Abstract
The human world is currently influenced largely by the outbreak of pandemic COVID‐19. At this moment, most researchers focus on developing treatment strategies and measures to work against COVID‐19. Treatment strategies specific for COVID‐19 are lacking. This article provides an overview of the life cycle and routes of transmission of SARS‐CoV‐2. The therapeutic effects of two drugs [i. e., remdesivir (RDV) and favipiravir (FPV)] which can potentially tackle COVID‐19 are discussed based on current published data. This review can serve as a reference for future studies.
Keywords: COVID-19, favipiravir, remdesivir, routes of transmission, pandemic
COVID‐19 is a pandemic that requires global action. This review presents the latest status of research on the use of two drugs (favipiravir and remdesivir) in tackling COVID‐19, and offers an overview of the life cycle and routes of transmission of SARS‐CoV‐2. It is hoped that the opportunities and challenges brought about by the use of these two drugs can be illuminated for future research.
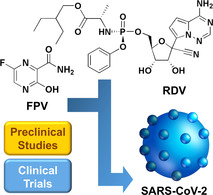
1. Introduction
Coronavirus disease 2019 (COVID‐19) emerges as an infectious disease whose mortality rate is ill‐elucidated because of the difficulty in quantifying the number of asymptomatic and undiagnosed deaths. [1] While some COVID‐19 patients display mild symptoms, others die within a few days after infection. The latter is due to the ability of COVID‐19 to cause critical pneumonia, which is hard to treat and may lead to organ failure. [2] In the search for effective regimens to tackle SARS and MERS, substantial preclinical research efforts have been reported previously. Yet, the COVID‐19 pandemic is different from SARS and MERS diseases. Vaccines or medications are currently lacking. Researchers worldwide, therefore, pay great efforts to drug repositioning and repurposing. [3] Few U. S. Food and Drug Administration (FDA)‐approved drugs or drug regimens (such as favipiravir, tocilizumab. arbidol, ribavirin, remdesivir, and lopinavir/ritonavir combination) are adopted to treat SARS‐CoV‐2 because they can target the RNA genome and angiotensin‐converting enzyme‐2 (ACE2) receptors to inhibit viral replication. These drugs have begun to be repurposed to tackle COVID‐19. Meanwhile, some new drugs (such as ivermectin and auranofin) are in the early stages of investigation, and may be used clinically to treat COVID‐19 in the future. One major method of identifying possible therapeutic agents against COVID‐19 is molecular docking, through which drug candidates can be screened and the one identified to show potential for development can be further tested in vitro and in vivo. [4]
Favipiravir (FPV) is one of the drugs that may potentially be used to tackle COVID‐19. This drug is a derivative of pyrazine carboxamide, showing antiviral activity against a variety of RNA viruses (including influenza virus, rhinovirus, and respiratory syncytial virus). [5] The generation of FPV was first reported by Toyama Chemical Company. This drug was later approved in Japan for use in influenza treatment.[ 6 , 7 ] It can be metabolised to an active metabolite, favipiravir ibofuranosyl 5′ triphosphate (T‐705‐RTP), which acts as a competitor with purine nucleosides and can interfere with viral replication.[ 8 , 9 ] Another drug showing high potential in COVID‐19 treatment is remdesivir (RDV, also known as GS‐5734). This drug was first developed by Gilead Sciences via collaboration with the U.S. Centres for Disease Control and Prevention (CDC) and the U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID). In this article, we will focus our discussions on the therapeutic effects of these two potential drugs after an overview of the virology, routes of transmission, and life cycle of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is provided. It is hoped that this article cannot only highlight the opportunities and challenges of using the two drugs to treat COVID‐19 but can also offer insights into drug development to tackle coronaviruses (CoVs) in the future.
2. Overview of SARS‐CoV‐2
The world is currently struggling to cope with COVID‐19, which is caused by SARS‐CoV‐2 emerged in the city of Wuhan in China in December 2019. Till October 7, 2020, over 35 000 000 cases have been reported throughout the world. More than 1 000 000 deaths in around 240 countries have resulted. [10] The World Health Organization (WHO) announced COVID‐19 as a pandemic on March 11, 2020. Currently, no drug or vaccine specifically targeting COVID‐19 is available, though related research is underway.
Structurally, SARS‐CoV‐2 is a single‐stranded RNA virus belonging to the family of Coronaviridae. CoVs can be classified into 4 genera: α‐CoV, β‐CoV, γ‐CoV and δ‐CoV. [11] SARS‐CoV‐2 belongs to β‐CoV. This virus has high transmissibility and infectivity compared to other viruses (e. g., SARS‐CoV, and MERS‐CoV) and has many natural, intermediate and final hosts.[ 12 , 13 ] Phylogenetic tree analysis reveals that SARS‐CoV‐2 is highly similar (with 96% identity) to bat SARS‐like CoV (SL‐CoV) at the genomic level. This leads to the thought that bats are the host of SARS‐CoV‐2.[ 14 , 15 , 16 ] The genome of SARS‐CoV‐2 comprises around 30 000 nucleotides. It encodes four specific proteins [i. e., membrane protein (M), envelope protein (E), nucleocapsid protein (N), and spike protein (S)]. The E‐protein is a small integral membrane protein consisting of 76 to 109 amino acids.[ 17 , 18 ] It, along with the M‐protein, [11] involves in viral assembly. The N‐protein is incorporated into the viral RNA and contributes to RNA replication and transcription. Finally, owing to the availability of the S‐protein on the CoV surface, the virus can bind to the angiotensin‐converting enzyme 2 (ACE‐2) receptor readily to get into the host cell.[ 19 , 20 ]
3. Life Cycle and Routes of Transmission
As mentioned in the preceding section, the S‐protein on the surface of SARS‐CoV‐2 can bind to the ACE‐2 receptor present on the host cell. After endocytosis, the virus is uncoated. It releases the viral genome, which subsequently binds to the rough endoplasmic reticulum for translation into the viral replicase polyproteins (PP1a and PP1ab) and RNA‐dependent RNA polymerase (RdRp). [21] Through replication and discontinuous transcription, the single‐stranded RNA (ssRNA) and viral proteins are generated. Upon the assembly of a virion from structural proteins and the viral RNA genome, the virion can be released by exocytosis to infect a new host cell. [22]
Upon infection, clinical manifestations of COVID‐19 are similar to those of SARS and MERS. Patients often get pneumonia and abnormal chest computed tomography (CT) scans,[ 23 , 24 , 25 ] but they seldom show serious upper respiratory symptoms (e. g., sore throat, and sneezing). This points to the possibility that the target cells locate mainly in the lower respiratory tract. Autopsy studies of COVID‐19 patients reveal that the infection results in diffuse alveolar damage.[ 26 , 27 ] Patients often experience pyrexia, fatigue, sputum development, headache, vomiting, diarrhoea, breathlessness, hemoptysis, and conjunctival congestion. [28] Although the origin of SARS‐CoV‐2 is thought to be bats, studies involving the detection and isolation of SARS‐CoV‐2 from environmental samples are lacking. [29] The situation has been changed recently by a study which has examined 585 environmental samples obtained from the Wuhan Seafood Market. 33 samples have been found to contain SARS‐CoV‐2. This suggests that the spread from nonhuman animals to humans may occur. [30] On the other hand, a substantial number of cases have been found not to have an exposure history to the market. This reveals the possibility of person‐to‐person transmission.
As suggested by epidemiological studies, there are three main reasons for SARS‐CoV‐2 transmission: (1) person‐to‐person interactions, (2) transmission by aerosols, and (3) transmission by touch. [31] To the best of our understanding, SARS‐CoV‐2 can spread from one person to another one when people are in close contact with each other (within around 6 feet). When an infected person coughs or sneezes, respiratory droplets may either get to the mouths or noses of people nearby or be inhaled by others. A person may also get COVID‐19 if he/she touches his/her mouth after touching a contaminated surface, although this might not be the major route of virus transmission. Rapid and early identification of COVID‐19 is vital if we need to control the progression of the disease. [32]
Transmission of COVID‐19 has been reported to be influenced by a number of environmental factors. [33] For instance, when the temperature is high, the rate of transmission is inhibited. [34] This is supported by a recent study, which has observed that SARS‐CoV‐2 has high resistance for a long time at 4 °C but the resistance of the virus lasts only for 5 min if the temperature is increased to 70 °C. [35] This is partially because high temperature may cause virus inactivation. The relationship between the transmission of the disease and environmental factors (including maximum relative humidity and maximum wind speed) has been further examined by Bhattacharjee, [36] though the impact of maximum air humidity and wind speed on the prevalence of COVID‐19 has been found not to be statistically significant.
More recently, the relationship between the severity of COVID‐19 and certain environmental factors (including the scarcity of non‐deciduous vegetation) has been investigated. [37] In the southern regions (e. g., Basilicata, Calabria, and Molise) where the rate of forest area per capita (0.34 hectares/inhabitant) is high, the effect of the pandemic is generally low. [37] On the contrary, in some northern regions (e. g., Emilia Romagna, Lombardy, and Veneto) where the forest is less than 0.20 hectares/inhabitant, the effect of COVID‐19 is severe. [37] This reveals that the evergreen Mediterranean forests and shrubland plants may help inhibit the transmission of the virus by emitting immunomodulating volatile organic compounds or by offering dietary sources of bioactive agents. This finding illustrates the protective role potentially played by nature and fuels the search for antiviral drug candidates from nature.
4. Drug Synthesis and Mechanisms of Action
The antiviral activity of RDV stems from its steric interaction with RdRp and its capacity of inducing premature termination of viral RNA replication. [38] In both in vitro and in vivo contexts, RDV has been reported to act against CoVs. [39] When RDV is administered intravenously to COVID‐19 patients, it is first converted ino an alanine metabolite upon cellular uptake, followed by conversion into nucleoside monophosphate (NMP) and then nucleoside triphosphate (NTP) via phosphorylation. NTP formed can be incorporated into RdRp, leading to the termination of replication of viral RNA. [40]
Scientists from Gilead Sciences, USAMRIID and CDC develop the first synthetic pathway for generating RDV (Figure 1). [41] During synthesis, tribenzyl protected lactol is oxidized to produce product 1. After that, glycosylation occurs, generating product 2, which is then treated with the bromo base in the presence of n‐butyllithium (n‐BuLi) to generate product 3. Upon a cyanation reaction, product 3 is converted into product 4. Product 5 is produced when benzyl groups are eliminated from product 4. It is then reacted with a diastereomeric mixture of the phosphoramidoylchloridate prodrug 7, leading to the formation of product 8. Upon separation by using chiral high pressure liquid chromatography (HPLC), purified isomers (9 a and 9 b) are obtained. Because this synthetic pathway involves the use of cryogenic temperature and chiral chromatography, it is hard to be scaled up.
Figure 1.
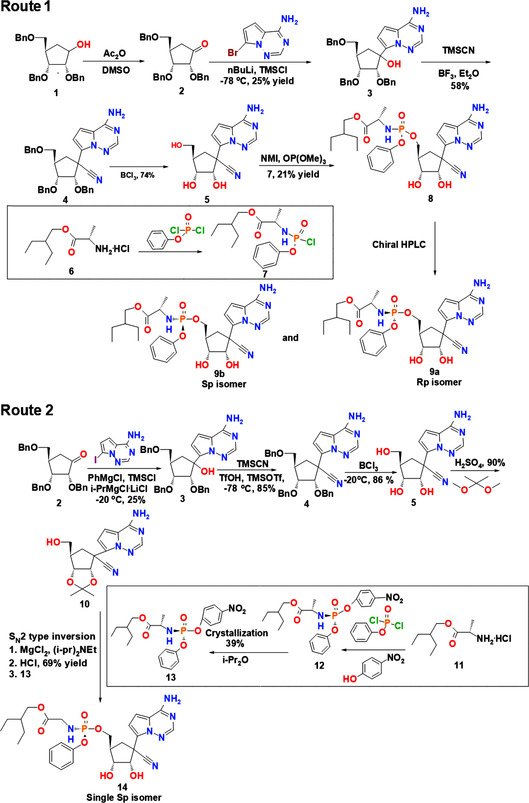
Synthetic routes of RDV. Abbreviations: Ac2O, acetic anhydride; DMSO, dimethyl sulfoxide; n‐BuLi, n‐butyllithium; TMSCl, trimethylsilyl chloride; TMSCN, trimethylsilyl cyanide; BF3, boron fluoride; Et2O, diethyl ether; BCl3, boron trichloride; MgCl2, magnesium chloride; NMI, N‐methylimidazole; OP(OMe)3, trimethyl phosphate; TfOH. triflic acid; TMSOTf, trimethylsilyl trifluoromethanesulfonate; HPLC, high pressure liquid chromatography; H2SO4, sulfuric acid; PhMgCl, phenylmagnesium chloride; i‐PrMgCl ⋅ LiCl, isopropylmagnesium chloride lithium chloride; i‐Pr2O, diisopropyl ether; i‐Pr2Net, N,N‐diisopropylethylamine; MgCl2, magnesium chloride; HCl, hydrochloric acid.
A recent method of RDV production enables diastereoselective synthesis of a single isomer. [41] In this method, the glycosylation step is mediated by using the iodo base. This allows for a more facile metal‐halogen exchange using isopropylmagnesium chloride lithium chloride (i‐PrMgCl ⋅ LiCl) to occur. The product generated can be subjected to treatment with trimethylsilyl cyanide (TMSCN), trimethylsilyl trifluoromethanesulfonate (TMSOTf), and triflic acid (TfOH) at −78 °C. This generates product 4. The use of TfOH in this method contributes to the high yield and high selectivity of the product. A protection‐deprotection strategy mediated first by a debenzylation reaction followed by protection of 2′,3′‐acetonide hydroxyl groups is applied to obtain product 10. Compared to the unprotected glycoside 5, the coupling of nucleoside 10 with the prodrug counterpart 13 provides a substantially higher yield (69 %). The final product of this reaction pathway is a single isomer, which can be obtained by solvent crystallization. This reaction pathway, therefore, enables stereoselective synthesis of RDV.
Similar to RDV, FPV inhibits the replication and transcription of viral RNA. [5] Mechanistically, upon oral administration, FPV can be metabolized to ribofuranosyl‐5′‐monophosphate (RMP), which is subsequently converted into a ribofuranosyl‐5′‐triphosphate (RTP) derivative by phosphorylation. The generated product can be incorporated into RdRp, leading to the formation of defective viral RNA and finally the termination of replication of viral RNA. [8] To generate FPV, methyl 3‐amino‐6‐bromopyrazine‐2‐carboxylate can be used as the starting material (Figure 2, Route 1). During synthesis, this compound is subjected to diazotization‐alcoholysis in the presence of concentrated H2SO4. After that, an amination step catalyzed by (S)‐(−)‐2,2′‐bis(diphenylphosphino)‐1,1′‐binaphthyl ((S)‐BINAP) is involved. The generated product undergoes fluorination mediated by using the Olah's reagent before demethylation occurs. The yield of the overall reaction is, however, estimated to be around 0.44 %. [42] This makes this route not favourable for large‐scale production of FPV.
Figure 2.
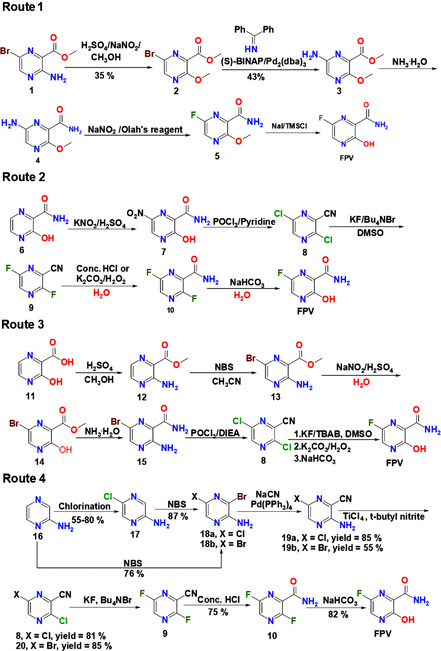
Synthetic routes of FPV. Abbreviations: NaNO2, sodium nitrite; H2SO4, sulfuric acid; CH3OH, methanol; S‐(BINAP), (S)‐(−)‐2,2′‐bis(diphenylphosphino)‐1,1′‐binaphthyl; Pd2(dba)3, tris(dibenzylideneacetone)dipalladium(0); NH3, ammonia; NaNO2, sodium nitrite; NaI, sodium iodide; TMSCl, trimethylsilyl chloride; POCl3, phosphoryl chloride; KNO3, potassium nitrate; KF, potassium fluoride; Bu4NBr, tetrabutylammonium bromide; K2CO3, potassium carbonate; H2O2, hydrogen peroxide; NaHCO3, sodium bicarbonate; NBS, N‐bromosuccinimide; CH3CN, methyl cyanide; DIEA, N,N‐diisopropylethylamine; KF, potassium fluoride; TBAB, tetra‐n‐butylammonium bromide; DMSO, dimethyl sulfoxide, NaCN, sodium cyanide; Pd (PPh3)4, tetrakis(triphenylphosphine)palladium(0); TiCl4, titanium tetrachloride; HCl, hydrochloric acid
In Routes 2 and 3, FPV is generated from an intermediate, namely 3,6‐dichloropyrazine‐2‐carbonitrile. This intermediate can be converted into product 9 by reacting with potassium fluoride. The product subsequently either undergoes nitrile hydration in the presence of concentrated HCl or is treated with an alkaline solution of hydrogen peroxide to give product 10. The fluorine atom is more reactive at C3 position of 10 (Figure 2)[ 43 , 44 ] and can be more readily replaced by the hydroxyl group to generate the final product. Route 2 has a lower step count, but is not economically feasible partly because of the high cost of the starting material 6. On the other hand, the starting material (viz., 3‐aminopyrazine‐2‐carboxylic acid 11) used in Route 3 is cheaper. Five steps are required to convert 11 into 8 with a yield of 37 %. In both routes, a large amount of phosphoryl chloride (POCl3) is used and this raises environmental concerns. More recently, Guo and co‐workers have synthesized FPV by using 2‐aminopyrazine as a starting material, providing a simple route for the generation of 8. [45] This new process does not involve the use of POCl3 and gives a good yield (Figure 2, Route 4). It is worth further exploration for large‐scale production of FPV in the future.
5. Preclinical and Clinical Evaluation of RDV
The therapeutic potential of RDV in tackling COVID‐19 is demonstrated both in vitro and in vivo. In the in vitro context, treatment with RDV has been shown to inhibit the replication process of SARS‐CoV‐like group 2b (HKU3, WIV1, and SHC014) and MERS‐CoV‐like group 2c (HKU5) bat CoVs. [46] With the use of only 1 mM of RDV, a 1.5‐2 log10 reduction in the production of the bat CoVs is observed. [46] More recently, RDV has been found to inhibit the replication of SARS‐CoV‐2 in human lung cells and primary airway epithelial cells (EC50=0.01 μM). [47] In the study, a chimeric SARS‐CoV encoding RdRp of SARS‐CoV‐2 has been constructed. [47] RDV has effectively reduced the viral load in the lung and has enhanced the pulmonary function in mice infected with the chimeric virus. [47] The therapeutic activity of RDV has been further corroborated by a recent study, [48] in which rhesus macaques infected with SARS‐CoV‐2 have been adopted as models. One group has been treated with RDV with a loading dose of 10 mg/kg and a daily maintenance dose of 5 mg/kg. [48] The other group has been treated with an equal volume of the vehicle solution. [48] Rhesus macaques treated with RDV have shown no respiratory symptoms. Compared to the vehicle‐treated counterparts, RDV‐treated rhesus macaques have displayed a substantially lower viral load in the lungs. [48] In addition, upon treatment with RDV, damage to lung tissues has been found to be significantly reduced. [48] This indicates the possible use of RDV in tackling COVID‐19. [48]
Apart from preclinical tests, the possible use of RDV in fighting against CoVs has been supported clinically. In an earlier clinical study, 36 out of 53 COVID‐19 patients treated with RDV (the dose on day 1 is 200 mg; whereas the daily dose on day 2‐10 is 100 mg) have shown improvement in symptoms. [49] Similar success has also been reported by Beigel and coworkers, [50] who have performed a double‐blind, randomized, placebo‐controlled clinical trial of RDV for the treatment of COVID‐19. Among 538 patients who have received RDV (the dose on day 1 is 200 mg and the daily dose on day 2‐10 is 100 mg) and 521 patients who have received a placebo, the median recovery time of those treated patients (11 days) has been found to be shorter than that of the controls (15 days). [50]
Network meta‐analysis of a randomized controlled trial (in which different treatment regimens have been adopted, including RDV 5‐day course, RDV 10‐day course, and standard care) has shown that, compared to the standard care group, COVID‐19 patients receiving the 5‐day or 10‐day treatment course have displayed a significantly higher rate of recovery. [51] A similar observation has been made by Antinori and coworkers, [52] who have conducted a clinical study on a 10‐day treatment course with RDV for severe COVID‐19 patients. In the study, 18 patients have been admitted to an intensive care unit (ICU) and 17 have been admitted to the infectious diseases ward (IDW). On day 28, 8 deaths have been recorded in the ICU group, with 6 ICU patients being discharged. On the other hand, only one death has been recorded in the IDW group, with 14 patients being discharged and two being hospitalized. This suggests that the use of RDV may lead to better clinical outcomes for patients who are hospitalized outside ICU.
As a matter of fact, RDV is the first agent recommended for authorization in the European Union (EU) for use in the treatment of COIVID‐19. A conditional marketing authorization has been granted by the European Medicines Agency (EMA) for treating COVID‐19 in adults (and adolescents over 12 years of age) who show signs of pneumonia and require oxygen supplementation. [53] The Emergency Use Authorization (EUA) makes RDV legally available for patients and physicians. This enables other clinical trials to incorporate RDV into the treatment protocols. Despite this, whether the scale of RDV production can meet the rapidly growing demand in the market is unknown. Furthermore, RDV is currently permitted only for a subgroup of COVID‐19 patients who require oxygen supplementation. Patient selection and drug allocation could be challenging at times of scarcity. [54] Finally, possible side‐effects of treatment with RDV are worth further investigation. This concern is justified by a recent study, [55] which has reported cases of patients who have to stop the treatment course because of alanine aminotransferase elevation and renal failure. A similar incidence of side effects has also been reported by Maldarelli and coworkers, [56] who have observed that one pregnant COVID‐19 patient has shown an increase in the transaminase level during the treatment course with RDV. At the moment the actual cause of her hypertransaminasemia is ill‐defined. It can be due to either the use of RDV or COVID‐19 per se; however, concerns have been raised on the safe use of RDV for tackling COVID‐19. This is an area that is worth extensive follow‐up investigation.
6. Preclinical and Clinical Evaluation of FPV
The effect of FPV in tackling CoVs has been verified by an open‐label control study, [57] in which 35 patients have been treated with FPV (the dose on day 1 is 3200 mg; whereas the daily dose on day 2‐14 is 1200 mg). [57] FPV has been found to be effective in controlling disease progression and in enhancing viral clearance in COVID‐19 patients. [57] The efficiency of FPV in tackling COVID‐19 has been further verified by another randomized, open‐label clinical study, [58] in which patients treated with FPV (the dose on day 1 is 3200 mg; whereas the daily dose on day 2‐10 is 1200 mg) have shown improvement in respiratory symptoms. [58] More recently, few clinical studies have been published to confirm the therapeutic potential of FPV. For instance, a case study has been published to document the recovery process of one COVID‐19 patient suffering from end‐stage renal disease. [59] The patient has been treated initially with lopinavir/ritonavir and ciclesonide but has developed severe pneumonia. [59] FPV has been used to replace those three drugs, and the patient has shown improvement in symptoms. [59] In addition, based on the observation made in severe COVID‐19 patients, Dauby and coworkers have reported that the use of FPV can improve the lymphocyte count. [60] All these findings evidence the high potential of FPV in treating COVID‐19.
Apart from being administered alone, FPV can be used along with other drugs or therapies to tackle COVID‐19. The feasibility of this has been demonstrated by Inoue and coworkers, [61] who have combined systemic corticosteroid therapy with FPV to treat COVID‐19 patients suffering from chronic obstructive pulmonary disease (COPD). Patients receiving the combined treatment have been extubated on day 5, showing negative results in the SARS‐CoV‐2 polymerase chain reaction (PCR) test on day 15 and being discharged on day 21. Similar success in treating COVID‐19 with the combined therapy has been documented by a recent study, [62] in which 11 severe COVID‐19 patients have been treated concomitantly with FPV and methylprednisolone. 10 out of the 11 subjects have reacted well during the course of the treatment, with no oxygen supplementation or ventilator therapy being required.
Despite the promising potential as mentioned above, it is worth noting that, owing to the lack of comparisons between the efficiency of FPV monotherapy and that of combined therapy, the effect of the incorporation of those additional therapeutic components on the therapeutic efficacy as a whole is unknown. More studies are required to verify how those components affect the safety and efficiency of the treatment course. In addition, the occurrence of pyrexia has been reported in COVID‐19 patients after treatment with FPV despite symptoms of COVID‐19 have been ameliorated. [63] That fever has subsided in patients when administration of FPV has been halted. [63] To the best of our knowledge, this is the first report of FPV‐induced fever. Clearly, more in‐depth studies are in dire need before both short ‐term and long‐term side‐effects caused by FPV can be better known.
7. Concluding Remarks and Future Outlooks
Over the last few months, COVID‐19 has emerged as a severe threat to public health and also becomes an economic burden worldwide. It is important to develop effective treatment strategies to control the pandemic as soon as possible. As mentioned in discussions above, multiple clinical trials have been started to evaluate the safety and efficacy of RDV and FPV in COVID‐19 patients.[ 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 ] Some of them are listed in Table 1. Recent findings suggest that RDV and FPV are antiviral agents that might show the potential to combat COVID‐19 in short term. On June 29, 2020, Gilead Sciences released a press note to set the price of RDV as $390 per 100 mg vial. [88] On the other hand, the cost of FPV varies from company to company. Glenmark Pharmaceuticals has launched FPV as FabiFlu, setting the price as around $1.0 per tablet. Sun Pharmaceuticals has also marketed FPV under the trade name of FluGuard. The price of each 200 mg tablet is set to be $0.5. Compared to other antiviral drugs, FPV appears to be the cheapest one and may serve as an option for emergency use in clinics, though more in‐depth research is needed to determine the safety and efficiency.
Table 1.
Some clinical trials devoted to examining the use of RDV and FPV in tackling COVID‐19.
|
Clinical Trial |
Description |
Patient's Gender* |
Status |
Ref. |
|---|---|---|---|---|
|
A phase 3 randomized clinical trial which evaluates the safety and antiviral activity of RDV in severe COVID‐19 patients. |
M/F |
Completed |
64 |
|
|
A phase 3 randomized clinical trial which evaluates the safety and antiviral activity of RDV in moderate COVID‐19 patients. |
M/F |
Completed |
65 |
|
|
A phase 3 randomized blinded controlled trial which determines the safety and efficacy of RDV (either alone or in combination with baricitinib) in hospitalized COVID‐19 patients. |
M/F |
Ongoing |
66 |
|
|
A multicenter, adaptive, randomized blinded controlled trial which evaluates the safety and efficacy of RDV in hospitalized COVID‐19 patients. |
M/F |
Ongoing |
67 |
|
|
A clinical trial which is held in Pakistan to evaluate the safety and efficiacy of RDV (and other treatment methods including therapeutic plasma exchange and stem cell therapy) in COVID‐19 patients. |
M/F |
Ongoing |
68 |
|
|
EUCTR2020‐000982‐18‐SE |
A solidarity multicenter trial which studies the efficacy of RDV and hydroxychloroquine in tackling COVID‐19. |
M/F |
Ongoing |
69 |
|
EUCTR2020‐001366‐11‐IE |
A randomized clinical trial which evaluates the efficacy of RDV and other antiviral agents (lopinavir and ritonavir) in COVID‐19 patients and compares the outcomes of RDV, lopinavir and ritonavir with standard care treatment. |
M/F |
Ongoing |
70 |
|
EUCTR2020‐001803‐17‐GB |
A phase 2/3 clinical trial which evaluates the safety, tolerability, pharmacokinetics, and efficacy of RDV in COVID‐19 patients aged from birth to <18 years. |
M/F |
Ongoing |
71 |
|
JPRN‐jRCT2031190264 |
A multicenter, adaptive, randomized blinded controlled trial which determines the efficacy of RDV in COVID‐19 patients. |
M/F |
Ongoing |
72 |
|
An open‐label, non‐randomized clinical study which evaluates the efficacy of FPV and hydroxychloroquine in COVID‐19 patients |
M/F |
Completed |
73 |
|
|
A phase 1 clinical trial which determines the bioequivalence of FPV in healthy volunteers under fasting conditions as compared with avigan. |
M |
Completed |
74 |
|
|
A phase 1 clinical trial which evaluates the bioequivalence of FPV in healthy volunteers under fasting conditions as compared with avigan. |
M |
Completed |
75 |
|
|
An open‐label, randomized, phase 1 clinical trial which evaluates the bioequivalence of FPV in healthy volunteers under fasting conditions as compared with avigan. |
M |
Completed |
76 |
|
|
An open‐label, randomized, phase 1 clinical trial which determines the bioequivalence of FPV in healthy volunteers under fasting conditions as compared with avigan. |
M |
Completed |
77 |
|
|
A phase 3 randomized clinical trial which evaluates the safety and efficacy of FPV in COVID‐19 patients and compares the efficiency with standard care treatment. |
M/F |
Ongoing |
78 |
|
|
ChiCTR2000029544 |
A randomized controlled trial which evaluates the safety and efficacy of FPV in COVID‐19 patients and compares the efficiency with baloxavir marboxil treatment. |
M/F |
Ongoing |
79 |
|
ChiCTR2000029548 |
An open‐label randomized controlled trial which evaluates the safety and efficacy of FPV, baloxavir marboxil, and lopinavir/ritonavir in COVID‐19 patients. |
M/F |
Ongoing |
80 |
|
ChiCTR2000029600 |
A clinical trial which studies the safety and efficacy of FPV in COVID‐19 patients |
M/F |
Ongoing |
81 |
|
ChiCTR2000029996 |
A phase 2, open‐label, randomized clinical trial which assesses the safety and efficacy of FPV in COVID‐19 patients. |
M/F |
Ongoing |
82 |
|
ChiCTR2000030113 |
A randomized controlled trial which evaluates the safety and efficacy of FPV in COVID‐19 patients who show low responsiveness to ritonavir treatment. |
M/F |
Ongoing |
83 |
|
ChiCTR2000030987 |
A randomized controlled trial which examines the safety and efficacy of FPV (in combination with chloroquine phosphate) in COVID‐19 patients. |
M/F |
Ongoing |
84 |
|
CTRI/2020/05/025114 |
A phase 3, open‐label, randomized clinical trial which evaluates the safety and efficacy of FPV in patients suffering from mild to moderate COVID‐19 and compares the efficacy with standard care treatment. |
M/F |
Ongoing |
85 |
|
CTRI/2020/06/025799 |
A phase 3, open‐label, multicenter, randomized clinical trial which evaluates the safety and efficacy of FPV in patients suffering from mild to moderate COVID‐19 and compares the efficacy with supportive care treatment. |
M/F |
Ongoing |
86 |
|
CTRI/2020/06/025957 |
A phase 3, open‐label, randomized clinical trial which evaluates the safety and efficacy of FPV in patients suffering from mild to moderate COVID‐19 and compares the efficacy with the combined FPV/umifenovir treatment. |
M/F |
Ongoing |
87 |
*Abbreviations: M, male; F, female
Similar to other drug therapies, a major challenge that may militate against the widespread use of RDV and FPV is the possible development of resistance among CoVs. The possibility of this has been partially supported by an earlier study, [89] which has found that mutations in the RNA polymerase enable the influenza A virus to be resistant to FPV. To combat the development of drug resistance, one possible strategy is to modify the structure of the drug; however, this takes time. Another possible strategy is to manipulate the dose adopted. The feasibility of this has been shown by Agostini and coworkers, [90] who have observed that resistance to RDV can be overcome by using a higher yet nontoxic dose of the drug. Although many more works are required to examine, evaluate, and optimize the use of both FPV and RDV, it is hard to refute the fact that FPV and RDV have been playing an important role in the treatment of COVID‐19 till now. The latest status of research on their use, as well as an overview of the life cycle and routes of transmission of SARS‐CoV‐2, has been presented in this article. It is hoped that the opportunities and challenges brought about by the use of these therapeutic options can be illuminated for future research.
Conflict of interest
The authors declare no conflict of interests. This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Biographical Information
Obireddy Sreekanth Reddy completed his Ph.D. in the Department of Chemistry at Sri Krishnadevaraya University, Andhra Pradesh (India). His research work focuses specifically on the fabrication of novel polymeric blend matrices for controlled drug delivery applications.

Biographical Information
Wing‐Fu Lai is an Assistant Professor in the School of Life and Health Sciences at the Chinese University of Hong Kong, Shenzhen. He is also an adjunct faculty member in the Department of Applied Biology and Chemical Technology at the Hong Kong Polytechnic University. His research interest lies in the design and engineering of both organic and inorganic materials for encapsulation and controlled release of bioactive agents.

O. Sreekanth Reddy, W.-F. Lai, ChemBioChem 2021, 22, 939.
References
- 1. Rothan H. A., Byrareddy S. N., J. Autoimmun. 2020, 109, 102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B., Lancet 2020, 395, 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morse J. S., Lalonde T., Xu S., Liu W. R., ChemBioChem 2020, 21, 730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singh T. U., Parida S., Lingaraju M. C., Kesavan M., Kumar D., Singh R. K., Pharmacol. Rep. 2020, 5, 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Furuta Y., Gowen B. B., Takahashi K., Shiraki K., Smee D. F., Barnard D. L., Antiviral Res. 2013, 100, 446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shiraki K., Daikoku T., Favipiravir, Pharmacol. Ther. 2020, 209, 107512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Furuta Y., Takahashi K., Kuno-Maekawa M., Sangawa H., Uehara S., Kozaki K., Nomura N., Egawa H., Shiraki K., Antimicrob. Agents Chemother. 2005, 49, 981–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Du Y. X., Chen X. P., Clin. Pharmacol. Ther. 2020, 108, 242–247. [DOI] [PubMed] [Google Scholar]
- 9. Furuta Y., Komeno T., Nakamura T., Proc. Jpn. Acad., Ser. B 2017, 93, 449–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Coronavirus disease (COVID-19). Retrieved August 14, 2020 from https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- 11. Boopathi S., Poma A. B., Kolandaivel P., J. Biomol. Struct. Dyn. 2020, 30, 1–10. [Google Scholar]
- 12. Wang L. S., Wang Y. R., Ye D. W., Liu Q. Q., Int. J. Antimicrob. Agents 2020, 55, 105948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu Y., Gayle A. A., Wilder-Smith A., Rocklöv J., J. Travel Med. 2020, 27, taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou P., Yang X. L., Wang X. G., Hu B., Zhang L., Zhang W., Si H. R., Zhu Y., Li B., Huang C. L., Chen H. D., Chen J., Luo Y., Guo H., Jiang R. D., Liu M. Q., Chen Y., Shen X. R., Wang X., Zheng X. S., Zhao K., Chen Q. J., Deng F., Liu L. L., Yan B., Zhan F. X., Wang Y. Y., Xiao G., Shi Z. L., bioRxiv 2020, DOI: 10.1101/2020.01.22.914952. [Google Scholar]
- 15. Li H., Yang L., Liu F. F., Ma X. N., He P. I., Tang W., Tong X. K., Zuo J. P., Acta Pharmacol. Sin. 2020, 41, 1133–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hu B., Ge X., Wang L. F., Shi Z., Virol. J. 2015, 12, 221–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Westerbeck J. W., Machamer C. E., J. Virol. 2019, 93, e00015–e00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P., Pan P., Wang W., Hu D., Liu X., Zhang Q., Wu J., J. Med. Virol. 2020, 92, 424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu X., Chen P., Wang J., Feng J., Zhou H., Li X., Zhong W., Hao P., Sci. China Life Sci. 2020, 63, 457–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Wit E., van Doremalen N., Falzarano D., Munster V. J., Nat. Rev. Microbiol. 2016, 14, 523–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eastman R. T., Roth J. S., Brimacombe K. R., Simeonov A., Shen M., Patnaik S., Hall M. D., ACS Cent. Sci. 2020, 6, 672–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cao Y. C., Deng Q. X., Dai S. X., Travel Med. Infect. Di. 2020, 35 101647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paules C. I., Marston H. D., Fauci A. S., JAMA 2020, 323, 707–708. [DOI] [PubMed] [Google Scholar]
- 24. Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B., Lancet 2020, 395, 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lei J., Li J., Li X., Qi X., Radiology 2020, 295, 18–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang X. Y., Huang H. J., Zhuang D. L., Nasser M. I., Yang M. H., Zhu P., Zhao M. Y., Biological, Infect. Dis. Poverty 2020, 9, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carsana L., Sonzogni A., Nasr A., Rossi R. S., Pellegrinelli A., Zerbi P., Rech R., Colombo R., Antinori S., Corbellino M., Galli M., Catena E., Tosoni A., Gianatti A., Nebuloni M., Lancet Infect. Dis. 2020, 20, 1135–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zu Z. Y., Jiang M. D., Xu P. P., Chen W., Ni Q. Q., Lu G. M., Zhang L. J., Radiology 2020, 296, E15–E25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. El Zowalaty M. E., Järhult J. D., One Health 2020, 9, 100124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cheng Z. J., Shan J., Infection 2020, 48, 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Malik M., Elkholy A., Khan W., Hassounah S., Abubakar A., Minh N. T., Mala P., E. Mediterr. Health J. 2016, 22, 533–542. [PubMed] [Google Scholar]
- 32. Yang Y., Peng F., Wang R., Yange M., Guan K., Jiang T., Xu G., Sun J., Chang C., J. Autoimmun. 2020, 109, 102434–102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eslami H., Jalili M., AMB Express, 2020, 10, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang M., Jiang A., Gong L., Luo L., Guo W., Li C., Zheng J., Li C., Yang B., Zeng J., Chen Y., Zheng K., Li H., medRxiv 2020, DOI: 10.1101/2020.02.22.20025791v1. [Google Scholar]
- 35. Chin A., Chu J., Perera M., Hui K., Yen H. L., Chan M., Peiris M., Poon L., medRxiv 2020, DOI: 10.1101/2020.03.15.20036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.S. Bhattacharjee, arXiv 2020, arXiv:2003.11277.
- 37. Roviello V., Roviello G. N., Environ. Chem. Lett. 2020, DOI: 10.1007/s10311-020-01063-0. [Google Scholar]
- 38. Sheahan T. P., Sims A. C., Graham R. L., Menachery V. D., Gralinski L. E., Case J. B., Leist S. R., Pyrc K., Feng J. Y., Trantcheva I., Bannister R., Park Y., Babusis D., Clarke M. O., Mackman R. L., Spahn J. E., Palmiotti C. A., Siegel D., Ray A. S., Cihlar T., Jordan R., Denison M. R., Baric R. S., Sci. Transl. Med. 2017, 9, eaal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M. L., Lescure F. X., Nicastri E., Oda R., Yo K., Quiros-Roldan E., Studemeister A., Redinski J., Ahmed S., Bernett J., Chelliah D., Chen D., Chihara S., Cohen S. H., Cunningham J., D'Arminio Monforte A., Ismail S., Kato H., Lapadula G., L'Her E., Maeno T., Majumder S., Massari M., Mora-Rillo M., Mutoh Y., Nguyen D., Verweij E., Zoufaly A., Osinusi A. O., DeZure A., Zhao Y., Zhong L., Chokkalingam A., Elboudwarej E., Telep L., Timbs L., Henne I., Sellers S., Cao H., Tan S. K., Winterbourne L., Desai P., Mera R., Gaggar A., Myers R. P., Brainard D. M., Childs R., Flanigan T., N. Engl. J. Med. 2020, 382, 2327–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gordon C. J., Tchesnokov E. P., Woolner E., Perry J. K., Feng J. Y., Porter D. P., Götte M., J. Biol. Chem. 2020, 295, 6785–6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Siegel D., Hui H. C., Doerffler E., Clarke M. O., Chun K., Zhang L., Neville S., Carra E., Lew W., Ross B., Wang Q., Wolfe L., Jordan R., Soloveva V., Knox J., Perry J., Perron M., Stray K. M., Barauskas O., Feng J. Y., Xu Y., Lee G., Rheingold A. L., Ray A. S., Bannister R., Strickley R., Swaminathan S., Lee W. A., Bavari S., Cihlar T., Lo M. K., Warren T. K., Mackman R. L., J. Med. Chem. 2017, 60, 1648–1661. [DOI] [PubMed] [Google Scholar]
- 42. Furuta Y., Egawa H., Nomura N., 2004, US20020013316A1.
- 43. Liu F. L., Li C. Q., Xiang H. Y., Feng S., Chem. Pap. 2017, 71, 2153-2158. [Google Scholar]
- 44. Hara T., Norimatsu N., Kurushima H., Kano T., 2013, US8835636B2.
- 45. Guo Q., Xu M., Guo S., Zhu F., Xie Y., Shen J., Chem. Pap. 2019, 73, 1043–1051. [Google Scholar]
- 46. Sheahan T. P., Sims A. C., Graham R. L., Menachery V. D., Gralinski L. E., Case J. B., Leist S. R., Pyrc K., Feng J. Y., Trantcheva I., Bannister R., Park Y., Babusis D., Clarke M. O., Mackman R. L., Spahn J. E., Palmiotti C. A., Siegel D., Ray A. S., Cihlar T., Jordan R., Denison M. R., Baric R. S., Sci. Transl. Med. 2017, 9, eaal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pruijssers A. J., George A. S., Schäfer A., Leist S. R., Gralinksi L. E., Dinnon K. H., Yount B. L., Agostini M. L., Stevens L. J., Chappell J. D., Lu X., Hughes T. M., Gully K., Martinez D. R., Brown A. J., Graham R. L., Perry J. K., Du Pont V., Pitts J., Ma B., Babusis D., Murakami E., Feng J. Y., Bilello J. P., Porter D. P., Cihlar T., Baric R. S., Denison M. R., Sheahan T. P., Cell Rep. 2020, 32, 107940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Williamson B. N., Feldmann F., Schwarz B., Meade-White K., Porter D. P., Schulz J., van Doremalen N., Leighton I., Kwe Yinda C., Pérez-Pérez L., Okumura A., Lovaglio J., Hanley P. W., Saturday G., Bosio C. M., Anzick S., Barbian K., Cihlar T., Martens C., Scott D. P., Munster V. J., de Wit E., bioRxiv 2020, DOI: 10.1101/2020.04.15.043166. [Google Scholar]
- 49. Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M. L., Lescure F. X., Nicastri E., Oda R., Yo K., Quiros-Roldan E., Studemeister A., Redinski J., Ahmed S., Bernett J., Chelliah D., Chen D., Chihara S., Cohen S. H., Cunningham J., D'Arminio Monforte A., Ismail S., Kato H., Lapadula G., L'Her E., Maeno T., Majumder S., Massari M., Mora-Rillo M., Mutoh Y., Nguyen D., Verweij E., Zoufaly A., Osinusi A. O., DeZure A., Zhao Y., Zhong L., Chokkalingam A., Elboudwarej E., Telep L., Timbs L., Henne I., Sellers S., Cao H., Tan S. K., Winterbourne L., Desai P., Mera R., Gaggar A., Myers R. P., Brainard D. M., Childs R., Flanigan T., N. Engl. J. Med. 2020, 382, 2327–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Beigel J. H., Tomashek K. M., Dodd L. E., Mehta A. K., Zingman B. S., Kalil A. C., Hohmann E., Chu H. Y., Luetkemeyer A., Kline S., Lopez de Castilla D., Finberg R. W., Dierberg K., Tapson V., Hsieh L., Patterson T. F., Paredes R., Sweeney D. A., Short W. R., Touloumi G., Lye D. C., Ohmagari N., Oh M. D., Ruiz-Palacios G. M., Benfield T., Fätkenheuer G., Kortepeter M. G., Atmar R. L., Creech C. B., Lundgren J., Babiker A. G., Pett S., Neaton J. D., Burgess T. H., Bonnett T., Green M., Makowski M., Osinusi A., Nayak S., Lane H. C., N. Engl. J. Med. 2020, 383, 992. [Google Scholar]
- 51. Yokoyama Y., Briasoulis A., Takagi H., Kuno T., Virus Res. 2020, 288, 198137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Antinori S., Cossu M. V., Ridolfo A. L., Rech R., Bonazzetti C., Pagani G., Gubertini G., Coen M., Magni C., Castelli A., Borghi B., Colombo R., Giorgi R., Angeli E., Mileto D., Milazzo L., Vimercati S., Pellicciotta M., Corbellino M., Torre A., Rusconi S., Oreni L., Gismondo M. R., Giacomelli A., Meroni L., Rizzardini G., Galli M., Pharmacol. Res. 2020, 158, 104899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wise J., BMJ 2020, 369, m2610. [DOI] [PubMed] [Google Scholar]
- 54. Ochoa Chaar C. I., Makuch R., Ther. Innov. Regul. Sci. 2020, DOI: 10.1007/s43441-020-00212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dubert M., Visseaux B., Isernia V., Bouadma L., Deconinck L., Patrier J., Wicky P. H., Le Pluart D., Kramer L., Rioux C., Le Hingrat Q., Houhou-Fidouh N., Yazdanpanah Y., Ghosn J., Lescure F. X., Int. J. Infect. Dis. 2020, 98, 290–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Maldarelli G. A., Savage M., Mazur S., Oxford-Horrey C., Salvatore M., Marks K. M., Open Forum Infect. Dis. 2020, 7, ofaa345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cai Q., Yang M., Liu D., Chen J., Shu D., Xia J., Liao X., Gu Y., Cai Q., Yang Y., Shen C., Li X., Peng L., Huang D., Zhang J., Zhang S., Wang F., Liu J., Chen L., Chen S., Wang Z., Zhang Z., Cao R., Zhong W., Liu Y., Liu L., Engineering 2020, DOI: 10.1016/j.eng.2020.03.007. [Google Scholar]
- 58. Chen C., Zhang Y., Huang J., Yin P., Cheng Z., Wu J., Chen S., Zhang Y., Chen B., Lu M., Luo Y., Ju L., Zhang J., Wang X., medRxiv 2020, DOI: 10.1101/2020.03.17.20037432. [Google Scholar]
- 59. Koshi E., Saito S., Okazaki M., Toyama Y., Ishimoto T., Kosugi T., Hiraiwa H., Jingushi N., Yamamoto T., Ozaki M., Goto Y., Numaguchi A., Miyagawa Y., Kato I., Tetsuka N., Yagi T., Maruyama S., CEN Case Rep. 2020, DOI: 10.1007/s13730-020-00534-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dauby N., Van Praet S., Vanhomwegen C., Veliziotis I., Konopnicki D., Roman A., J. Med. Virol. 2020, DOI: 10.1002/jmv.26488. [DOI] [PubMed] [Google Scholar]
- 61. Inoue H., Jinno M., Ohta S., Kishino Y., Kawahara T., Mikuni H., Sato H., Yamamoto M., Sato Y., Onitsuka C., Goto Y., Ikeda H., Sato H., Uno T., Uchida Y., Kimura T., Miyata Y., Hirai K., Homma T., Watanabe Y., Kusumoto S., Suzuki S., Tokimatsu I., Tanaka A., Sagara H., Respir. Med. Case Rep. 2020, 31, 101200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Murohashi K., Hagiwara E., Kitayama T., Yamaya T., Higa K., Sato Y., Otoshi R., Shintani R., Okabayashi H., Ikeda S., Niwa T., Nakazawa A., Oda T., Okuda R., Sekine A., Kitamura H., Baba T., Komatsu S., Iwasawa T., Kaneko T., Ogura T., Respir. Investig. 2020, DOI: 10.1016/j.resinv.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Takoi H., Togashi Y., Fujimori D., Kaizuka H., Otsuki S., Wada T., Takeuchi Y., Abe S., Int. J. Infect. Dis. 2020, DOI: 10.1016/j.ijid.2020.09.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.U. S. National Library of Medicine. 2020, A phase 3 randomized study to evaluate the safety and antiviral activity of remdesivir (GS-5734™) in participants with severe COVID-19. Retrieved August 14, 2020 from: https://clinicaltrials.gov/ct2/show/NCT04292899.
- 65.U. S. National Library of Medicine. 2020, A phase 3 randomized study to evaluate the safety and antiviral activity of remdesivir (GS-5734™) in participants with moderate COVID-19 compared to standard of care treatment. Retrieved August 14, 2020 from: https://clinicaltrials.gov/ct2/show/NCT04292730.
- 66.U. S. National Library of Medicine. 2020, A multicenter, adaptive, randomized blinded controlled trial of the safety and efficacy of investigational therapeutics for the treatment of COVID-19 in hospitalized adults (ACTT-2). Retrieved August 14, 2020 from: https://clinicaltrials.gov/ct2/show/NCT04401579.
- 67.U. S. National Library of Medicine. 2020, A multicenter, adaptive, randomized blinded controlled trial of the safety and efficacy of investigational therapeutics for the treatment of COVID-19 in hospitalized adults. Retrieved August 14, 2020 from: https://clinicaltrials.gov/ct2/show/NCT04280705.
- 68.U. S. National Library of Medicine. 2020, Role of investigational therapies alone or in combination to treat moderate, severe and critical COVID-19. Retrieved August 14, 2020 from: https://clinicaltrials.gov/ct2/show/NCT04492501.
- 69.European Union Clinical Trials Register. 2020, The NOR-SWE Solidarity multicenter trial on the efficacy of different anti-viral drugs in SARS-CoV-2 infected patients. Retrieved August 14, 2020 from: https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-000982-18/SE.
- 70.European Union Clinical Trials Register. 2020, An international randomised trial of additional treatments for COVID-19 in hospitalised patients who are all receiving the local standard of care. Retrieved August 14, 2020 from: https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-001366-11/IE.
- 71.European Union Clinical Trials Register. 2020, A phase 2/3 single-arm, open-label study to evaluate the safety, tolerability, pharmacokinetics, and efficacy of remdesivir (GS-5734™) in participants from birth to < 18 years of age with COVID-19. Retrieved August 14, 2020 from: https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-001803-17/GB.
- 72.Japan Registry of Clinical Trials. 2020, A multicenter, adaptive, randomized blinded controlled trial of the safety and efficacy of investigational therapeutics for the treatment of COVID-19 in hospitalized adults. Retrieved August 14, 2020 from: https://rctportal.niph.go.jp/en/detail?trial id=jRCT2031190264.
- 73.U. S. National Library of Medicine. 2020, The regimen of favipiravir plus hydroxychloroquine can accelerate recovery of the COVID-19 patients with moderate severity in comparison to lopinavir/ritonavir plus hydroxychloroquine regimen: an open-label, non-randomized clinical trial study. Retrieved August 14, 2020 from: https://clinicaltrials.gov/ct2/show/NCT04376814.
- 74.U. S. National Library of Medicine. 2020, Open-label, randomised, single oral dose, two-period, cross-over trial to assess the bioequivalence of Favir 200 Mg Ft in comparison with Avigan 200 Mg Ft in healthy male subjects under fasting conditions. Retrieved August 14, 2020 from: https://clinicaltrials.gov/ct2/show/NCT04444986.
- 75.U. S. National Library of Medicine. 2020, Open-label, randomised,single oral dose,two-period,cross-over trial to assess to bioequivalence of Favicovir 200 mg FT in comparison with Avigan 200 mg ft in healthy male subjects under fasting conditions. Retrieved August 14, 2020 from: https://clinicaltrials.gov/ct2/show/NCT04406194.
- 76.U. S. National Library of Medicine. 2020, Open-label, randomised, single oral dose, two-period, cross-over trial to assess to bioequivalence of Favira 200 mg FT in comparison with Avigan 200 mg FT in healthy male subjects under fasting conditions. Retrieved August 14, 2020 from: https://clinicaltrials.gov/ct2/show/NCT04400682.
- 77.U. S. National Library of Medicine. 2020, Open-label,randomised,single oral dose,two-period,cross-over trial to assess to bioequivalence of Loqular 200 mg FT in comparison with Avigan 200 mg FT in healthy male subjects under fasting conditions. Retrieved August 14, 2020 from: https://clinicaltrials.gov/ct2/show/NCT04407000.
- 78.U. S. National Library of Medicine. 2020, Efficacy and safety of favipiravir in management of COVID-19 (FAV-001). Retrieved August 14, 2020 from: https://clinicaltrials.gov/ct2/show/NCT04349241.
- 79.Chinese Clinical Trial Register. 2020, A randomized controlled trial for the efficacy and safety of baloxavir marboxil, favipiravir tablets in novel coronavirus pneumonia (COVID-19) patients who are still positive on virus detection under the current antiviral therapy. Retrieved August 14, 2020 from: http://www.chictr.org.cn/showprojen.aspx?proj=49013.
- 80.Chinese Clinical Trial Register. 2020, Randomized, open-label, controlled trial for evaluating of the efficacy and safety of baloxavir marboxil, favipiravir, and lopinavir-ritonavir in the treatment of novel coronavirus pneumonia (COVID-19) patients. Retrieved August 14, 2020 from: http://www.chictr.org.cn/showprojen.aspx?proj=49015.
- 81.Chinese Clinical Trial Register. 2020, Clinical study on safety and efficacy of favipiravir in the treatment of novel coronavirus pneumonia (COVID-19). Retrieved August 14, 2020 from: http://www.chictr.org.cn/showprojen.aspx?proj=49042.
- 82.Chinese Clinical Trial Register. 2020, A randomized, open-label, controlled trial for the efficacy and safety of Farpiravir Tablets in the treatment of patients with novel coronavirus pneumonia (COVID-19). Retrieved August 14, 2020 from: http://www.chictr.org.cn/showprojen.aspx?proj=49510.
- 83.Chinese Clinical Trial Register. 2020, Clinical study for safety and efficacy of Favipiravir in the treatment of novel coronavirus pneumonia (COVID-19) with poorly responsive ritonavir. Retrieved August 14, 2020 from: http://www.chictr.org.cn/showprojen.aspx?proj=49988.
- 84.Chinese Clinical Trial Register. 2020, Clinical trial of favipiravir tablets combine with chloroquine phosphate in the treatment of novel coronavirus pneumonia (COVID-19). Retrieved August 14, 2020 from: http://www.chictr.org.cn/showprojen.aspx?proj=51329.
- 85.Clinical Trials Registry India. 2020, A randomized, open-label, multicenter study to evaluate the efficacy and safety of favipiravir combined with standard supportive care in adult Indian patients with mild to moderate COVID-19. Identifier CTRI/2020/05/025114. Retrieved August 14, 2020 from: http://ctri.nic.in/Clinicaltrials/showallp.php?mid1=43504&EncHid=&userName=CTRI/2020/05/025114.
- 86.Clinical Trials Registry India. 2020, A randomized, open label, prospective, comparative, parallel group, multicentre study to evaluate efficacy and safety of favipiravir with supportive care versus supportive care alone in subjects with mild to moderate Coronavirus Disease (COVID-19). Retrieved August 14, 2020 from: http://ctri.nic.in/Clinicaltrials/showallp.php?mid1=44480&EncHid=&userName=CTRI/2020/06/025799.
- 87.Clinical Trials Registry India. 2020, A randomized open-label study to evaluate the efficacy and safety of favipiravir and umifenovir as compared to favipiravir alone in moderate hospitalized adult Indian COVID-19 patients. Retrieved August 14, 2020 from: http://ctri.nic.in/Clinicaltrials/showallp.php?mid1=44709&EncHid=&userName=CTRI/2020/06/025957.
- 88.Gilead Sciences. 2020, An open letter from Daniel O'Day, chairman and CEO. Retrieved August 14, 2020 from: https://www.gilead.com/news-and-press/press-room/press-releases/2020/6/an-open-letter-from-daniel-oday-chairman–ceo-gilead-sciences.
- 89. Goldhill D. H., Te Velthuis A. J. W., Fletcher R. A., Langat P., Zambon M., Lackenby A., Barclay W. S., Proc. Natl. Acad. Sci. U.S.A. 2018, 115, 11613–11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Agostini M. L., Andres E. L., Sims A. C., Graham R. L., Sheahan T. P., Lu X., Smith E. C., Case J. B., Feng J. Y., Jordan R., Ray A. S., Cihlar T., Siegel D., Mackman R. L., Clarke M. O., Baric R. S., Denison M. R., mBio. 2018, 9, e00221–00218. [DOI] [PMC free article] [PubMed] [Google Scholar]


