Abstract
Aims
Patients with cardiovascular disease appear particularly susceptible to severe COVID‐19 disease, but the impact of COVID‐19 infection on patients with heart failure (HF) is not known.
This study aimed to quantify the impact of COVID‐19 infection on mortality in hospitalized patients known to have HF.
Methods and results
We undertook a retrospective analysis of all patients admitted with a pre‐existing diagnosis of HF between 1 March and 6 May 2020 to our unit. We assessed the impact of concomitant COVID‐19 infection on in‐hospital mortality, incidence of acute kidney injury, and myocardial injury. One hundred and thirty‐four HF patients were hospitalized, 40 (29.9%) with concomitant COVID‐19 infection. Those with COVID‐19 infection had a significantly increased in‐hospital mortality {50.0% vs. 10.6%; relative risk [RR] 4.70 [95% confidence interval (CI) 2.42–9.12], P < 0.001} and were more likely to develop acute kidney injury [45% vs. 24.5%; RR 1.84 (95% CI 1.12–3.01), P = 0.02], have evidence of myocardial injury [57.5% vs. 31.9%; RR 1.81 (95% CI 1.21–2.68), P < 0.01], and be treated for a superadded bacterial infection [55% vs. 32.5%; RR 1.67 (95% CI 1.12–2.49), P = 0.01].
Conclusions
Patients with HF admitted to hospital with concomitant COVID‐19 infection have a very poor prognosis. This study highlights the need to regard patients with HF as a high‐risk group to be shielded to reduce the risks of COVID‐19 infection.
Keywords: COVID‐19, Heart failure, Acute kidney injury, Myocardial injury, Mortality
Background
The emergence of coronavirus disease 2019 (COVID‐19) has resulted in significant challenges to healthcare systems, including provision of care for those with heart failure (HF). Acute myocardial injury has been described and is associated with increased risk of in‐hospital mortality for patients with COVID‐19, 1 and as such, patients with hypertension, diabetes, and coronary artery disease are at risk of increased adverse outcomes with COVID‐19 infection. 2 , 3 However, there are limited data on outcomes in those with pre‐existing HF developing COVID‐19, and in the UK, patients with HF are not currently included on lists to be shielded.
Aims
This study sought to quantify the additional risk posed by COVID‐19 infection in hospitalized patients with chronic HF by assessing in‐hospital mortality.
Methods
We undertook a retrospective study examining all patients with chronic HF admitted to a large tertiary centre in London between 1 March and 6 May 2020. Patients with a new diagnosis of HF were excluded. For the purposes of this study, patients with both HF with reduced ejection fraction and HF with preserved ejection fraction were included.
Patients with a clinical suspicion of infection underwent laboratory testing for COVID‐19 during their admission, using nasopharyngeal swab PCR assay, and for analysis were divided into two groups—COVID‐19 positive or negative. Electronic health records were reviewed by clinicians in order to record the patient demographics, co‐morbidities, echocardiographic data, and admission data (Table 1 ).
Table 1.
Baseline characteristics of patient cohorts
| COVID‐19 positive | COVID‐19 negative | P value | |
|---|---|---|---|
| n = 40 | n = 94 | ||
| Age (years) (SD) | 79.37 (12.0) | 77.1 (13.6) | 0.36 |
| Male sex, n (%) | 18 (45.0) | 56 (59.6) | 0.12 |
| Ethnicity, n (%) | |||
| White/Caucasian | 16 (40.0) | 51 (54.3) | 0.13 |
| Asian/British Asian | 14 (35.0) | 18 (19.1) | 0.03 |
| Black/African/Caribbean | 8 (20.0) | 13 (13.8) | 0.28 |
| Other | 2 (5.0) | 12 (12.8) | 0.17 |
| Co‐morbidities, n (%) | |||
| Hypertension | 31 (77.0) | 63 (67.0) | 0.30 |
| Diabetes | 22 (55.0) | 48 (51.1) | 0.71 |
| Ischaemic heart disease | 19 (47.5.0) | 36 (38.3) | 0.27 |
| COPD | 8 (20.0) | 16 (17.0) | 0.81 |
| Asthma | 4 (10.0) | 19 (20.2) | 0.21 |
| Chronic kidney disease | 15 (37.5) | 18 (19.1) | 0.02 |
| Atrial fibrillation | 20 (50) | 40 (42.6) | 0.69 |
| Echocardiographic data | |||
| LVEF > 50% | 21 (52.5) | 45 (47.8) | 0.62 |
| LVEF < 50% | 19 (42.2) | 49 (52.2) | 0.62 |
| Admission data | |||
| Mean oxygen saturations (SD) | 91.5 (7.32) | 94 (5.9) | 0.07 |
| Mean RR (SD) | 25 (8.3) | 21 (4.9) | 0.02 |
| Presence of peripheral oedema | 22 (55.0) | 22 (63.8) | 0.34 |
| Supplemental oxygen given | 27 (67.5) | 35 (37.2) | 0.01 |
| Pre‐admission use of ACE‐I | 24 (60) | 51 (55) | 0.53 |
ACE‐I, angiotensin‐converting enzyme inhibitor; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; RR, respiratory rate; SD, standard deviation.
The primary outcome assessed was in‐hospital mortality. Secondary outcomes included prevalence of acute kidney injury (AKI), myocardial injury as measured by cardiac troponin elevation, respiratory compromise sufficient to require non‐invasive ventilation or continuous positive airway pressure, and lengths of stay in hospital. Myocardial injury was defined according to the Fourth Universal Definition of Myocardial Infarction guidance. 4 AKI was defined based on the Kidney Disease Improving Global Outcomes definition, with AKI recorded if the peak admission serum creatinine was a multiple of 1.5 above the patients' baseline. 5
Statistical analyses were performed using SPSS 2015 edition (IBM, New York). A multivariate Cox proportional hazards regression analysis was performed for the primary outcome. Variables between groups were compared using the two proportion Z‐test and Pearson χ2 test to compare in‐hospital outcomes. Relative risk (RR) ratios are given along with 95% confidence intervals (CIs). Statistical significance for P values was set at <0.05. The study conformed to the principles outlined in the Declaration of Helsinki and local ethics committees. Patient identifiable information was kept entirely confidential throughout.
Results
One hundred and thirty‐four HF‐associated hospitalizations occurred during the study period; 40 patients (29.8%) had a concomitant diagnosis of COVID‐19. Differentiation of HF or COVID‐19 as the primary cause of admission was not possible in all cases due to significant overlap of symptoms and exacerbation of HF in those with COVID‐19. Baseline characteristics are shown in Table 1 . There was no significant age (P = 0.36) or gender (P = 0.12) differences between those testing positive and negative for COVID‐19. There was a significantly higher proportion of patients of Asian origin in those testing positive for COVID‐19 (35.0% vs. 19.1%; P = 0.03) (Table 1 ).
Prevalence of most co‐morbidities, including diabetes and hypertension, was similar between groups; however, there was an increased prevalence of chronic kidney disease in the COVID‐19 cohort (37.5% vs. 19.1%, respectively; P = 0.02) (Table 1 ).
Patients with HF admitted with COVID‐19 had a significantly increased inpatient mortality compared with hospitalized HF patients without COVID‐19 infection [50% vs. 10.6%, respectively; RR 4.70 (95% CI 2.42–9.12), P < 0.001] (Figures 1 and 2 ).
Figure 1.
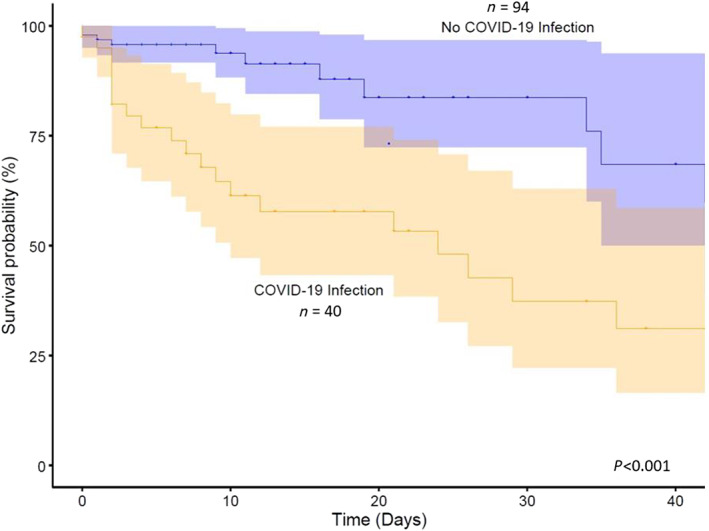
Proportional hazards regression analysis comparing survival probability between patients with COVID‐19 infection and those without COVID‐19 infection.
Figure 2.
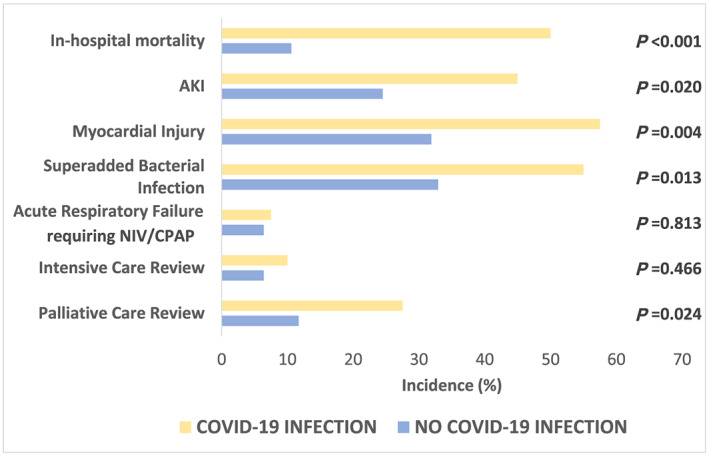
Primary and secondary outcomes in patients with COVID‐19 infection and patients without COVID‐19 infection. AKI, acute kidney injury; CPAP, continuous positive airway pressure; NIV, non‐invasive ventilation.
The documented cause of death in patients with COVID‐19 was COVID‐19 pneumonia in 60.0% (n = 12) and both COVID‐19 pneumonia and decompensated HF in 40.0% (n = 8). In the COVID‐19 negative patients, the documented cause of death was congestive cardiac failure (n = 8) and myocardial infarction (n = 2).
For the secondary outcomes, those with COVID‐19 infection had a significantly higher incidence of AKI [45% vs. 24.5%; RR 1.84 (95% CI 1.12–3.01), P = 0.02] and increased evidence of myocardial injury [57.5% vs. 31.9%; RR 1.81 (95% CI 1.21–2.68), P < 0.01] (Figure 2). Of those with myocardial injury, electrocardiogram showed lateral T‐wave inversion in 30.0% (n = 7) and lateral ST depression in 13.0% (n = 3).
Those with COVID‐19 were more likely to be tachypnoeic and require supplemental oxygen on admission and be treated for a superadded bacterial infection [55% vs. 32.5%; RR 1.67 (95% CI 1.12–2.49), P = 0.01] requiring antibiotic therapy. There was no significant difference in mean length of stay between the groups (mean 16.1 vs. 12.8 days, P = 0.22). None of the patients with COVID‐19 infection received inotropic or other circulatory support.
The risk of death was significantly higher in patients who required supplemental oxygen [RR 2.86 (95% CI 1.45–5.62), P = 0.001] or were lymphocytopenic on admission [RR 2.06 (95% CI 1.11–3.85), P = 0.03] (Table 2 ). There was no significant difference in pre‐admission angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker therapy between groups. In those testing positive for COVID‐19, angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker was discontinued in 50% (n = 12), but all of these patients had AKI.
Table 2.
Risk of in‐hospital death in heart failure patients with COVID‐19 according to specified variables
| Variable | RR (95% CI) |
|---|---|
| Black or Asian ethnicity | 1.35 (0.71–2.56), P = 0.366 |
| AKI | 1.244 (0.64–2.42), P = 0.516 |
| Myocardial injury | 1.86 (0.96–3.60), P = 0.061 |
| Superadded bacterial infection | 1.36 (0.71–2.60), P = 0.356 |
| BNP > 1000 pg/mL | 1.30 (0.52–3.29), P = 0.561 |
| Receiving supplemental oxygen on admission | 2.86 (1.45–5.62), P = 0.001 |
| Lymphocytopenia on admission (lymphocytes < 1.0 × 109/L) | 2.06 (1.11–3.85), P = 0.026 |
| C‐reactive protein > 100 mg/L on admission | 1.40 (0.70–2.81), P = 0.324 |
| LVEF < 50% pre‐admission | 1.10 (0.61–1.99), P = 0.75 |
AKI, acute kidney injury; BNP, brain natriuretic peptide; CI, confidence interval; LVEF, left ventricular ejection fraction; RR, relative risk.
In patients with COVID‐19 infection, 47.5% (n = 19) had classical chest radiographic findings characterized by multiple lower lobe and peripheral bilateral opacities, consistent with COVID pneumonia.
Conclusions
Infection with COVID‐19 is associated with significantly increased mortality in patients admitted with HF. This occurred despite similar rates of diabetes, hypertension, and coronary disease in those with and without COVID‐19 infection.
The risk of mortality in hospitalized HF patients with COVID‐19 is higher than for previously quoted overall inpatient mortality: 50% in our study vs. 23% overall. 6 This may be due to the high prevalence of co‐morbidities and frailty in patients with HF, which have a well‐established link with poorer outcomes from COVID‐19.
The significant risk of myocardial injury and AKI mirrors that seen in studies of other patient groups with COVID‐19 infection. 7 , 8 , 9 It is proposed that heightened activation of inflammatory and immunological pathways leads to acute cardiac dysfunction and renal hypoperfusion. 10 This may be exacerbated in our cohort by impaired underlying cardio‐renal physiological reserve. Further studies are required to assess whether the cause of AKI is due to HF, systemic vessel thrombosis, or a combination of both and whether early use of treatment with antiplatelet agents in high‐risk patients may prevent the development of myocardial injury and mortality associated with this.
Our study only includes those patients with HF who presented and were admitted to hospital. It is possible that a large cohort of HF patients contracted COVID‐19 in the community and had milder disease not requiring admission to hospital. The probability of survival was significantly less immediately after hospital admission. It is possible that patients may have been decompensating in the community and presented to hospital at an advanced stage of COVID‐19 illness. This highlights the importance of early recognition of COVID‐19 infection in patients with HF through widespread access to COVID‐19 laboratory testing and by encouraging patients to seek medical attention early to improve mortality in this subgroup of patients.
There was a significantly higher proportion of patients of Asian origin in those testing positive for COVID‐19. This subgroup of patients may be vulnerable to more severe forms of the infection requiring hospitalization, potentially due to the prevalence of co‐morbidities such as diabetes and hypertension. Further studies evaluating the link between ethnicity and outcomes from COVID‐19 are ongoing.
COVID‐19 infection is associated with markedly increased in‐hospital mortality in patients with HF. This study highlights the need to minimise the risk of contracting COVID‐19 for this high‐risk population and early discussions regarding ceilings of treatment if patients are to contract it. Patients with HF should be considered as being at risk, shielded from COVID‐19 infection where possible, and prioritized if a suitable vaccine is found.
Conflict of interest
None declared.
Funding
No grants, contracts, other forms of financial support, or relationship with industry declared.
Chatrath, N. , Kaza, N. , Pabari, P. A. , Fox, K. , Mayet, J. , Barton, C. , Cole, G. D. , and Plymen, C. M. (2020) The effect of concomitant COVID‐19 infection on outcomes in patients hospitalized with heart failure. ESC Heart Failure, 7: 4443–4447. 10.1002/ehf2.13059.
References
- 1. Shaobo S, Mu Q, Bo S, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol 2020; 5: 802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, Mcginn T, Davidson KW. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalised with COVID‐19 in the New York City Area. JAMA 2020; 323: 2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD, ESC Scientific Document Group . Fourth universal definition of myocardial infarction. Eur Heart J 2018; 40: 237–269. [DOI] [PubMed] [Google Scholar]
- 5. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group . KDIGO clinical practice guideline for acute kidney Injury. Kidney Int 2012; 2: 1–138. [Google Scholar]
- 6. Goel S, Jain T, Hooda A, Malhotra R, Johal G, Masoomi R, Kamran H, Krishnamoorthy PM, Senguttuvan NB, Sharma A, Gidwani U. Clinical characteristics and in‐hospital mortality for COVID‐19 across the globe. Cardiol Therapy 2020; 1–7. 10.1007/s40119-020-00189-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wei J, Huang F, Xiong T, Liu Q, Chen H, Huang H, Luo Y, Zhou X, Liu Z, Peng Y, Xu Y, Wang B, Yang Y, Liang Z, Lei X, Ge Y, Yang M, Zhang L, Zeng M, Yu H, Liu K, Jia Y, Prendergast B, Li W, Chen M. Acute myocardial injury is common in patients with COVID‐19 and impairs their prognosis. Heart 2020; 106: 1154–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kang Y, Chen T, Mui D, Ferrari V, Jagasia D, Scherrer‐Crosbie M, Chen Y, Han Y. Cardiovascular manifestations and treatment considerations in COVID‐19. Heart 2020; 106: 1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Durvasula R, Wellington T, McNamara E, Watnick S. COVID‐19 and kidney failure in the acute care setting: our experience from Seattle. Am J Kidney Dis 2020; 76: 4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mehra M, Ruschitzka F. COVID‐19 illness and heart failure: a missing link? J Am Coll Cardiol HF 2020; 8: 512–514. [DOI] [PMC free article] [PubMed] [Google Scholar]


