Abstract
The COVID-19 pandemic has brought unprecedented challenges to the transplant community. The reduction in transplantation volume during this time is partly due to concerns over potentially increased susceptibility and worsened outcomes of COVID-19 in immunosuppressed recipients. The consequences of COVID-19 on patients waitlisted for kidney transplantation, however, have not previously been characterized. We studied 56 waitlisted patients and 80 kidney transplant recipients diagnosed with COVID-19 between March 13 and May 20, 2020. Despite similar demographics and burden of comorbidities between waitlisted and transplant patients, waitlisted patients were more likely to require hospitalization (82% vs. 65%, P = .03) and were at a higher risk of mortality (34% vs. 16%, P = .02). Intubation was required in one third of hospitalized patients in each group, and portended a very poor prognosis. The vast majority of patients who died were male (84% waitlist, 100% transplant). Multivariate analysis demonstrated waitlist status, age, and male sex were independently associated with mortality. COVID-19 has had a dramatic impact on waitlisted patients, decreasing their opportunities for transplantation and posing significant mortality risk. Understanding the impact of COVID-19 on waitlist patients in comparison to transplant recipients may aid centers in weighing the risks and benefits of transplantation in the setting of ongoing COVID-19.
KEYWORDS: clinical decision-making, clinical research/practice, kidney transplantation/nephrology, patient survival, waitlist management
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; CI, confidence interval; COVID-19, coronavirus disease 2019; ESRD, end stage renal disease; HR, hazard ratio; IQR, interquartile range; NYP, New York Presbyterian; OR, odds ratio; PCR, polymerase chain reaction; PD, peritoneal dialysis; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
1. INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic has brought unprecedented challenges to the medical and transplantation community. During the pandemic, transplantation volume in the United States in March, April, and May of 2020 was severely curtailed, with an overall reduction in deceased donor transplantation of 51%.1 This effect was largely driven by kidney transplantation, and was echoed in other countries, including France and Spain where transplantation fell 90% and 87%, respectively.1 , 2 This severe decline in transplantation activity was borne, in part, out of concerns over potentially increased susceptibility and worsened outcomes of COVID-19 in transplant recipients, as well as concerns over impaired hospital resources for caring for posttransplant recipients. Additional challenges that contributed to reduced transplantation volumes included ensuring safe organ procurement in the setting of unknown risks of transmission, and difficulties in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing of donors and recipients. This was particularly challenging in the early stages of the pandemic due to limited testing capacity, unclear testing reliability, prolonged waiting for test results, and non-uniform protocols across transplant centers.
This overwhelming impact of COVID-19 on organ donation and transplantation may have potentially devastating effects on waitlisted transplant candidates. During this time, transplant programs have faced the difficult decision of carefully weighing the benefits of transplantation against the risks of newly immunosuppressing patients who may subsequently acquire COVID-19. The need for programs to temporarily cease or to temper transplantation activity has impacted an already growing national waitlist, with potentially detrimental effects on waitlist-associated mortality. While a number of studies have characterized COVID-19 disease in transplant recipients, the consequences of COVID-19 on waitlisted patients have not been defined. Simulation models have suggested that kidney transplantation in the context of COVID-19 will provide survival benefit, unless the COVID-19 mortality of transplant patients significantly exceeds that of waitlisted patients.3 Here, we describe our center’s experience with COVID-19 in waitlisted patients, and compare their presentation and outcomes to kidney transplant recipients.
2. MATERIALS AND METHODS
2.1. Patients
We studied all adult (>18 years) patients waitlisted for kidney transplantation at our center and kidney transplant recipients who tested positive for COVID-19 between March 13, 2020 and May 20, 2020. The establishment of a centralized dashboard that captured all patients tested for COVID-19 within any of the New York Presbyterian (NYP) hospital network facilitated the identification of COVID-19 infected waitlisted patients. Additional patients were identified by transplant coordinators performing telephone wellness checks and by waitlist patients notifying their coordinator. Kidney transplant recipients were initially evaluated by Telemedicine platform and triaged as previously described by Lubetzky et al.4 Those with significant or worsening symptomology were referred to the Weill Cornell Medicine Fever Clinic or the NYP/Weill Cornell Emergency Department. Patients were evaluated for COVID-19 infection by nasopharyngeal swab testing for SARS-CoV-2 via Polymerase Chain Reaction (PCR) using one of the following tests (antigen target): Cobas SARS-CoV-2 (ORF1ab and E gene), Xpert Xpress SARS-CoV-2 (E gene and N2), or Panther Fusion SARS-CoV-2 assay (ORF1ab region 1 and ORF1ab region 2). Only patients who tested positive by PCR were included for study. This study was approved by the Weill Cornell Medicine Institutional Review Board protocol # 1207012637 entitled Utilizing a Transplant Database for Quality Assessment and Performance Improvement and Clinical Outcomes and protocol # 20-05022154 entitled Impact of COVID-19 Illness on Kidney Transplant Candidates and Recipients.
2.2. Data collection
Baseline patient demographics and co-morbidities, transplantation characteristics, immunosuppressive regimens, concomitant infections, COVID-19 treatment approaches, and clinical course were extracted from the electronic medical record. For patients who were not admitted to our institution, information was obtained from electronic medical records, their dialysis center, and by Transplant provider and coordinator phone calls to patients and their family. As clinical practices and knowledge evolved during the pandemic, testing of inflammatory markers possibly associated with COVID-19 was more frequently performed; however, early in the course, laboratory testing was not uniformly employed, and laboratory data were not available for all patients admitted to other centers. The percentage of transplant and waitlisted patients who had data available for analysis is indicated in Table S1. Early findings of 54 of the 80 transplant patients with COVID-19 were included in a prior publication by Lubetzky et al.4
2.3. COVID-19 treatment
Hospitalized transplant patients were evaluated by a Transplant physician and a Transplant Infectious Disease specialist who guided anti-viral, anti-inflammatory, and anti-bacterial therapies. Likewise, hospitalized waitlisted patients were managed by Nephrology and Infectious Disease specialists. Patients were evaluated on a case-by-case basis by Infectious Disease physicians for inclusion in ongoing clinical trials at our institution, including for remdesivir, tocilizumab, selinexor, and convalescent plasma. The general approach to immunosuppressive therapy for hospitalized transplant patients involved decreasing mycophenolate mofetil dosage by 50% or more, and reducing tacrolimus dosage to trough levels of 4-6 ng/mL. Immunosuppressive dosing adjustments were generally not made for patients managed at home. Active (status 1) patients on the waitlist who were diagnosed with COVID-19 were made inactive (status 7) and monitored for symptom resolution prior to re-activation.
2.4. Statistical analysis
Baseline characteristics were compared between all COVID-19-positive waitlist and transplant patients ( Table 1). The subset of patients who required hospitalization were further analyzed in a more detailed study of their baseline characteristics, presenting symptoms and labs, COVID-19 treatments, and outcomes ( Tables 2, 3, 4). Continuous variables were expressed as median and interquartile range (IQR) and compared using Student’s t test or Mann-Whitney U test as appropriate. Categorical variables were reported as number (%) and compared using Fisher’s exact t test or chi-square test as appropriate. Univariate and multivariate logistic regression were performed to test the association between hospitalization or mortality and baseline patient characteristics. Factors that were significant on univariate analysis (P < .1) were entered into the multivariate model to identify independent predictors (P < .05). Results were expressed as odds ratios (OR) and 95% confidence intervals (CI). Patient cumulative mortality incidence following COVID-19 diagnosis of all waitlist patients was compared to that of all kidney transplant patients using Kaplan Meier survival estimates and log-rank test. The survival of the waitlist and transplant groups were compared after adjusting for age, sex, and diabetes using the Cox proportional hazards regression model. The United Network for Organ Sharing Standard Transplant Analysis and Research (STAR) file was used to determine waitlist removals due to death for Region 9.
TABLE 1.
Characteristics of all waitlisted and kidney transplant recipients with COVID-19
| Waitlisted (n = 56) | Transplant (n = 80) | P value | |
|---|---|---|---|
| Age, years (range) | 60 (38-86) | 57 (28-83) | .07 |
| Male | 37 (66%) | 56 (70%) | .71 |
| Race/Ethnicity | |||
| Asian | 9 (16%) | 9 (11%) | .45 |
| Black | 26 (46%) | 21 (26%) | .02 |
| Hispanic | 14 (25%) | 24 (30%) | .57 |
| White | 6 (11%) | 25 (31%) | .006 |
| BMI, kg/m2 | 27.9 | 28.1 | .91 |
| Cause of ESRD | |||
| Diabetes | 23 (41%) | 22 (27%) | .14 |
| Hypertension | 18 (32%) | 20 (25%) | .44 |
| Glomerulonephritis | 6 (11%) | 18 (22%) | .11 |
| Lupus | 0 (0%) | 3 (4%) | .27 |
| Polycystic Kidney Disease | 3 (5%) | 4 (5%) | 1.0 |
| Other | 6 (11%) | 13 (16%) | .45 |
| Cardiovascular disease | 26 (46%) | 26 (32%) | .11 |
| Pulmonary disease | 8 (14%) | 11 (14%) | 1.0 |
| Smoking history | 19 (34%) | 16 (20%) | .08 |
| Diabetes | 26 (46%) | 27 (34%) | .16 |
| History of stroke | 8 (14%) | 9 (11%) | .62 |
| ACE inhibitor or ARB | 19 (34%) | 26 (32%) | 1.0 |
| Presenting symptoms | |||
| Fever | 38/50 (76%) | 53/75 (71%) | .55 |
| Cough/upper respiratory symptoms | 34/44 (77%) | 44/74 (59%) | .07 |
| Shortness of breath | 25/44 (57%) | 36/74 (49%) | .45 |
| Fatigue/myalgia | 33/43 (77%) | 34/73 (47%) | .002 |
| Diarrhea | 18/44 (41%) | 25/73 (34%) | .55 |
| Nausea/vomiting | 6/42 (14%) | 5/73 (7%) | .21 |
| Headache/confusion | 10/45 (22%) | 13/73 (18%) | .63 |
| Hospitalized | 46 (82%) | 52 (65%) | .03 |
| Graft loss | — | 4 (5%) | |
| Died | 19 (34%) | 13 (16%) | .02 |
TABLE 2.
Admission laboratory values for waitlisted and transplant patients hospitalized with COVID-19
| Waitlisted (n = 46) | Transplant (n = 52) | P value | |
|---|---|---|---|
| Acute kidney injury | — | 25/45 (56%) | |
| Baseline creatinine (mg/dL) | — | 1.3 (1.0-1.9) | |
| Admission creatinine (mg/dL) | — | 1.9 (1.1-3.2) | |
| White blood cell count (x103/uL) | 6.3 (4.5-10.0) | 5.7 (4.0-7.4) | .16 |
| Absolute lymphocyte count (x103/uL) | 0.8 (0.5-1.1) | 0.6 (0.3-1.0) | .02 |
| Albumin (g/dL) | 3.4 (2.8-3.9) | 3.1 (2.7-3.6) | .48 |
| Troponin (ng/mL) | 0.07 (0.04-0.2) | 0.04 (0.03-0.08) | .05 |
| Lactate dehydrogenase (U/L) | 319.0 (233.5-444.0) | 329.5 (252.5-386.3) | .71 |
| Creatine kinase (U/L) | 334.0 (77.0-421.0) | 74.0 (47.5-155.5) | .38 |
| C-reactive protein (mg/dL) | 32.0 (8.1-63.5) | 12.3 (7.2-30.2) | .44 |
| Erythrocyte sedimentation rate (mm/hr) | 88.0 (63.0-117.0) | 73.5 (49.5-104.8) | .39 |
| Procalcitonin (ng/mL) | 1.7 (0.9-4.2) | 0.3 (0.1-0.6) | .002 |
| D-Dimer (ng/mL) | 507.0 (253.5-786.5) | 388.0 (244.5-558.5) | .39 |
| Ferritin (ng/mL) | 2317.0 (1662.6-3612.5) | 1524.0 (503.7-2428.0) | .005 |
| Interleukin 6 (pg/mL) | 30.8 (21.6-65.4) | 11.0 (5.3-48.3) | .88 |
| Chest X-ray with opacities c/w COVID–19 infection | 31/35 (89%) | 39/45 (87%) | 1.0 |
TABLE 3.
COVID-19 management and outcomes for hospitalized waitlist and kidney transplant patients
| Waitlisted (n = 46) | Transplant (n = 52) | |
|---|---|---|
| Respiratory supporta | ||
| None | 5/35 (14%) | 16 (31%) |
| Nasal Cannula | 16/33 (48%) | 16 (31%) |
| BiPap/Nonrebreather | 7/33 (21%) | 3 (6%) |
| Intubation | 11/38 (29%)b | 16 (31%) |
| Renal replacement therapy | — | 4/47 (9%) |
| Adjustment in Mycophenolate mofetil dose | ||
| No change | — | 5/47 (11%) |
| Reduced | — | 12/47 (26%) |
| Withheld | — | 30/47 (64%) |
| Azithromycin or Doxycycline | 16/33 (48%) | 18/45 (40%) |
| Additional antibiotics | 27/35 (77%) | 28/44 (64%) |
| Bolus steroids | 4/30 (13%) | 8/45 (18%) |
| Experimental therapies (%) | ||
| Hydroxychloroquine | 18/35 (51%) | 34/45 (76%) |
| Remdesivir | 0/30 (0%) | 6/45 (13%) |
| Tocilizumab | 1/30 (3%) | 2/45 (4%) |
| Selinexor | 0/30 (0%) | 2/45 (4%) |
| Convalescent plasma | 0/30 (0%) | 1/45 (2%) |
| Length of admission, days (IQR) | 11 (5-14) | 9 (5–19) |
| Remains inpatient | 2 (4%) | 1 (1.9%) |
| Graft loss | — | 4/47 (9%) |
| Died | 19 (41%) | 13 (25%) |
Indicates the highest level of respiratory support required during hospitalization
Included are all patients who were known to be intubated (n = 9) and who were recommended to be intubated but who elected to be DNR/DNI (n = 2). Not included are seven patients who died at other hospitals and presumably were intubated but confirmatory data are not available.
TABLE 4.
Characteristics of admitted COVID-19 patients stratified by death
| Waitlist survived (n = 27) | Waitlist died (n = 19) | Transplant survived (n = 39) | Transplant died (n = 13) | |
|---|---|---|---|---|
| Age, years (range) | 60.0 (39-74) | 64.0 (48-86) | 55.9 (29-83) | 67.8 (35-74) |
| Male | 17 (63%) | 16 (84%) | 29 (74%) | 13 (100%) |
| Race/Ethnicity | ||||
| Asian | 2 (7%) | 4 (21%) | 4 (10%) | 0 (0%) |
| Black | 14 (52%) | 6 (32%) | 11 (28%) | 4 (31%) |
| Hispanic | 6 (22%) | 7 (37%) | 14 (36%) | 2 (15%) |
| White | 4 (15%) | 1 (5%) | 10 (26%) | 7 (54%) |
| BMI, kg/m2 | 26.5 | 28.3 | 27.4 | 27.3 |
| Cardiovascular disease | 12 (44%) | 9 (47%) | 15 (38%) | 6 (46%) |
| Pulmonary disease | 3 (12%) | 2 (11%) | 7 (18%) | 1 (8%) |
| Smoking history | 10 (37%) | 6 (32%) | 7 (18%) | 4 (31%) |
| Diabetes | 11 (41%) | 12 (63%) | 12 (31%) | 8 (62%) |
| Thymoglobulin Induction | — | — | 31/36 (86%) | 9/13 (69%) |
| Prednisone maintenance | — | — | 17/38 (45%) | 5/13 (38%) |
| Acute kidney injury | — | — | 17/37 (46%) | 9/9 (100%) |
| Chest X-ray with opacities c/w COVID–19 infection | 17/21 (81%) | 14/14 (100%) | 32/38 (84%) | 7/7 (100%) |
| Intubated | 2 (7%) | 9/12 (75%)a | 7/38 (18%) | 11/13 (85%) |
| White blood cell count (x103/uL) | 6.1 (4.5-9.1) | 7.3 (4.5-10.7) | 5.7 (4.0-7.4) | 5.5 (4.2-8.0) |
| Absolute lymphocyte count (x103/uL) | 0.7 (0.5-1.1) | 0.8 (0.6-1.2) | 0.6 (0.4-1.0) | 0.6 (0.2-1.1) |
| D-Dimer (ng/mL) | 379.0 (202.0-576.0) | 782.9 (443.4-3036.0) | 380.0 (213.0-477.0) | 605.5 (390.5-784.5) |
| Ferritin (ng/mL) | 2317.0 (1832.0-3311.7) | 2567.8 (1612.5-5597.1) | 1843.8 (417.4-2464.4) | 1283.4 (701.1-1887.0) |
| Procalcitonin (ng/mL) | 1.5 (0.7-3.0) | 3.0 (1.4-4.8) | 0.3 (0.1-0.4) | 0.6 (0.4-0.9) |
| C-reactive protein (mg/dL) | 26.8 (4.7-39.5) | 62.7 (28.8-111.6) | 10.3 (5.3-34.8) | 16.5 (14.8-17.7) |
| Mycophenolate discontinued | — | — | 21/38 (55%) | 9/9 (100%) |
| Time from transplant | — | — | 4.6 (1.8-9.1) | 4.7 (2.4-6.7) |
| Waitlist qualifying time | 4.3 (2.6-7.0) | 6.0 (4.3-7.3) | — | — |
| Experimental Therapies (%) | ||||
| Hydroxychloroquine | 11/25 (44%) | 7/10 (70%) | 27/37 (73%) | 7/8 (87%) |
| Remdesivir | 0/18 (0%) | 0/12 (0%) | 5/37 (14%) | 1/8 (12%) |
| Tocilizumab | 0/18 (0%) | 1/12 (8%) | 1/37 (3%) | 1/8 (12%) |
| Selinexor | 0/18 (0%) | 0/12 (0%) | 2/37 (5%) | 0/8 (0%) |
| Convalescent plasma | 0/18 (0%) | 0/12 (0%) | 0/37 (0%) | 1/8 (12%) |
| Bolus steroids | 2/18 (11%) | 2/12 (17%) | 3/37 (8.1%) | 4/8 (50.0%) |
Includes seven patients who were intubated and two who were recommended to be intubated but elected to be DNR/DNI. For seven patients who were admitted to other hospitals and died, confirmation of intubation status was not available.
3. RESULTS
3.1. Characteristics of waitlisted and transplant patients with COVID-19
Fifty-six patients waitlisted for kidney transplantation and 80 kidney transplant recipients tested positive for COVID-19 between March 13 and May 20, 2020. COVID-19 infected waitlisted and transplant patients were of similar age (60 vs. 57 years) and were predominantly male (66% vs. 70%). African Americans comprised a higher proportion of the waitlisted group than of the transplant group (46% vs. 26%, P = .02). Baseline comorbidities of both groups are displayed in Table 1. Waitlisted patients tended to have a higher incidence of diabetes (46% vs. 34%) and end-stage renal disease (ESRD) secondary to diabetes (41% vs. 27%), although these differences did not reach statistical significance. Rates of cardiovascular disease were 46% waitlist versus 32% transplant (P = .11) and smoking history 34% waitlist versus 20% transplant (P = .08). Pulmonary disease, history of stroke, body mass index (BMI), and angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) use were similar.
Transplantation specific characteristics are displayed in Tables S2 and S3. The majority received a living donor transplant (64%), thymoglobulin induction (82%), and 17% had a history of a prior kidney transplant. The median time from transplantation to COVID-19 diagnosis was 4.7 years, with 21% of patients within 1 year of transplantation. Nearly all patients were on a tacrolimus and mycophenolate mofetil regimen, and 41% were on steroid maintenance. For the waitlist patients, the median waitlist qualifying time was 4.9 years (IQR 3.4-7.3 years). In-center hemodialysis was the regimen for 86% (n = 48), while five were on peritoneal dialysis (PD), one was switched from HD to PD during COVID-19 illness, and two were not yet on dialysis.
3.2. Clinical presentation
The most common presenting symptom reported in both groups was fever (76% waitlist, 71% transplant) and cough (77% waitlist, 59% transplant). More waitlisted patients reported fatigue and myalgias (77% vs. 47%), and the remaining symptoms were similar between the groups (Table 1). Hospitalization was required for 82% of the COVID-19-positive waitlist patients and for 65% of the kidney transplant patients (P = .03).
Laboratory values at the time of hospital admission are displayed in Table 2. While the median white blood cell count at presentation was normal and similar between waitlist and transplant groups, patients were generally lymphopenic (waitlist 0.8 × 103/uL vs. transplant 0.6 × 103/uL, P = .02). A number of inflammatory markers associated with COVID-19 severity, including procalcitonin, C-reactive protein, erythrocyte sedimentation rate, ferritin, D-dimer, and interleukin-6, were elevated in both groups of admitted patients. These laboratory markers trended higher in the waitlist group, with procalcitonin and ferritin significantly elevated compared to transplant patients (P = .002 and P = .005, respectively) (Table 2). Nearly all of the admitted waitlist and transplant patients with imaging available for review had pulmonary findings consistent with COVID-19 infection (89% and 87%, respectively).
3.3. COVID-19 treatment in hospitalized patients
Our general strategy for immunosuppressive therapy in hospitalized patients was to reduce tacrolimus dosage to target trough levels of 4-6 ng/mL and mycophenolate mofetil was discontinued in 64% and reduced in 26% of patients. Hydroxychloroquine was the most commonly used COVID-19 experimental therapy, with 76% of hospitalized transplant and 51% of hospitalized waitlist patients receiving it. Eleven transplant patients received additional experimental treatments, including remdesivir, tocilizumab, selinexor, and convalescent plasma, while only one waitlisted patient received tocilizumab. Approximately equal numbers received azithromycin or doxycycline (48% waitlist, 40% transplant) or bolus steroids (13% waitlist, 18% transplant).
3.4. Outcomes of COVID-19 patients
Intubation was required in 29% of waitlisted patients and 31% of transplant patients. Of the intubated waitlisted patients, 9/11 (82%) died, 1 remained hospitalized with a tracheostomy, and 1 was discharged to a long-term care facility after neurologically devastating strokes suffered during COVID-19 illness. Of the intubated transplant patients, 11/16 (69%) died, and 1 remained hospitalized with a tracheostomy. Of the transplant patients admitted to the hospital, 56% demonstrated acute kidney injury, four patients required initiation of renal replacement therapy during admission, and these four (4/47, 9%) remain dialysis-dependent and are considered to have had allograft loss (Table 3). Not included in this analysis are five patients who died at other hospitals and for whom graft status prior to death is not known.
The median hospital length of stay was 11 days for waitlist patients and 9 days for transplant patients. Currently two waitlisted patients and one transplant patient remain hospitalized, and they have lengths of stay >50 days. Of those who were hospitalized, the mortality rate was 41% for waitlist and 25% for transplant patients. An overwhelming number of those who died were male (100% in transplant group, 84% in waitlist group) (Table 4). Diabetes was present in 63% of waitlist patients and 62% of transplant who died versus 41% and 31% who survived. Waitlisted patients who died had a median qualifying waitlist time of 6.0 years, compared to 4.3 years of survivors. Time from transplant was similar among those who died versus those who survived; furthermore, the mortality rate was similar between those <1 year from transplant (12%) versus those >1 year from transplant (17%, P = .7). Among hospitalized transplant patients, those who had received thymoglobulin induction or who were on prednisone maintenance were not more likely to die (Table 4). All patients who died had pulmonary findings of infection on imaging, and all transplant patients who died had acute kidney injury. When laboratory values were stratified based on death, in both waitlist and transplant patients, those who died had higher presenting D-dimer, procalcitonin, and C-reactive protein (Table 4).
3.5. Risk factors associated with hospitalization and mortality
To evaluate the risk factors associated with hospitalization, we calculated the odds ratios (OR) and 95% confidence intervals (CI) from univariate logistic regression ( Table 5). Univariate analysis of all patients demonstrated that being a waitlist candidate (OR 2.47, 95% CI 1.09-5.64, P = .031), age >65 years (OR 3.07, 95% CI 1.09-9.49, P = .05), and male sex (OR 3.62, 95% CI 1.64-7.98, P = .001) were significantly associated with requiring hospitalization for COVID-19. Factors that were associated with hospitalization were entered into a multivariate logistic regression model to identify independent predictors of hospitalization for COVID-19. Waitlist status (OR 2.91, 95% CI 1.19-7.08, P = .02) and male sex (OR 3.56, 95% CI 1.53-8.27, P = 0.003) remained significantly associated with hospitalization.
TABLE 5.
Factors associated with hospitalization and with mortality for COVID-19
| Univariate odds ratio (95% CI) | P value | Multivariate odds ratio (95% CI) | P value | |
|---|---|---|---|---|
| (A) Factors associated with hospitalization for COVID–19 | ||||
| Waitlist status | 2.47 (1.09-5.64) | .031 | 2.91 (1.19-7.08) | .02 |
| Age >65 yearsa | 3.07 (0.99-9.49) | .05 | 2.83 (0.87-9.24) | .08 |
| Male | 3.62 (1.64-7.98) | .001 | 3.56 (1.53-8.27) | .003 |
| Black | 1.42 (0.63-3.21) | .85 | — | |
| BMI>30 kg/m2a | 1.36 (0.61-3.08) | .46 | — | |
| Diabetes | 2.19 (0.96-4.99) | .063 | 1.63 (0.66-3.91) | .29 |
| Cardiovascular disease | 1.56 (0.71-3.44) | .27 | — | |
| Pulmonary disease | 1.35 (0.55-3.33) | .51 | — | |
| History of stroke | 1.94 (0.53-7.19) | .32 | — | |
| Smoking history | 1.43 (0.58-3.49) | .78 | — | |
| ACE/ARB use | 0.79 (0.36-1.74) | .56 | — | |
| (B) Factors associated with mortality in patients with COVID–19 | ||||
| Waitlist status | 2.65 (1.18-5.96) | .02 | 3.60 (1.38-9.39) | .009 |
| Age >65 yearsa | 3.50 (1.45-8.41) | .004 | 3.99 (1.42-11.22) | .009 |
| Male | 6.04 (1.73-21.14) | .005 | 5.75 (1.52-21.66) | .010 |
| Black | 0.99 (0.43-2.28) | .98 | — | |
| BMI>30 kg/m2a | 1.23 (0.54-2.82) | .62 | — | |
| Diabetesb | 3.56 (1.57-8.19) | .002 | 2.97 (1.03-8.57) | .04 |
| Cardiovascular disease | 2.14 (0.96-4.78) | .063 | 0.76 (0.26-2.23) | .62 |
| Pulmonary disease | 1 (0.40-2.50) | 1.0 | — | |
| History of stroke | 1.95 (0.66-5.78) | .23 | — | |
| Smoking history | 1.43 (0.60-3.44) | .42 | — | |
| ACE/ARB use | 1.08 (0.47-2.49) | .86 | — | |
Age >65 years was used as the cut off as it represented the highest quartile, 75th percentile.
BMI >30 was used as the cut off as it represents the Centers for Disease Control and Prevention classification of obesity.
On univariate analysis of mortality, waitlist status (OR 2.65, 95% CI 1.18-5.96, P = .02), age (OR 3.50, 95% CI 1.45-8.41, P = .004), male sex (OR 6.04, 95% CI 1.73-21.14, P = .005), and diabetes (OR 3.56, 95% CI 1.57-8.19, P = .002) were associated with mortality after COVID-19 (Table 5). Multivariate logistic regression analysis demonstrated that waitlist status (OR 3.60, 95% CI 1.38-9.39, P = .009), age (OR 3.99, 95% CI 1.42-11.22, P = .009), male sex (OR 5.75, 95% CI 1.52-21.66, P = .010), and diabetes (OR 2.97, 95% CI 1.03-8.57, P = .04) were independently associated with mortality.
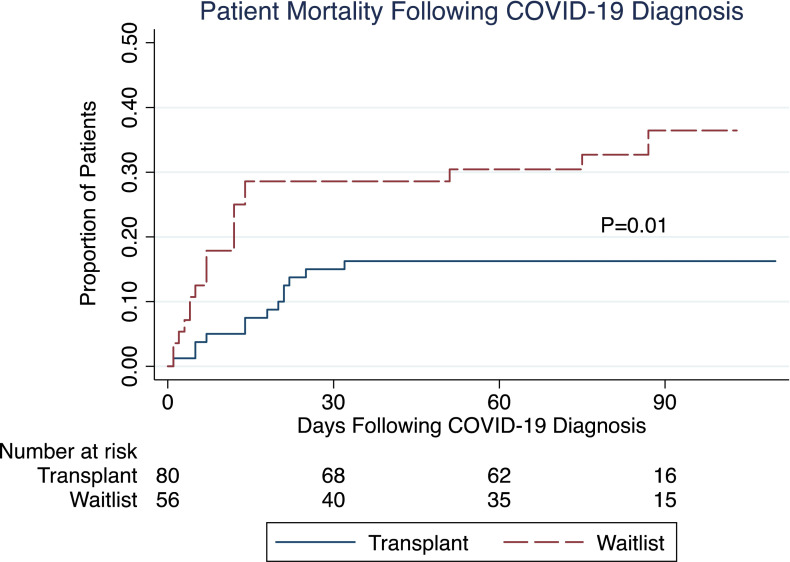
The overall case fatality rate was 34% for the waitlisted patients with COVID-19, compared to 16% for the transplant patients (P = .02). The median follow-up after COVID-19 diagnosis was similar between waitlisted and transplant patients (77 vs. 78 days). Cumulative mortality estimates of waitlisted and transplant patients with COVID-19 are shown in Figure 1. The mortality hazard ratio (HR) was 2.4 (95% CI 1.3-5.3, P = .01) for waitlist patients compared to transplant patients. The Cox proportional hazards model demonstrated that waitlist status (HR = 2.73, 95% CI 1.32-5.63, P = .007), age (HR = 2.56, 95% CI 1.24-5.29, P = .01), male sex (HR = 4.41, 95% CI 1.32-14.72, P = .02), and diabetes (HR = 2.23, 95% CI 1.07-4.63, P = .03) were independently associated with mortality following COVID-19 diagnosis.
FIGURE 1.
Cumulative mortality incidence for waitlist and transplant patients with COVID-19 [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
In recent months, many discussions within the transplant community have centered on the risks of kidney transplantation and immunosuppression initiation in patients who may subsequently develop COVID-19 infection versus the risks of delaying transplantation and increasing wait-time and waitlist-associated mortality. Informative to this discussion is an understanding of the course of COVID-19 infection in waitlisted and in transplant patients. To our knowledge, this is the first study of COVID-19 in patients waitlisted for kidney transplantation.
ESRD patients represent a unique cohort that is unable to fully comply with social distancing and quarantining recommendations, given their need for dialysis treatments three to four times per week that are administered in close proximity to other patients. Because comorbidities associated with poor COVID-19 prognosis, such as diabetes, hypertension, cardiovascular disease, and pulmonary disease, are commonly found in ESRD patients, they may be expected to have a higher COVID-19 mortality rate than the 1-8% of the general population.5 , 6 Several small case reports suggest a low COVID-19 mortality rate among ESRD patients,7 , 8 but this is in sharp contrast to the 30.5% mortality rate in a Spanish study of 36 hospitalized ESRD patients,9 and the 31% mortality rate in a US study of 59 hospitalized ESRD patients.10 Likewise, we found a high COVID-19 mortality rate in our cohort, with 41% of hospitalized waitlisted patients dying. Similar to Goichoechea et al, those with longer dialysis vintage had higher mortality. Patients who are waitlisted for a kidney transplant, however, reflect a specially selected subset of dialysis patients. They are generally healthier to meet candidacy criteria for transplantation, and thus may be anticipated to fare differently with COVID-19 infection. Nonetheless, we show here that these patients suffer great morbidity and mortality from COVID-19, in particular compared to the 10.2% mortality rate at our center for the general COVID population.11 It is worth noting that unlike a number of prior studies in which many patients were still hospitalized at the time of publication, in our series only 3 of the 136 patients remain hospitalized at this time and they have been followed for lengthy admissions of 85, 86, and 53 days.
Despite similar demographics and similar burden of comorbidities between waitlisted and transplant patients, waitlisted patients with COVID-19 were more likely to require hospitalization (82% vs. 65%) and were at a higher risk of mortality (overall: 34% vs. 16%, hospitalized: 41% vs. 25%). African Americans comprised a greater proportion of the waitlist group; however, race was not associated with hospitalization or mortality. In multivariate analysis, waitlist status was independently associated with need for hospitalization and with mortality after COVID-19. Waitlisted patients, like their transplanted counterparts, frequently have comorbidities associated with increased risk and worsened prognosis of COVID-19. However, they have not yet derived the health benefit of transplantation and being off dialysis, which may account for their worse outcomes with COVID-19 infection. Furthermore, ESRD patients have chronically impaired immune function due to their uremic state, which is associated with derangements in lymphocyte and granulocyte function.12 These impairments may alter waitlisted patients’ response to COVID-19. Lymphopenia on admission has been associated with poor outcomes in COVID-19,13 and while we observed lymphopenia in waitlisted patients, the degree was similar between survivors and non-survivors.
There was a striking male predominance of those who died, with males comprising 100% of the transplant and 84% of the waitlist deaths. This mirrors results in the general COVID-19 population in which men had up to 2.4 times the risk of dying from COVID-19.14 , 15 This gender disparity may be related to the fact that males have increased expression of ACE2, the receptor which SARS-CoV-2 binds for cellular entry.16 An additional factor hypothesized to mediate this gender bias is the difference in testosterone and dihydrotestosterone, which are necessary to mount antiviral immune responses.17 Another possibility is that males tend to have higher rates of diabetes, obesity, and cardiovascular disease, all of which are linked to COVID-19 severity. When stratifying COVID-19-infected waitlist and transplant patients by gender, we found no differences in body mass index. However, male waitlisted patients had a higher incidence of cardiovascular disease (51% vs. 37%) and diabetes (54% vs. 32%) than their female counterparts. Similar although less marked trends were seen for male versus female transplant patients, with respect to cardiovascular disease (34% vs. 29%) and diabetes (37% vs. 25%). Further studies may help to elucidate the multifactorial interplay between gender and COVID-19 risk.
Growing evidence suggests that inflammatory responses play a key role in the pathogenesis and outcome of COVID-19 infection. A number of studies have found inflammatory markers such as procalcitonin, C-reactive protein, erythrocyte sedimentation rate, ferritin, D-dimer, and interleukin-6 to be associated with worse risk of COVID-19 infection.18 , 19 In our series, hospitalized waitlist and transplant COVID-19 patients had elevations in all of these inflammatory markers, with waitlisted patients generally having higher levels than transplant patients. Chronic kidney disease is characterized as a chronic inflammatory state due to uremia, oxidative stress, elevated circulating inflammatory cytokines, protein-energy wasting, comorbid conditions, exposure to dialysis membranes and central venous catheters, gut dysbiosis, recurrent infections, among other factors.20 Derangements of C-reactive protein, ferritin, and interleukin-6 are commonly seen in chronic kidney disease.20 , 21 This could account for the higher levels of inflammatory markers we observed in the waitlist patients; however, when laboratory values were stratified based on death, in both waitlist and transplant patients, those who died had higher D-dimer, procalcitonin, and C-reactive protein at the time of presentation. Some have hypothesized that transplant immunosuppressive therapies may help prevent the cytokine storm implicated in acute respiratory distress syndrome in COVID-19. In vitro studies of mycophenolate mofetil and tacrolimus have shown promising anti-viral properties against SARS-CoV and MERS-CoV, although in vivo studies are lacking.22, 23, 24, 25 In the RECOVERY trial, corticosteroids reduced mortality in more severe COVID-19 disease.26 Whether immunosuppressive drugs provide any protection for transplant patients against the hyperinflammatory response in COVID-19 infection remains to be tested. Interestingly, transplant patients who may be considered the most immunosuppressed, those less than one year from transplantation, had a similar COVID-19 mortality rate compared to those greater than 1 year from transplantation (12% vs. 17%).
In areas hit particularly hard by COVID-19, such as our area of New York, the pandemic forced difficult decisions about the distribution of medical resources. Our center ceased kidney transplantation activity entirely during mid-March to June, as operating rooms were turned into COVID-19 intensive care units, transplant personnel were reassigned to care for COVID-19 patients, there were significant limitations in supplies of ventilators, dialysis machines and dialysate fluids, and shortages of pain and sedative medications and supplies such as gloves, gowns, and masks. These factors, as well as limited COVID-19 testing capacity, unclear testing reliability, and prolonged waiting for test results, led most New York City programs to feel it unsafe to continue kidney transplantation until these issues had been resolved. During March to June 2020, our center saw an increase in total waitlist deaths, with 44 candidates removed due to death, in comparison to 22 during the same time period in 2019, and 25 in 2018 ( Figure 2). Similar trends were seen in kidney waitlist deaths in the rest of Region 9, with a 79% increase from March to June, 2019 to 2020. In a national registry study of kidney transplant waitlist registrations, the number of waitlist deaths was 2.2 fold higher than expected in states with high prevalence of COVID-19.27 In an attempt to provide guidance to clinicians during the pandemic and to inform our understanding about the effects of a delay in transplantation on patient survival, Massie et. al. built a simulation model of waitlist and posttransplant mortality in the context of COVID-19.3 This showed that transplantation provided survival benefit in modeled scenarios, except when the case fatality rate of COVID-19 in transplant recipients greatly exceeded that of waitlisted patients. Our center’s data demonstrate that the mortality risk of COVID-19 appears to be greater in waitlisted patients than in transplant recipients.
FIGURE 2.
Waitlist deaths and kidney transplants performed between March and June, 2018-2020. The first panel shows the number of patients who received kidney transplants at our center (NYNY) between March 15 and June 30, 2018, 2019, 2020. The middle panel shows the number of patients who died while waitlisted for kidney transplantation at our center during the corresponding time periods. The third panel shows the number of patients who died on the kidney waitlist in Region 9 (New York and Western Vermont) during the corresponding time periods
Limitations of this study include those of a retrospective single-center study; however, the single-center nature of the study allows for increased granularity of data. National registry data of waitlist removals and deaths do not capture cause of death, and registry data do not capture COVID-19 outcomes. Our single-center experience, in an area hit particularly hard by COVID-19 during an evolving pandemic, may not be generalizable to all transplant patients or centers. It is possible that we have missed milder cases of COVID-19, as patients may have chosen to self-isolate at home without notifying our center. It is also possible that COVID-19-related waitlist deaths may be undercounted, as there can be delayed notification to our center of waitlisted patients who died while hospitalized elsewhere. During the peak months of the pandemic, our program made concerted efforts to follow our entire waitlist population and their COVID-19 status; transplant coordinators conducted phone call “Wellness Checks” of our waitlist patients to document history of a COVID-19 diagnosis, to screen for signs and symptoms of COVID-19, and to educate about COVID-19 precautions and the status of our transplant center. This likely resulted in a high capture rate within our cohort; however, it is possible that not all COVID-19 cases were captured and may have introduced a selection bias. At the time of reporting of these results, one transplant and two waitlisted patients remain hospitalized and thus their true outcomes are unknown. Compared to a number of prior studies where as many as one third of patients lacked final outcomes, our data represent resolutions of most cases in this cohort. Additionally, due to rapid changes during this time period in medical resources, available testing and therapies, and our understanding of COVID-19, there were heterogeneous approaches to patient care that may have impacted management and outcomes. Not all patients had COVID-19-associated laboratory values checked on admission, and data were incomplete on patients admitted and managed at other hospitals.
In conclusion, COVID-19 has had a dramatic impact on patients waitlisted for kidney transplantation, decreasing their opportunities for transplantation and posing significant mortality risk. Understanding the impact of COVID-19 on waitlist patients in comparison to transplant recipients and to the general population can help inform the management of waitlisted patients and aid transplant centers in determining the appropriateness of resuming transplant activity.
ACKNOWLEDGMENTS
We would like to acknowledge the contributions of our transplant coordinators, Jenna Vaughan, Rachel Martin, Arielle Nelson, Chelle Almirante, Dominique Zirino, Sophie Shachter, Tyler Chapman, and our transplant physicians Muthukumar Thangamani, Jun Lee, Choli Hartono, and Stuart Saal.
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. D.M.D. receives consulting fees from Veloxis Pharmaceutical, Inc. and Allovir, Inc. D.M.D. received no renumeration for the work presented here. J.R.L. receives research support from BioFire Diagnostics, LLC. D.M.D. and J.R.L. are inventors on patent US-2020-0048713-A1, entitled “Methods of detecting cell-free DNA in biological samples.” This research did not receive any specific grant funding.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, R.C.S., upon reasonable request.
Footnotes
Meredith J. Aull and Darshana M. Dadhania contributed equally to this work.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Loupy A, Aubert O, Reese PP, Bastien O, Bayer F, Jacquelinet C. Organ procurement and transplantation during the COVID-19 pandemic. Lancet. 2020;395(10237):e95–e96. doi: 10.1016/S0140-6736(20)31040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodrigo E, Minambres E, Gutierrez-Banos JL, Valero R, Belmar L, Ruiz JC. COVID-19-related collapse of transplantation systems: A heterogeneous recovery? Am J Transplant. 2020. [DOI] [PMC free article] [PubMed]
- 3.Massie AB, Boyarsky BJ, Werbel WA, et al. Identifying scenarios of benefit or harm from kidney transplantation during the COVID-19 pandemic: a stochastic simulation and machine learning study. Am J Transplant. 2020. [DOI] [PMC free article] [PubMed]
- 4.Lubetzky M, Aull MJ, Craig-Schapiro R, et al. Kidney allograft recipients, immunosuppression, and coronavirus disease-2019: a report of consecutive cases from a New York City transplant center. Nephrol Dial Transplant. 2020. [DOI] [PMC free article] [PubMed]
- 5.Li LQ, Huang T, Wang YQ, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(6):577–583. doi: 10.1002/jmv.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai CC, Wang CY, Wang YH, Hsueh SC, Ko WC, Hsueh PR. Global epidemiology of coronavirus disease 2019 (COVID-19): disease incidence, daily cumulative index, mortality, and their association with country healthcare resources and economic status. Int J Antimicrob Agents. 2020;55(4):105946. doi: 10.1016/j.ijantimicag.2020.105946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang R, Liao C, He H, et al. COVID-19 in hemodialysis patients: A report of 5 cases. Am J Kidney Dis. 2020;76(1):141–143. doi: 10.1053/j.ajkd.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma Y, Diao B, Lv X, et al. COVID-19 in hemodialysis (HD) patients: Report from one HD center in Wuhan, China. medRxiv. 2020: 2020.2002.2024.20027201.
- 9.Goicoechea M, Sanchez Camara LA, Macias N, et al. COVID-19: clinical course and outcomes of 36 hemodialysis patients in Spain. Kidney Int. 2020;98(1):27–34. doi: 10.1016/j.kint.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valeri AM, Robbins-Juarez SY, Stevens JS, et al. Presentation and Outcomes of Patients with ESKD and COVID-19. J Am Soc Nephrol. 2020;31(7):1409–1415. doi: 10.1681/ASN.2020040470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid-19 in New York city. N Engl J Med. 2020. [DOI] [PMC free article] [PubMed]
- 12.Betjes MG. Immune cell dysfunction and inflammation in end-stage renal disease. Nat Rev Nephrol. 2013;9(5):255–265. doi: 10.1038/nrneph.2013.44. [DOI] [PubMed] [Google Scholar]
- 13.Huang I, Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive Care. 2020;8:36. doi: 10.1186/s40560-020-00453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin JM, Bai P, He W, et al. Gender differences in patients With COVID-19: Focus on severity and mortality. Front Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Oudit GY, Pfeffer MA. Plasma angiotensin-converting enzyme 2: novel biomarker in heart failure with implications for COVID-19. Eur Heart J. 2020;41(19):1818–1820. doi: 10.1093/eurheartj/ehaa414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schroeder M, Tuku B, Jarczak D, et al. The majority of male patients with COVID-19 present low testosterone levels on admission to Intensive Care in Hamburg, Germany: a retrospective cohort study. medRxiv. 2020: 2020.2005.2007.20073817.
- 18.Zeng F, Huang Y, Guo Y, et al. Association of inflammatory markers with the severity of COVID-19: A meta-analysis. Int J Infect Dis. 2020;96:467–474. doi: 10.1016/j.ijid.2020.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cobo G, Lindholm B, Stenvinkel P. Chronic inflammation in end-stage renal disease and dialysis. Nephrol Dial Transplant. 2018;33(suppl_3) doi: 10.1093/ndt/gfy175. iii35-iii40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Achinger SG, Ayus JC. Inflammation from dialysis, can it be removed? Nephrol Dial Transplant. 2013;28(4):770–773. doi: 10.1093/ndt/gfs480. [DOI] [PubMed] [Google Scholar]
- 22.Carbajo-Lozoya J, Ma-Lauer Y, Malesevic M, et al. Human coronavirus NL63 replication is cyclophilin A-dependent and inhibited by non-immunosuppressive cyclosporine A-derivatives including Alisporivir. Virus Res. 2014;184:44–53. doi: 10.1016/j.virusres.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carbajo-Lozoya J, Muller MA, Kallies S, Thiel V, Drosten C, von Brunn A. Replication of human coronaviruses SARS-CoV, HCoV-NL63 and HCoV-229E is inhibited by the drug FK506. Virus Res. 2012;165(1):112–117. doi: 10.1016/j.virusres.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng KW, Cheng SC, Chen WY, et al. Thiopurine analogs and mycophenolic acid synergistically inhibit the papain-like protease of Middle East respiratory syndrome coronavirus. Antiviral Res. 2015;115:9–16. doi: 10.1016/j.antiviral.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin MH, Moses DC, Hsieh CH, et al. Disulfiram can inhibit MERS and SARS coronavirus papain-like proteases via different modes. Antiviral Res. 2018;150:155–163. doi: 10.1016/j.antiviral.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dexamethasone in Hospitalized Patients with Covid-19 — Preliminary Report. N Engl J Med. 2020. [DOI] [PMC free article] [PubMed]
- 27.Boyarsky BJ, Werbel WA, Durand CM, et al. Early national and center-level changes to kidney transplantation in the United States During COVID-19 spidemic. Am J Transplant. 2020. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, R.C.S., upon reasonable request.