addition, they have not examined the impact of potentially cofounding patients comorbidities like age, gender distribution, BMI, arterial hypertension, or atrial fibrillation. 10
Angiotensin‐converting enzyme/angiotensin II receptor blocker treatment increased cardiac angiotensin‐converting enzyme 2 mRNA expression in high‐risk patients
To improve our understanding of regulation of ACE2 expression, we have investigated cardiac ACE2 mRNA expression in a high‐risk patient population. We show here that ACE2 mRNA expression was increased in a large scale cohort of 81 patients undergoing heart surgery and in patients with end‐stage HF that were treated with ACEi/ARB. Importantly, we also found several other patient characteristics that are associated with differential ACE2 mRNA expression, like obesity or HF. Accounting for that, we conducted a multivariate logistic regression analysis showing that treatment with ACEi/ARB was independently associated with increased myocardial ACE2 mRNA expression. Interestingly, we observed a strong but non‐significant trend towards increased ACE2 mRNA expression in WBCs of patients treated with ACEi/ARB. Moreover, there was a significant correlation of cardiac and white blood cellular ACE2 mRNA expression. This may be in contrast to plasma ACE2 levels that are shed from various cell types including endothelial cells into the plasma. Plasma ACE2 levels have not been shown to correlate with ACEi/ARB treatment. 11 Nevertheless, this finding may offer a starting point for future studies investigating white blood cellular ACE2 expression as a potential biomarker for ACE2 expression in other organs and its biological impact, e.g. in lungs of COVID‐19 patients.
Importantly, we found the presence of HF as well as elevated NT‐proBNP levels to be significantly associated with increased atrial ACE2 mRNA expression (Figure 2 ), which is in accordance with previous studies. 16 , 17 To further investigate ACE2 regulation in end‐stage HF, we analysed ventricular ACE2 mRNA expression in 18 patients before and after cardiac unloading with LVAD therapy. Intriguingly, we were able to detect an increased cardiac ACE2 mRNA expression in end‐stage HF patients treated with ACEi/ARB therapy. Sama et al. 11 reported that patients with HF that were treated with MRAs exhibited increased plasma ACE2 protein levels. Therefore, we included treatment with MRAs in multivariate regression analysis showing a strong trend for increased ACE2 mRNA expression in patients treated with MRAs (P = 0.10; online supplementary Table S 2 ). However, when this regression analysis was corrected for multiple covariates including ACEi/ARB treatment, the association between MRA treatment and ACE2 mRNA expression levels was completely abolished (online supplementary Table S 2 ). In contrast, the association between ACEi/ARB treatment and ACE2 mRNA expression was maintained in multivariate regression analysis with similar slope and level of significance (online supplementary Table S 2 ). Therefore, it remains unclear if MRA treatment alone without accompanying ACEi/ARB treatment is able to influence cardiac ACE2 expression levels. At first glance, our data appear to be in contrast to Sama et al., 11 who showed a significant increase in ACE2 plasma protein levels in HF patients treated with MRA but reduced ACE2 plasma protein levels in patients treated with ACEi/ARB even after correction for concomitant drug treatment. A possible explanation for the discrepancies may arise from the methodological differences across the studies. Sama et al. 11 measured ACE2 protein in plasma and not in myocardium. Plasma ACE2 proteins are derived from proteolytic shedding of membrane‐bound ACE2 and are originated from different tissue sources (endothelium, myocardium, etc.). It has been shown that angiotensin II‐mediated activation of ADAM‐17 stimulates the proteolytic shedding of ACE2 into the plasma. 12 Thus, inhibition of angiotensin II‐mediated activation of ADAM‐17 by ACEi/ARB treatment reduces ACE2 plasma shedding, which would result in reduced plasma ACE2 but increased tissue ACE2 levels. MRAs, on the other hand, directly act on ACE2 transcription leading to an increase in ACE2 mRNA and a secondary increase in ACE2 protein both membrane‐bound and shed into plasma. 14 Therefore, our study is in accordance with Sama et al. We suggest here that ACEi/ARB treatment increases cardiac ACE2 levels also by stimulation of cardiac ACE2 transcription possibly by reducing circulating aldosterone levels. However, futures studies are required to exactly identify the mechanism of ACEi/ARB‐dependent transcriptional regulation of ACE2 and its impact on cardiac membrane‐bound ACE2 protein levels.
Regulation of angiotensin‐converting enzyme 2 mRNA expression by cardiac unloading with left ventricular assist device
We show here that cardiac ACE2 mRNA expression was dramatically reduced after LVAD implantation, indicating that improved haemodynamics following cardiac unloading may impact cardiac ACE2 expression. A part of this regulation may in fact result from altered levels of renin, angiotensin II and aldosterone. Interestingly, LVAD support, by normalizing blood pressure, has been shown to lower renin and aldosterone levels. However, in apparent contrast, cardiac angiotensin I and II levels were increased 5–10‐fold post‐LVAD implantation. 18 As stated above, angiotensin II stimulates the proteolytic shedding of ACE2 into the plasma, which would reduce cardiac membrane‐bound ACE2. 12 Aldosterone, on the other hand, increases ACE2 transcription. Therefore, the unique combination of reduced aldosterone but increased cardiac angiotensin II levels after cardiac unloading may be responsible for the observed reduction in cardiac ACE2 expression. Importantly, considering that 14 of 18 patients had been treated with ACEi/ARB, the cardiac increase in angiotensin II upon LVAD must have been dramatic exceeding the impact of ACEi/ARB treatment on cardiac ACE2 levels.
Limitations
While ACE2 is the main cell entry mechanism for SARS‐CoV‐2, 7 it is important to note that we have not measured directly the susceptibly for an infection or for a deleterious disease progression in vivo.
Another limitation originates from the methodology of the present paper. We measured ACE2 mRNA levels but not protein expression. An impaired translation into the functional relevant protein, an altered subcellular protein localization or an increased protein degradation may hamper the transferability of mRNA data to protein levels. Also, we have not differentiated mRNA expression between cardiac cell types. However, since more than 90% of cardiac tissue consists of cardiomyocytes, this issue may be less important.
The analysis of WBC ACE2 mRNA expression may also be subject to limitation. While we did find a significant correlation between cardiac and WBC ACE2 mRNA expression, we did not observe a significant increase in WBC ACE2 mRNA expression in patients treated with ACEi/ARB despite an almost doubled mean expression (online supplementary Figure S2C ). Although we cannot exclude a mechanistic explanation, we assume that the small number of patients – especially the relatively low number of patients without ACEi/ARB treatment (n = 5) – precludes statistical significance for this comparison between two patient groups. In contrast to the comparison between patients treated with or without ACEi/ARB, the correlation analysis of WBC ACE2 mRNA expression with cardiac ACE2 mRNA expression included all 22 patients into a linear model. The increased power of this type of analysis may explain why we were able to detect a significant correlation of cardiac and blood ACE2 mRNA expression.
This imbalance in the number of patients treated with or without ACEi/ARB may also apply to the main study cohort. From the 81 patients analysed, there were only 19 patients not treated with ACEi/ARB. Despite our multiple attempts to control for confounders, imbalances in patient characteristics cannot be completely eliminated.
Conclusion
Treatment with ACEi/ARB was associated with increased cardiac ACE2 mRNA expression independent of many comorbidities. In end‐stage HF patients, ACE2 mRNA expression was dramatically affected by ventricular unloading suggesting that haemodynamic factors may also be pivotal for regulation of cardiac ACE2 expression. Given the dramatic clinical benefit evoked by ACEi/ARB therapy especially in high‐risk patients with HF and the potential strong influence of other comorbidities on ACE2 expression, treatment with ACEi/ARB should not be withheld in these high‐risk patient populations, at least not until data from randomized clinical trials prove otherwise.
Supporting information
Appendix S1. Supporting Information.
Appendix S2. Figures.
Acknowledgements
We greatly appreciate the excellent and outstanding technical assistance of Gabriela Pietrzyk and Thomas Sowa. We acknowledge the expert statistical review of Florian Zeman (Center for Clinical Studies, University Medical Center Regensburg). Open access funding enabled and organized by Projekt DEAL.
Funding
S.W. is funded by DFG grants WA 2539/4–1, 5–1, 7–1, and 8–1. L.S.M. is funded by DFG grants MA 1982/5–1 and 7–1. S.W. and L.S.M. are also supported by the DFG SFB 1350 grant (project no. 387509280, TPA6), are funded by the ReForM C program of the faculty and by the DZHK (Deutsches Zentrum für Herz‐Kreislauf‐Forschung; German Centre for Cardiovascular Research). Philips Respironics.
Conflict of interest: M.A. has received research grants and lecture fees from Philips Respironics and ResMed. CONSIDER‐AF was supported by grants from Philips Respironics and the Medical Faculty at the University of Regensburg.
References
- 1. Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Biondi‐Zoccai G, Brown TS, Der Nigoghossian C, Zidar DA, Haythe J, Brodie D, Beckman JA, Kirtane AJ, Stone GW, Krumholz HM, Parikh SA. Cardiovascular considerations for patients, health care workers, and health systems during the COVID‐19 pandemic. J Am Coll Cardiol 2020;75:2352–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol 2020;5:802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020;46:846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kuster GM, Pfister O, Burkard T, Zhou Q, Twerenbold R, Haaf P, Widmer AF, Osswald S. SARS‐CoV2: should inhibitors of the renin–angiotensin system be withdrawn in patients with COVID‐19? Eur Heart J 2020;41:1801–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoffmann M, Kleine‐Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, O'Gallagher PE. Effect of angiotensin‐converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin‐converting enzyme 2. Circulation 2005;111:2605–2610. [DOI] [PubMed] [Google Scholar]
- 9. Furuhashi M, Moniwa N, Mita T, Fuseya T, Ishimura S, Ohno K, Shibata S, Tanaka M, Watanabe Y, Akasaka H, Ohnishi H, Yoshida H, Takizawa H, Saitoh S, Ura N, Shimamoto K, Miura T. Urinary angiotensin‐converting enzyme 2 in hypertensive patients may be increased by olmesartan, an angiotensin II receptor blocker. Am J Hypertens 2015;28:15–21. [DOI] [PubMed] [Google Scholar]
- 10. Nicin L, Abplanalp WT, Mellentin H, Kattih B, Tombor L, John D, Schmitto JD, Heineke J, Emrich F, Arsalan M, Holubec T, Walther T, Zeiher AM, Dimmeler S. Cell type‐specific expression of the putative SARS‐CoV‐2 receptor ACE2 in human hearts. Eur Heart J 2020;41:1804–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sama IE, Ravera A, Santema BT, van Goor H, Ter Maaten JM, Cleland JG, Rienstra M, Friedrich AW, Samani NJ, Ng LL, Dickstein K, Lang CC, Filippatos G, Anker SD, Ponikowski P, Metra M, van Veldhuisen DJ, Voors AA. Circulating plasma concentrations of angiotensin‐converting enzyme 2 in men and women with heart failure and effects of renin‐angiotensin‐aldosterone inhibitors. Eur Heart J 2020;41:1810–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patel VB, Clarke N, Wang Z, Fan D, Parajuli N, Basu R, Putko B, Kassiri Z, Turner AJ, Oudit GY. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM‐17: a positive feedback mechanism in the RAS. J Mol Cell Cardiol 2014;66:167–176. [DOI] [PubMed] [Google Scholar]
- 13. Monteil V, Kwon H, Prado P, Hagelkrüys A, Wimmer RA, Stahl M, Leopoldi A, Garreta E, Hurtado Del Pozo C, Prosper F, Romero JP, Wirnsberger G, Zhang H, Slutsky AS, Conder R, Montserrat N, Mirazimi A, Penninger JM. Inhibition of SARS‐CoV‐2 infections in engineered human tissues using clinical‐grade soluble human ACE2. Cell 2020;181:905–913.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, Raizada MK, Grant MB, Oudit GY. Angiotensin‐converting enzyme 2: SARS‐CoV‐2 receptor and regulator of the renin‐angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res 2020;126:1456–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID‐19 infection? Lancet Respir Med 2020;8:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goulter AB, Goddard MJ, Allen JC, Clark KL. ACE2 gene expression is up‐regulated in the human failing heart. BMC Med 2004;2:E1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McKinney CA, Fattah C, Loughrey CM, Milligan G, Nicklin SA. Angiotensin‐(1‐7) and angiotensin‐(1‐9): function in cardiac and vascular remodelling. Clin Sci 2014;126:815–827. [DOI] [PubMed] [Google Scholar]
- 18. Klotz S, Danser AHJ, Foronjy RF, Oz MC, Wang J, Mancini D, D'Armiento J, Burkhoff D. The impact of angiotensin‐converting enzyme inhibitor therapy on the extracellular collagen matrix during left ventricular assist device support in patients with end‐stage heart failure. J Am Coll Cardiol 2007;49:1166–1174. [DOI] [PubMed] [Google Scholar]
- 19. Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie J, Liu YM, Zhao YC, Huang X, Lin L, Xia M, Chen MM, Cheng X, Zhang X, Guo D, Peng Y, Ji YX, Chen J, She ZG, Wang Y, Xu Q, Tan R, Wang H, Lin J, Luo P, Fu S, Cai H, Ye P, Xiao B, Mao W, Liu L, Yan Y, Liu M, Chen M, Zhang XJ, Wang X, Touyz RM, Xia J, Zhang BH, Huang X, Yuan Y, Rohit L, Liu PP, Li H. Association of inpatient use of angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID‐19. Circ Res 2020;126:1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li J, Wang X, Chen J, Zhang H, Deng A. Association of renin‐angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID‐19) infection in Wuhan, China. JAMA Cardiol 2020;5:825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gao C, Cai Y, Zhang K, Zhou L, Zhang Y, Zhang X, Li Q, Li W, Yang S, Zhao X, Zhao Y, Wang H, Liu Y, Yin Z, Zhang R, Wang R, Yang M, Hui C, Wijns W, McEvoy JW, Soliman O, Onuma Y, Serruys PW, Tao L, Li F. Association of hypertension and antihypertensive treatment with COVID‐19 mortality: a retrospective observational study. Eur Heart J 2020;41:2058–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bangalore S, Sharma A, Slotwiner A, Yatskar L, Harari R, Shah B, Ibrahim H, Friedman GH, Thompson C, Alviar CL, Chadow HL, Fishman GI, Reynolds HR, Keller N, Hochman JS. ST‐segment elevation in patients with Covid‐19 – a case series. N Engl J Med 2020;382:2478–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gu H, Xie Z, Li T, Zhang S, Lai C, Zhu P, Wang K, Han L, Duan Y, Zhao Z, Yang X, Xing L, Zhang P, Wang Z, Li R, Yu JJ, Wang X, Yang P. Angiotensin‐converting enzyme 2 inhibits lung injury induced by respiratory syncytial virus. Sci Rep 2016;6:19840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zou Z, Yan Y, Shu Y, Gao R, Sun Y, Li X, Ju X, Liang Z, Liu Q, Zhao Y, Guo F, Bai T, Han Z, Zhu J, Zhou H, Huang F, Li C, Lu H, Li N, Li D, Jin N, Penninger JM, Jiang C. Angiotensin‐converting enzyme 2 protects from lethal avian influenza a H5N1 infections. Nat Commun 2014;5:3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus‐induced lung injury. Nat Med 2005;11:875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, Hausvater A, Newman JD, Berger JS, Bangalore S, Katz SD, Fishman GI, Kunichoff D, Chen Y, Ogedegbe G, Hochman JS. Renin‐angiotensin‐aldosterone system inhibitors and risk of Covid‐19. N Engl J Med 2020;382:2441–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bean DM, Kraljevic Z, Searle T, Bendayan R, O'Gallagher K , Pickles A, Folarin A, Roguski L, Noor K, Shek A, Zakeri R, Shah AM, Teo JT, Dobson RJ. Angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers are not associated with severe COVID‐19 infection in a multi‐site UK acute hospital trust. Eur J Heart Fail 2020;22:967–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Soler MJ, Ye M, Wysocki J, William J, Lloveras J, Batlle D. Localization of ACE2 in the renal vasculature: amplification by angiotensin II type 1 receptor blockade using telmisartan. Am J Physiol Renal Physiol 2009;296:F398–F405. [DOI] [PubMed] [Google Scholar]
- 29. Igase M, Strawn WB, Gallagher PE, Geary RL, Ferrario CM. Angiotensin II AT1 receptors regulate ACE2 and angiotensin‐(1‐7) expression in the aorta of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 2005;289:H1013–H1019. [DOI] [PubMed] [Google Scholar]
- 30. Burchill LJ, Velkoska E, Dean RG, Griggs K, Patel SK, Burrell LM. Combination renin‐angiotensin system blockade and angiotensin‐converting enzyme 2 in experimental myocardial infarction: implications for future therapeutic directions. Clin Sci 2012;123:649–658. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.
Appendix S2. Figures.


