Abstract
Critically ill patients with coronavirus disease 2019 (COVID‐19) present with hypoxaemia and are mechanically ventilated to support gas exchange. We performed a retrospective, observational study of blood gas analyses (n = 3518) obtained from patients with COVID‐19 to investigate changes in haemoglobin oxygen (Hb–O2) affinity. Calculated oxygen tension at half‐saturation (p50) was on average (±SD) 3·3 (3·13) mmHg lower than the normal p50 value (23·4 vs. 26·7 mmHg; P < 0·0001). Compared to an unmatched historic control of patients with other causes of severe respiratory failure, patients with COVID‐19 had a significantly higher Hb–O2 affinity (mean [SD] p50 23·4 [3·13] vs. 24·6 [5.4] mmHg; P < 0·0001). We hypothesise that, due to the long disease process, acclimatisation to hypoxaemia could play a role.
Keywords: haemoglobin, oxygen affinity, infection
Coronavirus disease 2019 (COVID‐19) is characterised by hypoxaemia that can precede radiological changes or other clinical symptoms including dyspnoea. 1 Given that the severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) has a vascular tropism, 2 the physiological manifestation of the altered pulmonary perfusion, hypoxaemia is to a degree disproportionate to the severity of parenchymal lung disease. In addition, direct (midbrain 3 ) or indirect (via metabolism of angiotensin II on the carotid bodies 4 ) viral actions can affect respiratory drive and response to hypoxaemia. The duration of the disease is generally more prolonged compared to acute respiratory distress syndrome (ARDS) from other aetiologies. 1 As reported by Daniel et al. 5 in this Journal, haemoglobin (Hb) oxygen (O2) affinity in 14 patients with COVID‐19 was not different from 11 control participants, when affinity was measured in vitro with a Hemox analyser, with a standardised pH (7·4) and temperature. These oxygen tensions at half‐saturation (p50) values were obtained directly from the blood gas analyser, without adjustments for physiological changes in CO2 or pH in vivo, which could be important in COVID‐19. We hypothesised that in vivo Hb–O2 affinity could be affected by other factors in COVID‐19.
Methods
To assess alterations in in vivo Hb–O2 affinity, we performed a retrospective, observational analysis of all arterial and venous blood gases (n = 3518) obtained from all intubated and ventilated patients (n = 43) with severe COVID‐19 in one intensive care unit (ICU), at Guy’s and St Thomas’ Hospital (London, UK) between 15 April and 15 May 2020. Institutional approval was gained from the local audit committee (project reference: 11013). The need for individual informed consent was waived for this retrospective analysis of data collected prospectively for routine care, with no breach of privacy or anonymity. The study qualified as a service evaluation as defined by the UK NHS Health Research Authority (NHS HRA) and therefore did not require review by a research ethics committee.
Measured values of partial pressure of oxygen (pO2) and oxygen saturation (SO2) were compared to the standard oxyhaemoglobin dissociation curve (ODC) for normal Hb–O2 affinity. 6 The p50 values were calculated using the Hill equation (Eq.) 7 , 8 (after correcting for pH, temperature and base excess 9 ; Hill Eq. 1, see below) and derived from Roche blood gas analyser (Cobas system, F. Hoffmann‐La Roche Ltd; 10 Roche Eq. 2, see below), and compared to the normal value (for pH 7·4, 37·0°C and pCO2 40 mmHg) of 26·7 mmHg respectively. 11 , 12 , 13 Results were compared to a historic, unmatched control cohort with an overall total of 15 945 arterial and venous blood gas samples obtained from 828 critically ill patients with acute respiratory failure (pneumonia/pneumonitis, or secondary ARDS, but presumed COVID‐19 negative, as these samples were obtained in 2017 and earlier). The one‐sample t‐test was used for comparison between actual means (Eq. 1 and Eq. 2, see below) and normal value. The two‐tailed, unpaired t‐test was used for comparison between means of COVID‐19 and control samples. Statistical analysis was performed using Prism (GraphPad Software Inc., La Jolla, CA, USA).
We calculated the p50 using two methods:
Hill Eq. 1
Roche Eq. 2
Results
A total of 3518 blood gas analyses of 43 patients [34 (79%) male; mean (range) age 53 (26–77) years] were obtained (Table I). Figure S1 presents pO2 and SO2 values. Figure 1 shows the distribution of p50 values derived by the Hill equation (Eq. 1). Compared to the standard p50 value of 26·7 mmHg, Eq. 1 presented a difference of 3·3 mmHg [mean p50 23·4 mmHg, 99% confidence interval (CI) 23·23–23·50; P < 0·0001] and Eq. 2 a difference of 1·9 mmHg (mean p50 24·8 mmHg, 99% CI 24·68–25·00; P < 0·0001). Table I shows the comparison to the control group data. Data on intra‐ and inter‐subject variability, as well as data on temporal trends is shown in the supplement.
Table 1.
Summary of results from patients with COVID‐19 and from an unmatched control group of critically ill patients without COVID‐19. The ratio of arterial oxygen partial pressure to fractional inspired oxygen (PaO2/FiO2) was calculated for arterial samples only. Values are reported as mean, standard deviation (SD) and 99% confidence interval (99% CI).
| COVID‐19 | CONTROL | Group with COVID‐19 showed | Unpaired t‐test (two‐tailed P value) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | 99% CI | n | Mean | SD | 99% CI | |||||
| p50 using Eq. 1, mmHg | 3518 | 23·37 | 3·13 | 23·23 | 23·50 | 15 945 | 24·59 | 5·42 | 24·48 | 24·70 | Lower p50 | <0·0001 |
| p50 using Eq. 2, mmHg | 3518 | 24·8 | 3·7 | 24·7 | 25·0 | 15 945 | 25·7 | 6·0 | 25·6 | 25·8 | Lower p50 | <0·0001 |
| pH | 3518 | 7·382 | 0·077 | 7·379 | 7·386 | 15 932 | 7·397 | 0·072 | 7·395 | 7·398 | Lower pH | <0·0001 |
| pO2, mmHg | 3518 | 77·9 | 28·0 | 76·7 | 79·1 | 15 945 | 86·6 | 62·5 | 85·3 | 87·8 | Lower pO2 | <0·0001 |
| SO2, % | 3518 | 94·2 | 7·9 | 93·9 | 94·6 | 15 945 | 93·1 | 10·4 | 92·9 | 93·3 | Higher SO2 | <0·0001 |
| pCO2, mmHg | 3518 | 46·1 | 11·8 | 45·5 | 46·6 | 15 936 | 43·1 | 10·1 | 42·9 | 43·4 | Higher pCO2 | <0·0001 |
| BE, mmol/l | 3518 | 1·2 | 4·3 | 1·0 | 1·3 | 15 925 | 0·7 | 4·2 | 0·6 | 0·8 | Higher BE | <0·0001 |
| Hct, % | 3483 | 26·5 | 4·0 | 26·4 | 26·7 | 15 703 | 29·7 | 6·7 | 29·6 | 29·8 | Lower Hct | <0·0001 |
| Hb, g/l | 3518 | 81·2 | 12·4 | 80·6 | 81·7 | 15 941 | 93·8 | 20·2 | 93·4 | 94·2 | Lower Hb | <0·0001 |
| PaO2/FiO2, mmHg | 2627 | 215·7 | 111·5 | 210·1 | 221·3 | 13,941 | 299·4 | 236·5 | 294·2 | 304·6 | Lower PaO2/FiO2 | <0·0001 |
| Temperature, °C | 3518 | 36·8 | 0·8 | 36·8 | 36·8 | 15 945 | 36·8 | 0·8 | 36·8 | 36·8 | NS | 0·057 |
BE, base excess; Hct, haematocrit; NS, no significant difference.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Fig 1.
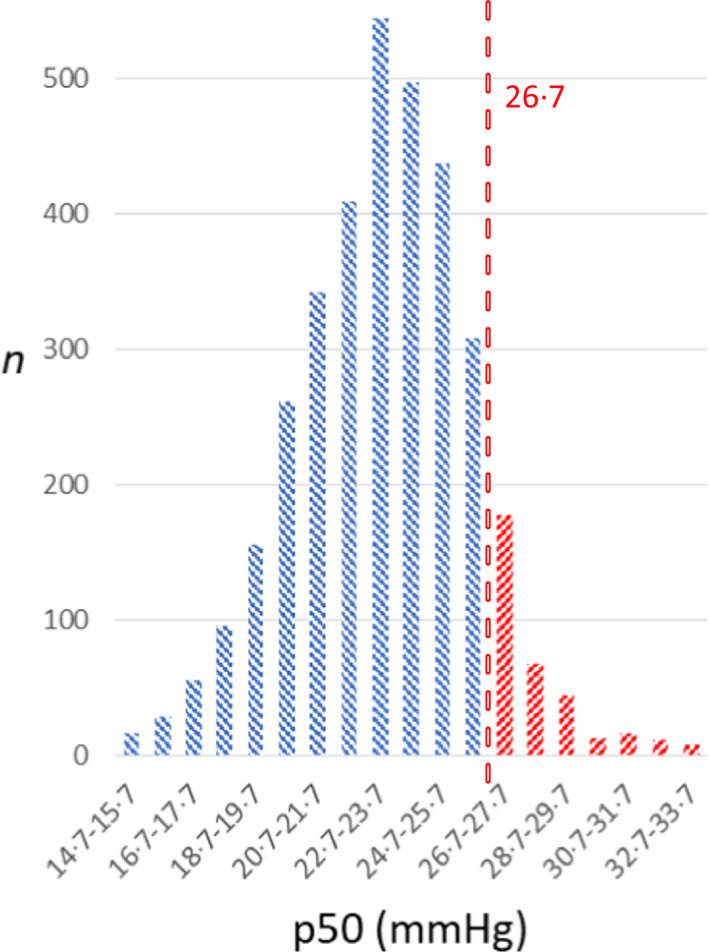
Distribution of p50 values calculated using Hill equation (Eq. 1) from measured pO2 and SO2 (n = 3518). Blue indicates left shift and red indicates right shift of oxyhaemoglobin affinity from the standard p50 value. Dashed line at 26·7 mmHg indicates standard value for p50. [Colour figure can be viewed at wileyonlinelibrary.com]
Discussion
When compared to the control group, patients with COVID‐19 had a lower pH and higher pCO2, but not significantly different temperature. Thus, a lower Hb–O2 affinity (i.e. a right shift of the ODC) would have been expected. However, we found a lower p50 (i.e. a left shift of the ODC) in the COVID‐19 group (for both equations). We included two different equations in order to strengthen the methodology, and the results obtained from these equations were in good agreement (Pearson’s R 2 0·65). The left shift in the ODC is reflected in the opposing alterations in pO2 and SO2. Patients with COVID‐19 had a significantly lower pO2, while showing a higher SO2. In contrast with typical ARDS, changes in Hb–O2 affinity could reflect the severity and the duration of hypoxaemia prior to presentation to critical care.
The Hb–O2 affinity is an important link between alveolar O2 tension and tissue oxygen supply. The Hb–O2 affinity is characterised in terms of p50 (the O2 tension where 50% of the Hb is oxygenated), and is the defining factor for binding the O2 that diffuses from the pulmonary alveoli into the blood and its release in peripheral tissues. Siggaard‐Andersen et al. 14 have shown agreement between the arterial and the venous values for standard p50 based on widely different SO2 levels. Changes in Hb–O2 affinity are crucial ways of adjusting both arterial O2 loading and peripheral O2 unloading in order to ensure aerobic metabolism when inspired pO2 decreases and/or O2 demand increases. The functional properties of Hb may improve tissue O2 supply with ODC shifts in either direction – increased Hb–O2 affinity increases O2‐loading under conditions such as severe hypoxia, 15 , 16 while decreased Hb–O2 affinity favours the release of bound oxygen from the Hb molecule.
Hb–O2 binding is considered co‐operative, that is, binding of the first molecule of O2 to Hb causes an increase in the O2 affinity of the remaining Hb subunits. According to mathematical modelling, 17 an increase in Hb–O2 affinity resulting from a p50 change of −3 mmHg (as seen in our present data using Eq. 1) only slightly increases SO2 (by 1%) in arterial blood in normoxia [arterial oxygen partial pressure (PaO2) 90 mmHg]. However, in hypoxia (PaO2 45 mmHg), the increased Hb–O2 affinity increases arterial SO2 by ~4·5%. While being at a disadvantage under normoxaemia, humans with a high Hb–O2 affinity (adolescents from a family with Hb Andrew‐Minneapolis, a stable β‐chain mutant with whole blood p50 ~17 mmHg) respond more appropriately to altitude‐induced hypoxia. 16 An increased Hb–O2 affinity results in oxygenation benefits during severe hypoxia and increases survival during acute hypoxia in several animal models. 15 , 18 Thus, a high Hb–O2 affinity may be of particular importance for O2 loading in hypoxic conditions. 19 There are well described existing strategies of shifting the ODC to the left and increasing SO2 at a given pO2. A fast increase in Hb–O2 affinity is mediated by a reduction of CO2 and increase of pH via hyperventilation under environmental hypoxia. This reversible alteration can occur rapidly within seconds to minutes. A slower mechanism is a decrease in 2,3‐diphosphoglycerate (DPG) or other organic phosphates. 17 Reduced 2,3‐DPG levels were observed in critically ill normoxaemic patients; however, the effect on p50 was diminished potentially due to acidaemia in this cohort. 20
A hypothetical explanation for our present findings in patients with COVID‐19 could be the response to prolonged periods of hypoxia. Patients with COVID‐19 often present to hospital after a period lasting on average 15 days, during which patients may suffer from ‘happy hypoxia’, a term coined for the phenomenon that profoundly low SO2 levels are found in individuals with relatively little subjective sensation of dyspnoea. 21 It has been hypothesised that SARS‐CoV‐2 may exert an idiosyncratic effect on the respiratory system via angiotensin‐converting‐enzyme 2 receptors in the carotid body and the midbrain, and this may lead to attenuation of the perceived dyspnoea. 3 , 4 , 22 The patients in our present COVID‐19 group might have had unrecognised hypoxia for a significant period prior to their hospital admission. Furthermore, even after hospital admission, many patients with COVID‐19 remain relatively stable for a few days before they deteriorate and are admitted to the ICU. 1 Thus, when compared to a general critical care population (e.g. patients with ARDS in whom hypoxia has to develop within 7 days, as per the Berlin definition), patients with respiratory failure secondary to COVID‐19 may have a much longer time to ‘acclimatise’ to hypoxaemia. These changes in p50 continued to be present during the length of the stay in ICU, therefore we suspect a sustained response, which could be explained by reduced 2,3‐DPG levels. 23 The mechanisms and the importance of this phenomenon require further studies.
Author contributions
Dominik J. Vogel designed the study, collected the data, analysed the data, interpreted the data and drafted the first version of the manuscript. Federico Formenti interpreted the data. Andrew J. Retter interpreted the data. Francesco Vasques interpreted the data. Luigi Camporota designed the study and interpreted the data. All authors read and approved the final manuscript.
Conflict of interests
The authors declare no competing interests.
Supporting information
Fig S1. SO2 with respective pO2 for 3518 blood gas analyses (circles) and the sigmoid fitting line (green line). For comparison standard oxyhaemoglobin dissociation curve is shown (dashed red line).1
Fig S2. Hill plot showing SO2 with respective pO2 for 3518 blood gas analyses (circles) and the sigmoid fitting line (green line). For comparison standard oxyhaemoglobin dissociation curve is shown (dashed red line).1
Fig S3. Distribution of the mean p50 calculated for each subject according to the following equation.
Fig S4. Distribution of absolute difference between each measurement and the respective individual’s mean p50 calculated with Hill Eq. 1.
Fig S5. Delta between each individual measurement from the mean p50 of that subject plotted over time starting from the first measurement.
Fig S6. Distribution of the individual patients’ mean p50 calculated with Eq. 1 and Eq. 2 plotted according to their length of stay during the observation period.
Data S1. Supplemental results.
References
- 1. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid‐19. N Engl J Med. 2020;383:120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Manganelli F, Vargas M, Iovino A, Iacovazzo C, Santoro L, Servillo G. Brainstem involvement and respiratory failure in COVID‐19. Neurol Sci. 2020;41(7):1663–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leung PS. Novel roles of a local angiotensin‐generating system in the carotid body. J Physiol. 2006;575:4. DOI: 10.1113/jphysiol.2006.115550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Daniel Y, Hunt BJ, Retter A, Henderson K, Wilson S, Sharpe CC, et al. Haemoglobin oxygen affinity in patients with severe COVID‐19 infection. Br J Haematol. 2020;190;e126–e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Severinghaus JW. Simple, accurate equations for human blood O2 dissociation computations. J Appl Physiol Respir Environ Exerc Physiol. 1979;46:599–602. [DOI] [PubMed] [Google Scholar]
- 7. Hill AV. The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curves. (PROCEEDINGS OF THE PHYSIOLOGICAL SOCIETY: January 22, 1910). J Physiol. 1910;40(Suppl):iv–vii. [Google Scholar]
- 8. Thomas LJ Jr. Algorithms for selected blood acid‐base and blood gas calculations. J Appl Physiol. 1972;33:154–8. [DOI] [PubMed] [Google Scholar]
- 9. Kelman GR, Nunn JF. Nomograms for correction of blood Po2, Pco2, pH, and base excess for time and temperature. J Appl Physiol. 1966;21:1484–90. [DOI] [PubMed] [Google Scholar]
- 10. Cobas b 221 system ‐ Instructions for Use. Revision 10.0. Mannheim, Germany: Roche Diagnostics GmbH; 2009. p. A‐100. [Google Scholar]
- 11. Aberman A, Cavanilles JM, Trotter J, Erbeck D, Weil MH, Shubin H. An equation for the oxygen hemoglobin dissociation curve. J Appl Physiol. 1973;35:570–1. [DOI] [PubMed] [Google Scholar]
- 12. Severinghaus JW. Blood gas calculator. J Appl Physiol. 1966;21:1108–16. [DOI] [PubMed] [Google Scholar]
- 13. Winslow RM, Samaja M, Winslow NJ, Rossi‐Bernardi L, Shrager RI. Simulation of continuous blood O2 equilibrium curve over physiological pH, DPG, and Pco2 range. J Appl Physiol Respir Environ Exerc Physiol. 1983;54:524–9. [DOI] [PubMed] [Google Scholar]
- 14. Siggaard‐Andersen O, Wimberley PD, Fogh‐Andersen N, Gothgen IH. Arterial oxygen status determined with routine pH/blood gas equipment and multi‐wavelength hemoximetry: reference values, precision, and accuracy. Scand J Clin Lab Invest Suppl. 1990;203:57–66. [DOI] [PubMed] [Google Scholar]
- 15. Eaton JW, Skelton TD, Berger E. Survival at extreme altitude: protective effect of increased hemoglobin‐oxygen affinity. Science. 1974;183:743–4. [DOI] [PubMed] [Google Scholar]
- 16. Hebbel RP, Eaton JW, Kronenberg RS, Zanjani ED, Moore LG, Berger EM. Human llamas: adaptation to altitude in subjects with high hemoglobin oxygen affinity. J Clin Invest. 1978;62:593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mairbaurl H, Weber RE. Oxygen transport by hemoglobin. Compr Physiol. 2012;2:1463–89. [DOI] [PubMed] [Google Scholar]
- 18. Yalcin O, Cabrales P. Increased hemoglobin O2 affinity protects during acute hypoxia. Am J Physiol Heart Circ Physiol. 2012;303:H271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Turek Z, Kreuzer F, Hoofd LJ. Advantage or disadvantage of a decrease of blood oxygen affinity for tissue oxygen supply at hypoxia. A theoretical study comparing man and rat. Pflugers Arch. 1973;342:185–97. [DOI] [PubMed] [Google Scholar]
- 20. Morgan TJ, Koch D, Morris D, Clague A, Purdie DM. Reduced red cell 2,3‐diphosphoglycerate concentrations in critical illness without decreased in vivo P50. Anaesth Intensive Care. 2001;29:479–83. [DOI] [PubMed] [Google Scholar]
- 21. Couzin‐Frankel J. The mystery of the pandemic's 'happy hypoxia'. Science. 2020;368:455–6. [DOI] [PubMed] [Google Scholar]
- 22. Tobin MJ, Laghi F, Jubran A. Why COVID‐19 silent hypoxemia is baffling to physicians. Am. J. Respir. Crit. Care Med.. 2020;202:356–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Willford DC, Hill EP, Moores WY. Theoretical analysis of optimal P50. J Appl Physiol Respir Environ Exerc Physiol. 1982;52:1043–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. SO2 with respective pO2 for 3518 blood gas analyses (circles) and the sigmoid fitting line (green line). For comparison standard oxyhaemoglobin dissociation curve is shown (dashed red line).1
Fig S2. Hill plot showing SO2 with respective pO2 for 3518 blood gas analyses (circles) and the sigmoid fitting line (green line). For comparison standard oxyhaemoglobin dissociation curve is shown (dashed red line).1
Fig S3. Distribution of the mean p50 calculated for each subject according to the following equation.
Fig S4. Distribution of absolute difference between each measurement and the respective individual’s mean p50 calculated with Hill Eq. 1.
Fig S5. Delta between each individual measurement from the mean p50 of that subject plotted over time starting from the first measurement.
Fig S6. Distribution of the individual patients’ mean p50 calculated with Eq. 1 and Eq. 2 plotted according to their length of stay during the observation period.
Data S1. Supplemental results.


