Abstract
Background
The effect of timing of exposure to first Plasmodium falciparum infections during early childhood on the induction of innate and adaptive cytokine responses and their contribution to the development of clinical malaria immunity is not well established.
Methods
As part of a double-blind, randomized, placebo-controlled trial in Mozambique using monthly chemoprophylaxis with sulfadoxine-pyrimethamine plus artesunate to selectively control timing of malaria exposure during infancy, peripheral blood mononuclear cells collected from participants at age 2.5, 5.5, 10.5, 15, and 24 months were stimulated ex vivo with parasite schizont and erythrocyte lysates. Cytokine messenger RNA expressed in cell pellets and proteins secreted in supernatants were quantified by reverse-transcription quantitative polymerase chain reaction and multiplex flow cytometry, respectively. Children were followed up for clinical malaria from birth until 4 years of age.
Results
Higher proinflammatory (interleukin [IL] 1, IL-6, tumor necrosis factor) and regulatory (IL-10) cytokine concentrations during the second year of life were associated with reduced incidence of clinical malaria up to 4 years of age, adjusting by chemoprophylaxis and prior malaria exposure. Significantly lower concentrations of antigen-specific T-helper 1 (IL-2, IL-12, interferon-γ) and T-helper 2 (IL-4, IL-5) cytokines by 2 years of age were measured in children undergoing chemoprophylaxis compared to children receiving placebo (P < .03).
Conclusions
Selective chemoprophylaxis altering early natural exposure to malaria blood stage antigens during infancy had a significant effect on T-helper lymphocyte cytokine production >1 year later. Importantly, a balanced proinflammatory and anti-inflammatory cytokine signature, probably by innate cells, around age 2 years was associated with protective clinical immunity during childhood.
Clinical Trials Registration
Keywords: Plasmodium falciparum, cytokines, age, exposure, immunity
Prevention of exposure to Plasmodium falciparum infection during infancy significantly impacted antigen-specific T-helper 1 and 2 cytokine responses at age 2 years, and protection from clinical malaria was associated with balanced proinflammatory and regulatory cytokine/chemokine signatures characteristic of innate immune cells.
In endemic areas, clinical malaria primarily affects children <5 years of age [1]. Exposure to repeated Plasmodium falciparum infections from birth leads to the development of naturally acquired immunity, which is attained faster against the most severe forms of malaria and takes longer against milder forms but is never sterilizing [2]. Young children exposed to P. falciparum are at high risk from suffering malaria complications until they have developed partial clinical immunity, but the immune mechanisms involved and their determinants are not fully elucidated. Specifically, cellular immune correlates of protection against P. falciparum have been less characterized [3], in contrast to antibodies that are known to exert an antiparasitic effect [4].
Cytokines and chemokines mediate cellular immune responses and contribute in part to some of the symptoms and pathological alterations during malarial disease [5]. The outcome of the infection depends on the regulation of a network of proinflammatory and regulatory immune responses, leading to protection or immunopathology [6, 7]. There are few field data available on the relevance of individual cytokines or chemokines in acquired immunity to malaria. To date, the factors shown to be potentially implicated in protective immunity include interferon gamma (IFN-γ) and tumor necrosis factor (TNF) produced by T cells that may inhibit parasite development and destroy infected hepatocytes [8, 9]; IFN-γ and memory T cells that activate macrophages to phagocyte parasitized erythrocytes and merozoites [10]; and interleukin (IL) 10, which is produced by regulatory T lymphocytes and other cells that control pathogenesis [11]. Antigen-specific T-helper 1 (TH1) responses are clearly involved in protection against malaria in animal models [12], but human data are scarcer. IL-12, a key TH1 cytokine produced mainly by antigen-presenting cells, induces and regulates dendritic cell maturation and function, in addition to promoting the activation and IFN-γ production of T cells and natural killer (NK) cells [13]. IFN-γ and IL-2 T-helper cell responses, as well as γδ T cells, are induced after P. falciparum experimental infection in naive individuals; IFN-γ has been associated with malaria protection [14], and IL-2 may be key for the generation of effector responses to malaria [15]. Although proinflammatory and TH1 signatures correlate with immunity, it is not clear if they reflect innate rather than protective adaptive immune responses, particularly in the immature immune system of a child [16]. Reexposure to P. falciparum has been associated with acquisition of antigen-specific IL-10 immunoregulatory responses that dampen pathogenic inflammation while enhancing antiparasite effector mechanisms [11, 17].
Most previous studies of cytokine responses and malaria immunity have been done in newborn cord blood samples [18, 19], in adult populations [20], or in cross-sectional studies after the onset of clinical symptoms [21–23]. Few have investigated the early acquisition of P. falciparum–specific cytokine responses in asymptomatic or healthy young infants and their relationship with development of clinical immunity in prospective cohorts [7, 9, 11, 24, 25] or how they are affected by malaria chemoprevention or therapeutic tools [26, 27]. Data reported have not shown consistent patterns. For example, in Gambian children, chemoprophylaxis resulted in higher lymphoproliferative responses and IFN-γ production [28]. In Ugandan children, chemoprophylaxis was associated with higher production of IL-2 and TNF, which was associated with malaria protection, and lower production of IL-10 and IFN-γ, which was associated with malaria risk [27]. Also, Kenyan children sleeping under bednets had decreased production of proinflammatory cytokines TNF, IL-1, and IL-6 [29].
We conducted a double-blind, randomized, placebo-controlled trial in Mozambique administering monthly chemoprophylaxis with sulfadoxine-pyrimethamine (SP) plus artesunate (AS) to selectively control the age of first infection by blood stage P. falciparum during infancy, in order to understand the role of parasite exposure in the acquisition of immunity to malaria [30]. This study set out to elucidate the role of age and exposure to P. falciparum in the induction of cytokine responses and their role in immunity in young children. To this end, we measured cellular mediators produced by blood leukocytes after parasite antigen or mock stimulation to identify those associated with prospective risk of malaria.
MATERIALS AND METHODS
Study Design
The study was conducted at the Centro de Investigação em Saúde de Manhiça, Maputo Province, southern Mozambique, from September 2005 to March 2009, and has been described in detail elsewhere [30]. In brief, it consisted of a double-blind, randomized placebo-controlled trial (RCT) including 349 newborns from the Maragra village receiving monthly chemoprophylaxis with SP plus AS or placebo administered during different periods of the first year of life according to the randomization group: late exposure, early exposure, or control (Supplementary Methods and Figure 1). Study participants were followed up until age 24 months as part of the RCT. Weekly active case detection was conducted from birth to approximately age 10.5 months and monthly home visits from 10.5 to 24 months of age. Children presenting fever were taken to the Maragra Health Post, where they were examined and parasitemia and hematocrit were determined. Additionally, passive case detection was carried out at the Maragra Health Post and Manhiça District Hospital through the continuous morbidity surveillance system to monitor attendance to the outpatient clinics and admissions to hospital; data were analyzed until children were 4 years of age. A 1-mL blood sample was collected into ethylenediaminetetraacetic acid microtainers by finger-prick at the 5 cross-sectional visits by month [M] 2.5, 5.5, 10.5, 15, and 24; at the first clinical malaria episode (if any, acute); and 1 month later (convalescence). Approval for the study was obtained from the national ethical review committee of Mozambique and the ethical review committee of Hospital Clínic in Barcelona, Spain. Children were enrolled in the study after their guardians provided written informed consent.
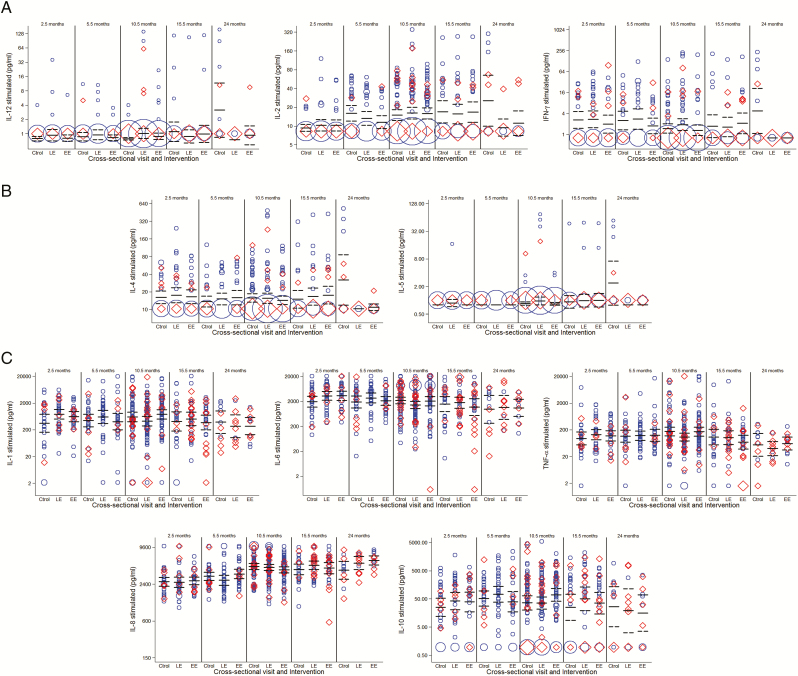
Figure 1.
Weighted scattered plots of Plasmodium falciparum antigen-specific cytokine concentrations (pg/mL) in each study group and at each cross-sectional visit, showing geometric means and 95% confidence intervals. A, T-helper 1 (TH1) cytokines: interleukin (IL) 12, IL-2, interferon-γ. B, T-helper 2 (TH2) cytokines: IL-4, IL-5. C, Proinflammatory and regulatory cytokines: IL-1, IL-6, tumor necrosis factor, IL-8, and IL-10. Tables 1 and 2 show the outcomes of the statistical tests for the comparisons that had significantly different cytokine responses. The area of the symbol is proportional to the number of observations; those with previous or current P. falciparum infections are shown in red. In the case of TH1 and TH2 cytokines at the cross-sectional visit at 24 months, most concentration values were low or undetectable for the late-exposure and early-exposure groups (ie, larger area of red and blue symbols at the bottom). Abbreviations: Ctrol, control; EE, early exposure; IFN, interferon; IL, interleukin; LE, late exposure; TNF, tumor necrosis factor.
Laboratory Procedures
Standard laboratory methods were used to assess parasitological and hematological parameters [30, 31]. Peripheral blood mononuclear cells (PBMCs) were isolated using a Lymphoprep gradient and resuspended in complete medium. A total of 1.2 million fresh PBMCs were stimulated with 20 μL of a P. falciparum (3D7 strain) schizont extract corresponding to lysate from 2 million synchronized infected red blood cells (iRBCs) or with 20 μL of uninfected red blood cells (uRBCs). After incubation for 24 hours, 48 hours, or 72 hours, the supernatants were collected and frozen at –80ºC. The PBMC pellets were collected in Trizol and frozen at –80ºC. At the end of the field study, supernatants were thawed and cytokine concentrations (IL-12p70, IFN-γ, IL-2, IL-10, IL-8, IL-6, IL-4, IL-5, IL-1β, TNF, TNF-β) were measured with the Bender MedSystems Human Th1/Th2 11plex FlowCytomix Multiplex Kit [32]. Cytokine messenger RNA (mRNA) levels (IL-2, IL-4, IL-6, IL-10, IFN-γ, TNF, IL-13), normalized to the reference gene RPL13a, were measured in a subsample by reverse-transcription quantitative polymerase chain reaction. A high level of cytokine production detected in culture supernatants of unstimulated infant samples are considered biologically relevant and were therefore not subtracted from the stimulated samples, but shown side by side [19, 33]. Cytokine concentrations were analyzed in relation to plasma antibody responses to P. falciparum blood stage antigens [34].
Definitions and Statistical Methods
Clinical malaria was defined as axillary temperature ≥37.5°C or a reported fever in the preceding 24 hours with any positive parasitemia from the blood slide microscopy, using a case definition previously validated in the area for this age group [35]. Cytokine concentrations (pg/mL) were logarithmically transformed and the average within the groups was presented as geometric mean with 95% confidence interval. Differences among treatment groups at different timepoints were estimated by analysis of variance and evaluated using a likelihood ratio test and a global P value for significance. Correlations with antibody levels within and between visits were done by Spearman. To analyze factors independently associated with cytokine concentrations, we used mixed-effects regression models including relevant covariates. Cytokine concentrations in relation to incidence of malaria were assessed by negative binomial regressions, unadjusted and after adjusting by relevant covariates. Data analysis was performed using Stata version 11 (StataCorp, College Station, Texas) and R studio. Statistical significance was defined at P < .05.
RESULTS
A total of 1712 blood samples were collected from children and processed during the field study: 318 at M2.5, 295 at M5.5, 290 at M10.5, 273 at M15, 301 at M24, 129 at first acute malaria episode, and 106 at convalescence. Data presented here are based on the analysis of a random subgroup of 643 sets of supernatants (iRBC and uRBC stimulated: M2.5, 93; M5.5, 95; M10.5, 220; M15, 89; M24, 35; 59 acute and 52 convalescent). In addition, 316 sets of cell pellets in Trizol (iRBC and uRBC stimulated, 632 total) were processed for mRNA cytokine analysis (M2.5, 59; M5.5, 60; M10.5, 192; M15, 59; M24, 11; 10 acute and 9 convalescent). A pilot study was conducted to select the optimal timepoint among 24, 48, and 72 hours for cytokine detection in supernatants and in Trizol pellets after PBMC stimulation with iRBC using samples from 24 immune adults and 28 infants. Data indicated that the 24-hour timepoint was the optimal for cytokine detection [19]. The correlations between mRNA expression in PBMC pellets and concentration in culture supernatants for quantifiable cytokines were moderate-high for IL-6, TNF, and IL-10, which had the highest production, but low for the others (Supplementary Figure 2).
Factors Affecting Cytokine Concentrations
SP plus AS chemoprophylaxis during M2.5–M5.5 or M5.5–M10.5 significantly affected the production of TH1 and T-helper 2 (TH2) cytokines in PBMCs collected at M24 following iRBC antigen stimulation but not upon uRBC mock stimulation. Thus, children who had been chemotherapy-suppressed in year 1 had significantly lower supernatant concentrations of IL-12 (P = .01), IFN-γ (P = .002), IL-2 (P = .03), IL-4 (P = .005), and IL-5 (P = .01) at M24 compared with continuously exposed controls (Figure 1). Chemoprophylaxis had no significant impact on the proinflammatory (IL-1, IL-6, TNF, TNF-β, IL-8) or the regulatory (IL-10) cytokine concentrations (Tables 1 and 2).
Table 1.
Effect of Chemoprophylaxis on the Magnitude of Cytokine Production at Cross-Sectional Visits
| Month | ||||||
|---|---|---|---|---|---|---|
| Cytokine | 2.5 | 5.5 | 10.5 | 15 | 24 | |
| TH1 cytokines | ||||||
| IL - 12 | iRBC | X | ||||
| uRBC | ||||||
| IL - 2 | iRBC | X | ||||
| uRBC | ||||||
| IFN - γ | iRBC | X | ||||
| uRBC | ||||||
| TH2 cytokines | ||||||
| IL - 4 | iRBC | X | ||||
| uRBC | ||||||
| IL - 5 | iRBC | X | ||||
| uRBC | ||||||
| Proinflammatory cytokines | ||||||
| IL - 1β | iRBC | |||||
| uRBC | ||||||
| IL - 6 | iRBC | |||||
| uRBC | ||||||
| TNF | iRBC | |||||
| uRBC | ||||||
| TNF - β | iRBC | |||||
| uRBC | ||||||
| Regulatory cytokines | ||||||
| IL - 10 | iRBC | |||||
| uRBC | ||||||
| Proinflammatory chemokine | ||||||
| IL - 8 | iRBC | |||||
| uRBC |
Heatmap of all cytokines with statistically significant differences shown with “x.” All other empty fields indicate non-statistically significant differences.
Abbreviations: IFN, interferon; IL, interleukin; iRBC, infected red blood cell; TH1, T-helper 1; TH2, T-helper 2; TNF, tumor necrosis factor; uRBC, uninfected red blood cell.
Table 2.
Effect of Chemoprophylaxis on the Magnitude of Cytokine Production at Cross-Sectional Visits
| Cytokine | Proportional Differencea (95% CI) vs Control | P Value | ||
|---|---|---|---|---|
| Late Exposure | Early Exposure | |||
| TH1 | ||||
| IL - 12 | iRBC | 0.25 (.08–.75) | 0.29 (.10–.83) | .0178 |
| uRBC | NS | |||
| IL - 2 | iRBC | 0.35 (.15–.84) | 0.45 (.20–1.01) | .0337 |
| uRBC | NS | |||
| IFN-γ | iRBC | 0.20 (.06–.66) | 0.15 (.05–.47) | .0024 |
| uRBC | NS | |||
| TH2 | ||||
| IL - 4 | iRBC | 0.35 (.16–.77) | 0.33 (.16–.70) | .0055 |
| uRBC | NS | |||
| IL - 5 | iRBC | 0.38 (.16–.90) | 0.31 (.14–.71) | .0116 |
| uRBC | NS |
Statistically significant proportional differences (95% CIs) in chemoprophylaxis groups compared to control group in the case of TH1 and TH2 cytokines at month 24. P value from linear regression model using likelihood ratio test.
Abbreviations: CI, confidence interval; IFN, interferon; IL, interleukin; iRBC, infected red blood cell; NS, not significant; TH1, T - helper 1; TH2, T - helper 2; uRBC, uninfected red blood cell.
aDifference adjusted by clinical malaria episodes before visit.
Clinical malaria episodes significantly affected the magnitude of some cytokine responses. Overall, proinflammatory cytokines and IL-10 were higher during the acute phase compared to levels preceding clinical disease and declined at convalescence. Differences were significant for the secreted proteins and mRNA transcripts (Table 3).
Table 3.
Effect of a Clinical Malaria Episode on the Magnitude of Cytokine Production After Stimulation With Plasmodium falciparum Lysate at the Acute and Convalescent Visits in Relation to the Preacute Visit
| Cytokine | Timepoint | No. | Geometric Mean | Proportional Difference | (95% CI) | P Value |
|---|---|---|---|---|---|---|
| IFN - γ | Preacute | 40 | 1.77 | 1 | … | .0022 |
| Secreted | Acute | 59 | 4.05 | 2.25 | (1.19–4.28) | |
| Convalescent | 51 | 1.46 | 0.81 | (.42–1.57) | ||
| IL - 2 | Preacute | 52 | 13.98 | 1 | … | .0117 |
| Secreted | Acute | 59 | 10.05 | 0.72 | (.57–.91) | |
| Convalescent | 52 | 10.22 | 0.73 | (.57–.93) | ||
| IL - 4 | Preacute | 52 | 15.43 | 1 | … | .0240 |
| Secreted | Acute | 59 | 12.69 | 0.82 | (.66–1.02) | |
| Convalescent | 52 | 11.22 | 0.72 | (.58–.91) | ||
| IL - 2 | Preacute | 31 | 0.00 | 1 | … | .0140 |
| mRNA | Acute | 10 | 0.00 | 1.05 | (.46–2.43) | |
| Convalescent | 16 | 0.01 | 2.85 | (1.40–5.81) | ||
| IFN - γ | Preacute | 36 | 0.62 | 1 | … | <.0001 |
| mRNA | Acute | 13 | 3.90 | 5.41 | (3.04–9.64) | |
| Convalescent | 16 | 0.91 | 1.44 | (.83–2.48) | ||
| TNF | Preacute | 37 | 3.29 | 1 | … | <.0001 |
| mRNA | Acute | 13 | 13.13 | 3.67 | (2.18–6.16) | |
| Convalescent | 16 | 6.36 | 1.86 | (1.15–3.02) | ||
| IL - 10 | Preacute | 37 | 8.11 | 1 | … | .0057 |
| mRNA | Acute | 13 | 24.18 | 2.63 | (1.41–4.91) | |
| Convalescent | 16 | 7.48 | 0.97 | (.54–1.75) |
P values are from regression models using likelihood ratio test. Only significant differences are shown.
Abbreviations: CI, confidence interval; IFN, interferon; IL, interleukin; mRNA, messenger RNA; TNF, tumor necrosis factor.
Other factors evaluated in relation to cytokine production (maternal infection, parity, season, birth weight, etc) did not show any specific or consistent association, except for weight-for-age z score that was negatively associated with levels of proinflammatory and anti-inflammatory cytokines (Supplementary Table 1). Exposure to previous or current P. falciparum infection was not associated with cytokine concentrations. Age significantly affected the production of some cytokines during the first year of life. IL-6 levels were higher at M2.5 and declined gradually (Figure 1). IL-2 and IL-8 showed a steady increase with age, whereas IL-1 and TNF did not vary during most of the first year.
Effect of Cytokine Concentrations on Clinical Malaria
Higher concentrations of proinflammatory cytokines in PBMC supernatants by the end of the first year of life were associated with reduced incidence of clinical malaria up to M24. Tables 4 and 5 show the effect of a 2-fold increment in cytokine levels at M10.5 on the incidence of clinical malaria up to M24, adjusted by treatment, season, neighborhood, malaria infection at visit and before visit, maternal infection, congenital infection, inflammation in the placenta, use of insecticide-treated nets and indoor residual spraying. This was statistically significant for TNF and a trend was observed for IL-1, IL-6, and IL-8. Furthermore, as part of an extended follow-up analysis (Supplementary Figure 1), higher secretion of proinflammatory and also regulatory cytokines by PBMCs at M24, either spontaneous production or following P. falciparum antigen stimulation, was associated with lower risk of subsequent clinical malaria up to M36 and M48 (Tables 4 and 5).
Table 4.
Effect of the Magnitude of Cytokine Production on Subsequent Incidence of Clinical Malaria: Heatmap of All Cytokines and Chemokines in Supernatants
| Month | |||||||
|---|---|---|---|---|---|---|---|
| Cytokine | 2.5–24 | 5.5–24 | 10.5–24 | 15–24 | 24–36 | 24–48 | |
| TH1 cytokines | |||||||
| IL - 12 | iRBC | ||||||
| uRBC | |||||||
| IL - 2 | iRBC | ||||||
| uRBC | |||||||
| IFN-γ | iRBC | ||||||
| uRBC | |||||||
| TH2 cytokines | |||||||
| IL4 | iRBC | ||||||
| uRBC | |||||||
| IL5 | iRBC | ||||||
| uRBC | |||||||
| Proinflammatory cytokines | |||||||
| IL - 1 | iRBC | X | X | X | |||
| uRBC | X | X | X | ||||
| IL - 6 | iRBC | X | X | X | |||
| uRBC | X | X | X | ||||
| TNF | iRBC | X | |||||
| uRBC | X | X | X | ||||
| Proinflammatory chemokine | |||||||
| IL - 8 | iRBC | X | |||||
| uRBC | X | X | X | ||||
| Regulatory cytokine | |||||||
| IL - 10 | iRBC | X | X | ||||
| uRBC | X | X |
Statistically significant or trend differences indicating a reduction in the incidence of clinical malaria are shown in marked boxes, and nonsignificant differences are empty. Incidence rate ratios with 95% confidence intervals and p values for statistically significant or trend differences for pro-inflammatory and regulatory cytokines and chemokines are shown on Table 5.
Abbreviations: IFN, interferon; IL, interleukin; iRBC, infected red blood cell; TH1, T-helper 1; TH2, T-helper 2; TNF, tumor necrosis factor; uRBC, uninfected red blood cell.
Table 5.
Effect of the Magnitude of Cytokine Production on Subsequent Incidence of Clinical Malaria
| Cytokine | Cytokines at 10.5 mo on Malaria Risk up to 24 mo | Cytokines at 24 mo on Malaria Risk up to 36 mo | Cytokines at 24 mo on Malaria Risk up to 48 mo | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IRR | (95% CI) | P Value | IRR | (95% CI) | P Value | IRR | (95% CI) | P Value | ||
| IL - 1 | iRBC | 0.93 | (.85–1.01) | .078 | 0.69 | (.48–.99) | .030 | 0.72 | (.51–1.02) | .077 |
| uRBC | 0.92 | (.84–1.01) | .072 | 0.74 | (.58–.95) | .018 | 0.76 | (.59–.98) | .049 | |
| IL - 6 | iRBC | 0.93 | (.84–1.03) | .152 | 0.73 | (.54–1.00) | .034 | 0.77 | (.56–1.04) | .112 |
| uRBC | 0.92 | (.83–1.02) | .107 | 0.73 | (.55–.97) | .016 | 0.77 | (.59–1.01) | .076 | |
| TNF | iRBC | 0.88 | (.79–.97) | .014 | … | … | NS | … | … | NS |
| uRBC | 0.89 | (.80–.99) | .037 | 0.6 | (.41–.88) | .006 | 0.62 | (.43–.89) | .022 | |
| IL - 10 | iRBC | … | … | NS | 0.82 | (.67–1.00) | .044 | 0.85 | (.72–1.00) | .089 |
| uRBC | … | … | NS | 0.79 | (.66–.94) | .005 | 0.77 | (.66–.90) | .002 | |
| IL - 8 | iRBC | 0.74 | (.53–1.03) | .073 | … | … | NS | … | … | NS |
| uRBC | 0.73 | (.52–1.03) | .068 | 0.23 | (.04–1.30) | .072 | 0.17 | (.02–1.13) | .031 |
Shown are IRRs with 95% CI and P values for statistically significant or trend differences, for proinflammatory and regulatory cytokines and chemokines.
Abbreviations: CI, confidence interval; IL, interleukin; iRBC, infected red blood cell; IRR, incidence rate ratio; mo, months; NS, not significant; TNF, tumor necrosis factor; uRBC, uninfected red blood cell.
Correlations Among Cytokine and Antibody Responses
Different patterns were observed at each visit. Pro- and anti-inflammatory cytokines were highly and positively correlated from the younger ages, except for IL-8, which was negatively correlated to the rest, while TH1 and TH2 cytokines correlated positively among themselves only as age increased (Supplementary Figure 3). With time, weak-moderate correlations of inflammatory with T-helper cytokines transitioned from negative to positive. In addition, TNF and IL-6 at M10.5 weakly correlated positively with TH2 cytokines at M24 (Supplementary Figure 4).
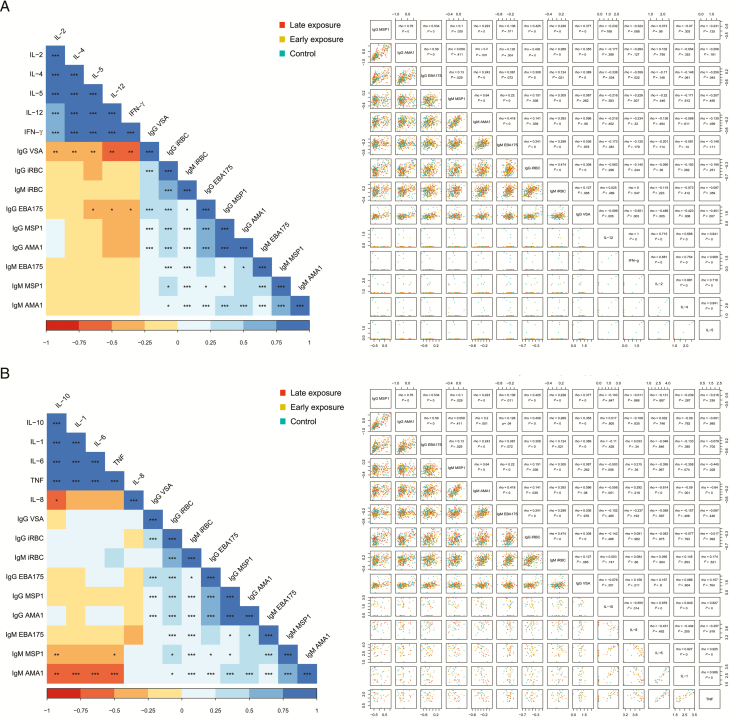
We tested associations of cytokines affected by chemoprophylaxis or involved in malaria protection with antibody responses [34]. At M24, T-helper cytokines correlated positively with M5.5 immunoglobulin M (IgM) to P. falciparum lysate, and negatively with M5.5 immunoglobulin G (IgG) to merozoite surface protein 1 (MSP1) and erythrocyte-binding antigen 175 (EBA175), M10.5 IgG to variant surface antigen (VSA), and various antimalarial antibodies at M15 (Supplementary Figure 5A and 5B) and M24, particularly IgG to VSA and EBA175 (Figure 2A).
Figure 2.
Heatmaps and scatterplots of the correlations between antibody levels and cytokine concentrations after stimulation with Plasmodium falciparum schizont lysate (log-transformed) at the indicated study visits. A, Antibodies vs T-helper 1 and 2 cytokines at month 24. B, Antibodies vs proinflammatory and regulatory cytokines at month 24. Spearman coefficients and P values: *<.05, **<.01, ***<.001. Abbreviations: AMA1, apical membrane antigen; EBA175, erythrocyte-binding antigen; IFN, interferon; IgG, immunoglobulin G; IgM, immunoglobulin M; IL, interleukin; iRBC, infected red blood cell; MSP1, merozoite surface protein; TH1, T-helper 1; TH2, T-helper 2; TNF, tumor necrosis factor; VSA, variant surface antigen.
Regarding inflammation cytokines, TNF, IL-1, IL-6, and IL-10 at M10.5 correlated negatively with IL-8 and IgM to malarial antigens at M5.5 (Supplementary Figure 5C). TNF correlated positively with M10.5 iRBC IgM whereas IL-6 correlated negatively with iRBC IgG (Supplementary Figure 5D). At M24, IL-10 correlated negatively with M10.5 AMA1 IgG; TNF, IL-1, IL-6, and IL-10 correlated negatively with M15 iRBC antibodies (Supplementary Figure 5E and 5F) and M24 AMA1 and MSP1 IgM (Figure 2B), whereas they correlated positively with M15 AMA1, MSP1, and EBA175 IgG.
DISCUSSION
This study shows that a proinflammatory (IL-1, IL-6, TNF) and regulatory (IL-10) cytokine signature between 1 and 2 years of age is associated with less incidence of malaria up to ages 3 and 4 years, having adjusted by chemoprophylaxis and prior malaria exposure. In terms of what responses correlate with timing of first infection in infancy, children receiving 3- to 5-month malaria chemoprophylaxis in the first year of life (early and late exposure groups) had significantly lower concentrations of antigen-specific TH1 (IL-2, IL-12, and IFN-γ) and TH2 (IL-4, IL-5) cytokines at 2 years of age compared to children under continuous P. falciparum exposure. Because our analysis was done in a RCT with longitudinal design [30], selectively controlling by monthly chemoprophylaxis exposure to blood stage P. falciparum, results can more reliably shed light into the determinants of the acquisition of cellular immune responses in early childhood. However, the 2 subsets of cytokines were nonoverlapping and thus we found no evidence that altering the timing of initial P. falciparum exposure impacts subsequent development of clinical immunity, consistent with the main trial results [30].
Remarkably, a chemoprophylactic intervention altering natural exposure to P. falciparum blood stages in infancy had an effect on the T-cell adaptive response that was apparent >1 year later. Lower T-helper cytokine concentrations at M24 correlated with higher anti–P. falciparum IgG levels at M24 and prior visits, and lower anti–P. falciparum IgM levels at M5.5, indicating that antibodies could be markers of exposure in children who received prophylaxis in year 1. Indeed, in the RCT, early and late exposure groups had lower incidence of malaria in year 1 but higher in year 2 than the control group [30]; although a potential malaria “rebound” in year 2 was not statistically significant, this could be reflected immunologically. Thus, higher malaria exposure between M10.5 and M24 as a result of chemoprophylaxis between M2.5 and M10.5 could have dampened the production of T-helper cytokines at M24.
Higher concentrations of inflammatory cytokines at M10.5 and M24, associated here with malaria protection, correlated with lower IL-8 and lower IgM to P. falciparum antigens, indicative of less recent/current exposure, at prior timepoint visits. The fact that the association between proinflammatory cytokines and protection was observed in iRBC- and uRBC-stimulated PBMCs suggests that these responses were produced by innate rather than adaptive cells [36, 37]. In contrast, the T-helper signature at M24 was only detected in iRBC-stimulated PBMCs, suggesting that these cytokines were produced by memory T cells. Infants recruited were initially naive, and the nonspecific innate immune response has been reported as the key defense mechanism in this population [38, 39]. TNF has correlated with immunity to clinical malaria in children [9], while IL-1 and IL-6 in the mothers from this cohort were also associated with less malaria [19].
Furthermore, the proinflammatory protective signature was accompanied by a regulatory protective IL-10 response at age 2 years, when TNF levels diminished, showing that effective immunity against clinical malaria requires a proinflammatory followed by an anti-inflammatory response. In previous studies, IL-10 was elevated as a result of poor immune regulatory ability typically seen in children, and as a result of the absence of acquired immunity due to a lack of previous exposure to malaria [22]. During acute malaria, proinflammatory and TH1 cytokines had higher concentrations, which were more significant for IFN-γ, TNF, and IL-10. IFN-γ is produced by NK cells, γδ T cells, CD8+ T cells, and CD4+ T cells; therefore, it is both an innate and adaptive cytokine. Increased IFN-γ during acute malaria is related to protection against malaria [8, 9, 14]. The fact that regulatory IL-10 was higher during acute phase and declined at convalescence further supports that regulatory balanced responses are acquired in adequate immunity to control excessive inflammation [11].
In contrast with antibody responses [34], age or prior/present infection had no prominent influence on the magnitude of the cytokine response, consistent with previous studies by our group [26]. Cytokine concentrations except IL-2 and IL-8 declined by M24. At the age of 2 years, when the spleen matures and the incidence of severe malaria declines [2], there appears to be an inflection point in the acquisition of immunity. Indeed, key responses for immunity such as VSA and EBA175 antibodies attained higher levels [34], which have been associated with malaria protection in children from the same area [40]. A limitation of our study is that it was not designed to assess cytokines after M24, a time when a protective adaptive T-helper signature correlated with immunity could have emerged. Another limitation is that we did not phenotype the cells producing the cytokines. Also, a larger sample size could have established more statistically conclusive associations between T-helper/inflammatory cytokines and malaria.
Finally, this study could portray, on a small scale, the impact of drug usage during malaria elimination programs, including partial or temporary interruption of exposure by artemisinin-based combination therapies, on acquisition of protective immunity to malaria. Suppressive doses of SP plus AS during a defined period prevented infection and altered development of natural immunity but not completely. It remains to be assessed how quickly sustained chemoprophylaxis or mass drug administration would slow down or interrupt acquisition of immunity and/or could cause a loss of immunity at a population level [41].
In conclusion, a balanced proinflammatory and anti-inflammatory cytokine response at age 2 years may be required for the acquisition of protective clinical immunity to malaria in childhood. In addition, timing of malarial antigen immune priming in infancy did not impact development of clinical immunity within the first 2 years, but it may affect the subsequent acquisition of adaptive T-helper responses that may be relevant later in life.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank all children and their families for their participation in the study; the field workers, field supervisors, laboratory staff, data managers, and other staff at Centro de Investigação em Saúde de Manhiça for their work during the study; Laura Puyol, Diana Barrios, and Pau Cisteró for laboratory support; Jaume Ordi for the training and quality control for the placental histology readings; Lázaro Mussacate Quimice for reading all placental histologies; Gemma Moncunill for critically reviewing the manuscript; and Sònia Tomàs and Patricia García for their work as project managers.
Financial support. The study was funded by a European Union Framework Programme 6 STREP (Specific Targeted Research Project) (Malaria age exposure, project reference number 18902); Instituto de Salud Carlos III (Ayuda de incentivación a la participación en proyectos del Espacio Europeo de Investigación); and the Spanish Ministerio de Educación y Ciencia (project reference number A107190024). C. D. was supported by a Ramón y Cajal grant from the Spanish Ministerio de Ciencia e Innovación (grant number RYC-2008-02631); M. N. M. was supported by a PhD scholarship from Fundació Marfà; C. G. and Q. B. were supported by a grant from the Instituto de Salud Carlos III (Contrato post-Formación Sanitaria Especializada, Fondo de Investigaciones Sanitarias, reference numbers CM04/00028 and CM05/00134, respectively); and D. L. D. was supported by a Pfizer Australia Senior Research Fellowship. The Manhiça Health Research Centre receives core funding from the Spanish Agency for International Cooperation and Development. ISGlobal is a member of the Centres de Recerca de Catalunya program, Generalitat de Catalunya.
Potential conflicts of interest. All authors: No potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. World malaria report 2017. Available at: http://wwwwhoint/malaria/publications/world-malaria-report-2017/en/2017. Accessed 14 December 2017.
- 2. Doolan DL, Dobaño C, Baird JK Acquired immunity to malaria. Clin Microbiol Rev 2009; 22:13–36, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hoffman SL, Oster CN, Mason C, et al. Human lymphocyte proliferative response to a sporozoite T cell epitope correlates with resistance to falciparum malaria. J Immunol 1989; 142:1299–303. [PubMed] [Google Scholar]
- 4. Cohen S, McGregor IA, Carrington S Gamma-globulin and acquired immunity to human malaria. Nature 1961; 192:733–7. [DOI] [PubMed] [Google Scholar]
- 5. Clark IA, Budd AC, Alleva LM, Cowden WB Human malarial disease: a consequence of inflammatory cytokine release. Malar J 2006; 5:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Farrington L, Vance H, Rek J, et al. Both inflammatory and regulatory cytokine responses to malaria are blunted with increasing age in highly exposed children. Malar J 2017; 16:499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dodoo D, Omer FM, Todd J, Akanmori BD, Koram KA, Riley EM Absolute levels and ratios of proinflammatory and anti-inflammatory cytokine production in vitro predict clinical immunity to Plasmodium falciparum malaria. J Infect Dis 2002; 185:971–9. [DOI] [PubMed] [Google Scholar]
- 8. Luty AJ, Lell B, Schmidt-Ott R, et al. Interferon-gamma responses are associated with resistance to reinfection with Plasmodium falciparum in young African children. J Infect Dis 1999; 179:980–8. [DOI] [PubMed] [Google Scholar]
- 9. Robinson LJ, D’Ombrain MC, Stanisic DI, et al. Cellular tumor necrosis factor, gamma interferon, and interleukin-6 responses as correlates of immunity and risk of clinical Plasmodium falciparum malaria in children from Papua New Guinea. Infect Immun 2009; 77:3033–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ockenhouse CF, Schulman S, Shear HL Induction of crisis forms in the human malaria parasite Plasmodium falciparum by gamma-interferon-activated, monocyte-derived macrophages. J Immunol 1984; 133:1601–8. [PubMed] [Google Scholar]
- 11. Boyle MJ, Jagannathan P, Bowen K, et al. The development of Plasmodium falciparum-specific IL10 CD4 T cells and protection from malaria in children in an area of high malaria transmission. Front Immunol 2017; 8:1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Doolan DL, Hoffman SL The complexity of protective immunity against liver-stage malaria. J Immunol 2000; 165:1453–62. [DOI] [PubMed] [Google Scholar]
- 13. Torre D Early production of gamma-interferon in clinical malaria: role of interleukin-18 and interleukin-12. Clin Infect Dis 2009; 48:1481–2. [DOI] [PubMed] [Google Scholar]
- 14. D’Ombrain MC, Robinson LJ, Stanisic DI, et al. Association of early interferon-gamma production with immunity to clinical malaria: a longitudinal study among Papua New Guinean children. Clin Infect Dis 2008; 47:1380–7. [DOI] [PubMed] [Google Scholar]
- 15. Horowitz A, Newman KC, Evans JH, Korbel DS, Davis DM, Riley EM Cross-talk between T cells and NK cells generates rapid effector responses to Plasmodium falciparum-infected erythrocytes. J Immunol 2010; 184:6043–52. [DOI] [PubMed] [Google Scholar]
- 16. PrabhuDas M, Adkins B, Gans H, et al. Challenges in infant immunity: implications for responses to infection and vaccines. Nat Immunol 2011; 12:189–94. [DOI] [PubMed] [Google Scholar]
- 17. Portugal S, Moebius J, Skinner J, et al. Exposure-dependent control of malaria-induced inflammation in children. PLoS Pathog 2014; 10:e1004079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fievet N, Ringwald P, Bickii J, et al. Malaria cellular immune responses in neonates from Cameroon. Parasite Immunol 1996; 18:483–90. [DOI] [PubMed] [Google Scholar]
- 19. Dobaño C, Berthoud T, Manaca MN, et al. High production of pro-inflammatory cytokines by maternal blood mononuclear cells is associated with reduced maternal malaria but increased cord blood infection. Malar J 2018; 17:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anum D, Kusi KA, Ganeshan H, et al. Measuring naturally acquired ex vivo IFN-γ responses to Plasmodium falciparum cell-traversal protein for ookinetes and sporozoites (CelTOS) in Ghanaian adults. Malar J 2015; 14:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wroczyńska A, Nahorski W, Bakowska A, Pietkiewicz H Cytokines and clinical manifestations of malaria in adults with severe and uncomplicated disease. Int Marit Health 2005; 56:103–14. [PubMed] [Google Scholar]
- 22. Mandala WL, Msefula CL, Gondwe EN, et al. Cytokine profiles in Malawian children presenting with uncomplicated malaria, severe malarial anemia, and cerebral malaria. Clin Vaccine Immunol 2017; 24. doi: 10.1128/CVI.00533-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rovira-Vallbona E, Moncunill G, Bassat Q, et al. Low antibodies against Plasmodium falciparum and imbalanced pro-inflammatory cytokines are associated with severe malaria in Mozambican children: a case-control study. Malar J 2012; 11:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Le Hesran JY, Fiévet N, Thioulouse J, et al. Development of cellular immune responses to Plasmodium falciparum blood stage antigens from birth to 36 months of age in Cameroon. Acta Trop 2006; 98:261–9. [DOI] [PubMed] [Google Scholar]
- 25. Schofield L, Ioannidis LJ, Karl S, et al. Synergistic effect of IL-12 and IL-18 induces TIM3 regulation of γδ T cell function and decreases the risk of clinical malaria in children living in Papua New Guinea. BMC Med 2017; 15:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Quelhas D, Puyol L, Quintó L, et al. Intermittent preventive treatment with sulfadoxine-pyrimethamine does not modify plasma cytokines and chemokines or intracellular cytokine responses to Plasmodium falciparum in Mozambican children. BMC Immunol 2012; 13:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jagannathan P, Bowen K, Nankya F, et al. Effective antimalarial chemoprevention in childhood enhances the quality of CD4+ T cells and limits their production of immunoregulatory interleukin 10. J Infect Dis 2016; 214:329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Otoo LN, Riley EM, Menon A, Byass P, Greenwood BM Cellular immune responses to Plasmodium falciparum antigens in children receiving long term anti-malarial chemoprophylaxis. Trans R Soc Trop Med Hyg 1989; 83:778–82. [DOI] [PubMed] [Google Scholar]
- 29. Friedman JF, Phillips-Howard PA, Hawley WA, et al. Impact of permethrin-treated bed nets on growth, nutritional status, and body composition of primary school children in western Kenya. Am J Trop Med Hyg 2003; 68:78–85. [PubMed] [Google Scholar]
- 30. Guinovart C, Dobaño C, Bassat Q, et al. The role of age and exposure to Plasmodium falciparum in the rate of acquisition of naturally acquired immunity: a randomized controlled trial. PLoS One 2012; 7:e32362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mayor A, Serra-Casas E, Bardají A, et al. Sub-microscopic infections and long-term recrudescence of Plasmodium falciparum in Mozambican pregnant women. Malar J 2009; 8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Berthoud TK, Manaca MN, Quelhas D, et al. Comparison of commercial kits to measure cytokine responses to Plasmodium falciparum by multiplex microsphere suspension array technology. Malar J 2011; 10:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kollmann TR, Crabtree J, Rein-Weston A, et al. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol 2009; 183:7150–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nhabomba AJ, Guinovart C, Jiménez A, et al. Impact of age of first exposure to Plasmodium falciparum on antibody responses to malaria in children: a randomized, controlled trial in Mozambique. Malar J 2014; 13:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saúte F, Aponte J, Almeda J, et al. Malaria in southern Mozambique: malariometric indicators and malaria case definition in Manhiça district. Trans R Soc Trop Med Hyg 2003; 97:661–6. [DOI] [PubMed] [Google Scholar]
- 36. McCall MB, Netea MG, Hermsen CC, et al. Plasmodium falciparum infection causes proinflammatory priming of human TLR responses. J Immunol 2007; 179:162–71. [DOI] [PubMed] [Google Scholar]
- 37. Walther M, Woodruff J, Edele F, et al. Innate immune responses to human malaria: heterogeneous cytokine responses to blood-stage Plasmodium falciparum correlate with parasitological and clinical outcomes. J Immunol 2006; 177:5736–45. [DOI] [PubMed] [Google Scholar]
- 38. Kumar SK, Bhat BV Distinct mechanisms of the newborn innate immunity. Immunol Lett 2016; 173:42–54. [DOI] [PubMed] [Google Scholar]
- 39. Schrum JE, Crabtree JN, Dobbs KR, et al. Cutting edge: Plasmodium falciparum induces trained innate immunity. J Immunol 2018; 200:1243–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Campo JJ, Dobaño C, Sacarlal J, et al. Impact of the RTS,S malaria vaccine candidate on naturally acquired antibody responses to multiple asexual blood stage antigens. PLoS One 2011; 6:e25779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aponte JJ, Menendez C, Schellenberg D, et al. Age interactions in the development of naturally acquired immunity to Plasmodium falciparum and its clinical presentation. PLoS Med 2007; 4:e242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.