To the Editor
We read with interest the letter by Dzik and colleagues, 1 who compared the ABO blood group distribution between 957 COVID‐19 patients admitted in the Boston area during the 2020 pandemic for whom ABO typing was available and 5840 historical controls from the same period of 2019 and found no significant difference. The investigation was prompted by evidence from previous studies conducted in Asia and Mediterranean Europe highlighting a higher risk of severe SARS‐CoV‐2 infection in individuals carrying the A blood group, and relative protection in those carrying the O group,2, 3, 4 although evidence is still controversial. 5 By examining Italian and Spanish cohorts, we detected a cross‐replicating association between rs657152 at the ABO locus with severe COVID‐19 with respiratory failure that was significant at genomewide level. 2 The analysis was restricted to unrelated individuals of European descent and was independent of age, sex and the genetic background. A blood group–specific analysis showed a higher risk of severe COVID‐19 in blood group A (odds ratio [OR], 1.45 95% confidence interval [95% CI], 1.20‐1.75; P = .028) and a protective effect in blood group O compared with other blood groups (OR, 0.65; 95% CI, 0.53‐0.79; P = .004). By analyzing a local control group of 14 658 first‐time Italian blood donors from Milan, it was shown that the association was not driven by a selection bias in the controls due to selective retention of blood group O individuals in the blood donation program. 2
Several factors may account for the discrepant results obtained by Dzik et al. 1 Indeed, the Boston cohort included a different range of COVID‐19 severity (including mild infections), and the analysis was not adjusted for confounders; in particular, it did not account for genetic background differences between cases and controls. On the other side, previous studies could not discriminate whether the association between ABO and COVID‐19 was explained by an increased risk of infection by SARS‐CoV‐2 or by an increased risk of progression toward the development of more severe symptoms. 2
Supporting the association between ABO and the risk of severe COVID‐19, we now provide data on the ABO blood frequency among patients admitted at the Fondazione IRCCS Caʼ Granda Milan during the outbreak. In Table 1 (top panel), we report the impact of ABO group on the risk of severe COVID‐19 with respiratory failure in a case–control study conducted in unrelated European individuals for whom genetic typing is available. 2 We confirmed the findings in the overall cohort (shown in Table 1, middle panel) that carriage of group A was associated with higher risk of severe COVID‐19 than group O independently of confounders (OR, 1.43; 95% CI, 1.04‐1.96; P = .032), while group B was not. 2 We also observed a marked increase in the risk of severe disease in carriers of the AB group (OR, 2.78; 95% CI, 1.38‐5.58; P = .005). To further validate the association, as suggested by Dzik et al.,1 we next examined the impact of ABO on severe COVID‐19 by comparing the same patients to a further control group of European patients evaluated at the Milan blood bank during 2019, where ABO blood group could not be biased by inclusion in a blood donation program (Table 1, middle panel). The overall ABO distribution in this control group was similar than that of Milan blood donors. We confirmed a higher risk of severe COVID‐19 in carriers of group A (OR, 1.24; 95% CI, 1.02‐1.52) and AB (OR, 1.72; 95% CI, 1.18‐2.51) compared to group O, although the effect size was smaller than in the previous analysis. When we evaluated the impact of ABO on disease severity (admission to intensive care unit due to the requirement of mechanical ventilation) within the severe COVID‐19 cohort of patients with respiratory failure, we observed a nonsignificant trend for a higher risk in carriers of type B blood (P = .051), while group A did not further increase the risk (Table 1, bottom panel).
TABLE 1.
Frequency distribution of ABO blood groups between unrelated European patients with COVID‐19 admitted at the Fondazione IRCCS Cà Granda during March and April 2019 and two different control groups
| Blood group | ||||
|---|---|---|---|---|
| O | A | B | AB | |
| GWAS case–control study | ||||
| Severe COVID‐19 (n = 505) | 190 (37.6) | 225 (44.6) | 53 (10.5) | 37 (7.3) |
| Blood donors (n = 890) | 416 (46.7) | 339 (38.1) | 106 (11.9) | 29 (3.3) |
| OR | REF | 1.43 | 1.05 | 2.78 |
| 95% CI | 1.04‐1.96 | 0.64‐1.75 | 1.38‐5.58 | |
| P value a | 0.028 | 0.83 | 0.004 | |
| Expanded case–control study | ||||
| Transfused 2019 (n = 18 097) | 7831 (43.3) | 7291 (40.3) | 2174 (12.0) | 801 (4.4) |
| OR | REF | 1.24 | 0.96 | 1.72 |
| 95% CI | 1.02‐1.52 | 0.70‐1.32 | 1.18‐2.51 | |
| P value b | 0.032 | 0.80 | 0.005 | |
| Severe COVID‐19 patients | ||||
| ICU | 32 (33.0) | 42 (43.3) | 14 (14.4) | 9 (9.3) |
| Medicine | 158 (38.7) | 183 (44.9) | 39 (9.6) | 28 (6.9) |
| OR | REF | 1.19 | 2.08 | 1.78 |
| 95% CI | 0.71‐2.00 | 0.99‐4.37 | 0.75‐4.22 | |
| P value c | 0.51 | 0.051 | 0.34 | |
Note: Unrelated European healthy blood donors evaluated at the Fondazione during the same period (top panel) and patients evaluated at the Fondazione Blood Bank during 2019 for blood transfusion (middle panel). The impact of ABO blood groups on the risk of admission to the ICU is reported in the bottom panel.
Abbreviations: GWAS, Genomewide association study; ICU, intensive care unit; REF, reference.
OR of severe COVID‐19 with respiratory failure; at logistic regression analysis adjusted for age, sex, smoking status, arterial hypertension, and carriage of rs11385942, the top COVID‐19 risk variant at the Chromosome 3 gene cluster 2 .
OR of severe COVID‐19 with respiratory failure; at logistic regression analysis adjusted for age and sex.
OR of admission to ICU due to requirement of mechanical ventilation in COVID‐19 with respiratory failure; at logistic regression analysis adjusted for age, sex, smoking status, arterial hypertension, and carriage of rs11385942, the top COVID‐19 risk variant at the Chromosome 3 gene cluster 2 .
Potential biologic mechanisms underlying the epidemiologic association encompass the neutralizing activity of natural anti‐A against SARS‐CoV spike protein 6 and/or the known impact of non‐O blood groups on von Willebrand factor levels, which predisposes to thrombotic disorders.7, 8 Consistently with the latter hypothesis, carriage of blood group A was previously associated with development of acute respiratory distress syndrome after severe sepsis and major trauma. 9 The secretor ABO phenotype has also been robustly linked to protection against other RNA virus infections affecting mucous membranes, namely, rotaviruses and enteroviruses. 10 Therefore, we next assessed the impact of the rs601338 G>A FUT2 variant, the main determinant of nonsecretor phenotype in Europeans, on the main study outcomes in the models reported in Table 1. Although the variant was not associated with development of severe COVID‐19 with respiratory failure (P = .62), it protected against the requirement of mechanical ventilation and ICU admission (adjusted OR, 0.57; 95% CI, 0.37‐0.87; P = .007). This was related to a specific interaction and protection in carriers of blood group A (P = .035). Although there was no significant association with the risk of thrombotic events (stroke, myocardial infarction, and venous thromboembolism), remarkably the nonsecretor phenotype was associated with lower in‐hospital mortality (adjusted OR, 0.53; 95% CI, 0.32‐0.83; P = .014). Mortality data according to O vs non‐O blood group and rs601338 variant are shown in Figure 1. Therefore, secretion of A/B antigens may promote COVID‐19 progression, but further studies will be necessary to confirm the protective effect of nonsecretor phenotype.
FIGURE 1.
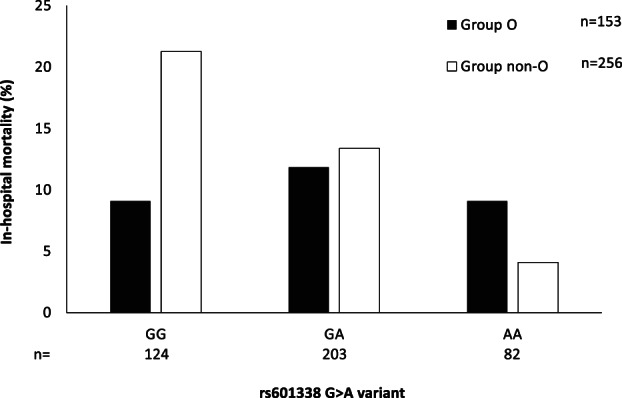
Association between FUT2 rs601338 G>A variant (encoding for the nonsecretor phenotype) and in‐hospital mortality (% values) among 409 patients with severe COVID‐19 with respiratory failure, for whom complete data and follow‐up were available. P = .007 for the impact of the A variant on mortality in group non‐O patients; P = NS in group O patients
These data are consistent with the hypothesis that carriage of non‐O blood groups predisposes to severe COVID‐19 with respiratory failure in European individuals and that the secretor phenotype may moderate disease progression. However, we definitively agree with Dzik and colleagues that additional studies, taking into account genetic and acquired cofactors, are required to confirm the association of ABO blood group and the secretor phenotype with the risk of COVID‐19 worldwide and to investigate the underlying mechanisms and the possible translational implications.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Funding information Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Grant/Award Numbers: PR‐0391, RC100017A; H2020 European Research Council, Grant/Award Number: IMI2‐777377; Ministero della Salute, Grant/Award Number: RF‐2016‐02364358
REFERENCES
- 1. Dzik S, Eliason K, Morris EB, Kaufman RM, North CM. COVID‐19 and ABO blood groups. Transfusion. 2020;60:1883–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ellinghaus D, Degenhardt F, Bujanda L, et al. Genomewide association study of severe Covid‐19 with respiratory failure. N Engl J Med. 2020;383(16):1522–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhao J, Yang Y, Huang H, et al. Relationship between the ABO blood group and the COVID‐19 susceptibility. Clin Infect Dis. 2020; [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu Y, Feng Z, Li P, Yu Q. Relationship between ABO blood group distribution and clinical characteristics in patients with COVID‐19. Clin Chim Acta. 2020;509:220–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boudin L, Janvier F, Bylicki O, Dutasta F. ABO blood groups are not associated with risk of acquiring the SARS‐CoV‐2 infection in young adults. Haematologica. 2020; [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guillon P, Clement M, Sebille V, et al. Inhibition of the interaction between the SARS‐CoV spike protein and its cellular receptor by anti‐histo‐blood group antibodies. Glycobiology. 2008;18:1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Franchini M, Crestani S, Frattini F, Sissa C, Bonfanti C. ABO blood group and von Willebrand factor: Biological implications. Clin Chem Lab Med. 2014;52:1273–1276. [DOI] [PubMed] [Google Scholar]
- 8. Zalba Marcos S, Antelo ML, Galbete A, Etayo M, Ongay E, Garcia‐Erce JA. Infection and thrombosis associated with COVID‐19: Possible role of the ABO blood group. Med Clin (Barc). 2020; [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reilly JP, Meyer NJ, Shashaty MGS, et al. ABO blood type A is associated with increased risk of ARDS in whites following both major trauma and severe sepsis. Chest. 2014;145:753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Le Pendu J, Ruvoen‐Clouet N. Fondness for sugars of enteric viruses confronts them with human glycans genetic diversity. Hum Genet. 2020;139:903–910. [DOI] [PubMed] [Google Scholar]


