Abstract
Background
The missing asymptomatic COVID‐19 infections have been overlooked because of the imperfect sensitivity of the nucleic acid testing (NAT). Globally understanding the humoral immunity in asymptomatic carriers will provide scientific knowledge for developing serological tests, improving early identification, and implementing more rational control strategies against the pandemic.
Measure
Utilizing both NAT and commercial kits for serum IgM and IgG antibodies, we extensively screened 11 766 epidemiologically suspected individuals on enrollment and 63 asymptomatic individuals were detected and recruited. Sixty‐three healthy individuals and 51 mild patients without any preexisting conditions were set as controls. Serum IgM and IgG profiles were further probed using a SARS‐CoV‐2 proteome microarray, and neutralizing antibody was detected by a pseudotyped virus neutralization assay system. The dynamics of antibodies were analyzed with exposure time or symptoms onset.
Results
A combination test of NAT and serological testing for IgM antibody discovered 55.5% of the total of 63 asymptomatic infections, which significantly raises the detection sensitivity when compared with the NAT alone (19%). Serum proteome microarray analysis demonstrated that asymptomatics mainly produced IgM and IgG antibodies against S1 and N proteins out of 20 proteins of SARS‐CoV‐2. Different from strong and persistent N‐specific antibodies, S1‐specific IgM responses, which evolved in asymptomatic individuals as early as the seventh day after exposure, peaked on days from 17 days to 25 days, and then disappeared in two months, might be used as an early diagnostic biomarker. 11.8% (6/51) mild patients and 38.1% (24/63) asymptomatic individuals did not produce neutralizing antibody. In particular, neutralizing antibody in asymptomatics gradually vanished in two months.
Conclusion
Our findings might have important implications for the definition of asymptomatic COVID‐19 infections, diagnosis, serological survey, public health, and immunization strategies.
Keywords: antibody dynamics, asymptomatic, COVID‐19, neutralizing antibody, SARS‐CoV‐2
The combination of NAT and serological testing for IgM antibody significantly improves the detection sensitivity of asymptomatic COVID‐19 infections, compared with NAT alone. S1‐specific IgM antibody response with rapid emergence and disappearance might be helpful to assist NAT for early identification of infectious individuals. A majority of asymptomatics induce very low levels of neutralizing antibody that disappear in two months. Abbreviations: NAT, nucleic acid testing; FI, fluorescence intensity; NT50, half‐maximal neutralizing titer.

Abbreviations
- FI
fluorescence intensity
- NAT
nucleic acid testing
- NT50
half‐maximal neutralizing titer
1. INTRODUCTION
SARS‐CoV‐2 is an emerging coronavirus, which was first recognized as the causative agent of COVID‐19 in December 2019, 1 and has rapidly spread around the world. On March 11, 2020, the WHO has declared COVID‐19 a global pandemic. 2 As of June 28, 2020, there have been 9 843 073 confirmed cases and 495 760 deaths, reported in 215 countries and territories worldwide. 3 Unlike the epidemic of SARS‐CoV and MERS‐CoV, the sharp rise in the number of global COVID‐19 cases in a short epidemic episode brings the fear of having viral transmission from asymptomatic individuals. On January 28, 2020, the National Health Commission of China (NHCC) updated the COVID‐19 Prevention and Control Plan (3rd edition) and first emphasized the identification and quarantine of asymptomatic infections. 4 Asymptomatic COVID‐19 infection has been defined as a person infected with SARS‐CoV‐2 who has no clinical symptoms (such as fever, cough, or sore throat) and no radiological changes of the lung, yet nucleic acid testing (NAT) positive for SARS‐CoV‐2. 4 As of February 11, 2020, there were 72 314 COVID‐19 cases reported in China, and 889 cases (1.2%) belonged to asymptomatic infections. 5 As of April 14, 2020, a total of 6764 asymptomatic infections reported in China, which accounts for about 5.9% of all registered cases. Among these, 1297 asymptomatic individuals, in fact presymptomatics and subsequently, developed to the confirmed cases with different severities of illness, while the others remained asymptomatic. 6 Beyond doubt, presymptomatics are really infectious. 7 , 8 , 9 Interestingly, several studies reported that asymptomatic COVID‐19 infections also play important roles in the transmission. 10 , 11 Therefore, both types of asymptomatics contribute significantly to disease transmission. To better control the pandemic of COVID‐19, actively discovering, as well as early identifying and quarantining asymptomatics are urgently needed.
Until now, detection of asymptomatic infections has been relied on extensive NAT screening of quarantined individuals. The test sensitivity of NAT highly depends on the course and the type of clinical COVID‐19 syndromes, the collection site, the transportation, and storage of specimens. About 30% false‐negative rates of NAT have been reported in COVID‐19 patients. 12 In particular, recent seroprevalence investigations strongly suggested that COVID‐19 cases, especially asymptomatics are greatly underestimated in different countries and regions. SARS‐CoV‐2‐specific IgG response in blood donors reached 3.08% during lockdown of Wuhan city, 13 consistent with the other report of the seropositivity in healthcare workers in Wuhan ranging from 3.2% to 3.8%. 14 Both studies indicate that the number of actual infections is at least five times higher than that of the reported cases in Wuhan. In Spain, there were 5% serological positive individuals of national population and 1/3 of them did not report symptoms. 15 Similar situation occurred in different regions of the United States. 16 Therefore, these missing asymptomatic cases that are infectious in the community have been substantially overlooked because of the limited sensitivity of NAT and passive approaches to discover them.
The sensitivity of SARS‐CoV‐2 IgG response can be near 100% when serum samples from COVID‐19 patients are acquired within 19 days after symptoms onset. 17 However, asymptomatic individuals have a much longer median duration of viral shedding (19d) than the symptomatic group, 18 which indicate asymptomatics may develop different immune responses to SARA‐CoV‐2 infection when compared to symptomatic COVID‐19 patients. Unfortunately, humoral immunity in asymptomatic infections with SARS‐CoV‐2 has not been established. It is of significant importance for developing serological tests and improving early identification, and providing more rational control strategies for the pandemic, in order to establish dynamics of IgG and IgM responses and neutralizing antibodies in asymptomatic COVID‐19 infections.
2. MATERIALS
2.1. Definitions
In order to identify and report SARS‐CoV‐2 infected cases in time, the NHCC updated the COVID‐19 Prevention and Control Plan (3rd edition) on January 28, 2020, which first emphasized the identification and quarantine of asymptomatic infections. 4 Specifically, close contacts with confirmed cases and persons with close social distance during extensive investigation of clusters and tracing infectious sources were required to screen by Real‐time PCR (RT‐qPCR) testing SARS‐CoV‐2 genes in nasopharyngeal swabs. On April 8, 2020, the lockdown had been lifted in Wuhan. Personnel returning to work were also required to screen by RT‐qPCR. All NAT‐positive individuals were asked to provide detail information, including demography, preexisting conditions, exposure history, symptoms, as well as screening records, and accepted centralized isolation for the preceding 14 days. If clinical symptoms and/or lung damage occurred, they should be transferred to the general hospital such as Tongji Hospital for further treatment. Contacts with negative NATs also complied with home quarantine for 14 days. Asymptomatic infection was defined as an individual with a positive NAT result but without any relevant clinical symptoms and radiological changes of the lung during quarantine. Mild COVID‐19 patient was defined as an individual with nasopharyngeal swabs that were NAT‐positive for SARS‐CoV‐2, with mild symptoms (such as fever, cough, or sore throat) yet without radiological changes of the lung. A close social distance was defined as (a) anyone who had been within approximately 6 feet (2 meters) of a person infected with SARS‐CoV‐2 for longer than 10 minutes; and (b) those who had direct contact with the infectious secretions of a confirmed COVID‐19 patient. At present, the NHCC updated the latest COVID‐19 Prevention and Control Plan (6th edition), but continued to use the previous definitions. 19
2.2. Study design and participants
Between February 17, 2020, and April 28, 2020, 1056 confirmed COVID‐19 patients with different severities of illness were hospitalized in Tongji Hospital, Wuhan, China. Only 51 mild COVID‐19 patients who had serial serum samples yet not any preexisting conditions were selected and collected for a total of 87 samples. After extensively screening, 11 766 epidemiologically suspected individuals, 63 asymptomatic infections, and 63 healthy controls were informed in the study. Among these, 48 healthy controls and 36 asymptomatics had clear exposure history, respectively. All participants were traced consecutively for 65 days. Serum specimens were collected from each individuals and were stored at −80°C until use.
2.3. Real‐time PCR
Nasopharyngeal swabs of all participants on enrollment were collected and maintained in viral transport medium. Before detection, all specimens were thermally inactivated in 56°C for 30 minutes before detection. SARS‐CoV‐2 infection was confirmed using TaqMan One‐Step RT‐qPCR Kits (DAAN Gene) which detected ORF1ab and N genes and approved by the China Food and Drug Administration (CFDA). The RT‐qPCR assay kits were performed according to manufacturers’ instructions, and the cutoff Ct value was 40 for both genes. The Ct values of both genes were less than 40 and were defined as positive.
2.4. SARS‐CoV‐2 antibody detection
The IgM and IgG antibodies against recombinant both nucleoprotein (N) and spike (S) proteins of SARS‐CoV‐2 in serum specimens were detected by a chemiluminescence method according to the manufacturer's instructions (YHLO Biotech), respectively. The antibody levels ≥10 AU/mL are reactive (positive), and the results <10 AU/mL are negative.
2.5. Protein microarray fabrication
The SARS‐CoV‐2 proteome microarray was prepared as our previous study with minor modifications. 20 Three more proteins, that is, ORF3a, ORF3b, and ORF7b, were expressed by our laboratory, and another protein RdRp was provided by H. Eric Xu's Lab. 21 The proteins printed on the microarray were listed in Table S1. The proteins with indicated concentrations, along with the negative (GST, Biotin‐control, and eGFP) and positive controls (Human IgG, Human IgM, and ACE2‐Fc), were printed in triplicate on PATH substrate slide (Grace Bio‐Labs, Oregon, USA) to generate identical arrays in a 2 × 7 subarray format using Super Marathon printer (Arrayjet). Protein microarrays were stored at −80°C until use.
2.6. Microarray‐based serum analysis
The prepared SARS‐CoV‐2 proteome microarray was conducted to probe all serum samples, as previously described. 20 Briefly, a 14‐chamber rubber gasket was mounted onto each slide to create individual chambers for the 14 identical subarrays. The arrays stored at −80°C were warmed to room temperature and then incubated in blocking buffer (3% BSA in 1 × PBS buffer with 0.1% Tween 20) for 3 hours. Serum samples were diluted 1:200 in PBS containing 0.1% Tween 20, added with 0.5 mg/mL total E coli lysate. 200 μL of diluted serum or buffer only was incubated with each subarray for 2 hours at room temperature. After washed with 1 × PBST, the secondary antibodies Cy3‐conjugated goat anti‐human IgG and Alexa Fluor 647‐conjugated donkey anti‐human IgM (Jackson ImmunoResearch), which were diluted 1:1000 in 1 × PBST, were added on the subarray and incubated at room temperature for another 1 hour. The microarrays were then washed again, dried by centrifugation at room temperature, and scanned by LuxScan 10K‐A (CapitalBio Corporation) with the parameters set as 95% laser power/PMT 550 and 95% laser power/ PMT 480 for IgM and IgG, respectively. Data of fluorescent intensity (FI) from each microarray were extracted by GenePix Pro 6.0 software (Molecular Devices). The quantitative result of FI for antibody responses to the protein in the serum was defined as the median of the foreground subtracted by the median of background for each spot and then averaged the triplicate spots for each protein. Serum IgG and IgM responses to the protein were analyzed separately and expressed as mean (Log2FI) ± SD in different groups. Overall visualization of IgG and IgM profiles was built by clustering analysis to generate heatmaps.
2.7. Neutralization antibody detection using pseudovirus neutralization assay
A full‐length codon‐optimized s gene of SARS‐CoV‐2 was first synthesized and cloned into the lentivirus vector GV367 (Genechem) and then used to generate a eGFP‐coexpressing pseudovirus by cotransfected into HEK293T cells (CRL‐11268) with the other two viral packaging help vectors pHelper1.0 and pHelper2.0 (Genechem). 48 hours after transfection, the supernatants were collected after centrifugation with 4000 g for 10 minutes at 4°C and further filtrated with 0.45 μm filter. The recombinant pseudovirus was further purified by centrifugation with 25 000 rpm for 2 hours at 4°C and diluted with PBS. The titer of recombinant pseudovirus was quantified by fluorometry and RT‐qPCR targeting s gene. The SARS‐CoV‐2 pseudovirus neutralization assay was carried out on Vero E6 cells (CRL‐1586) in a 96‐well plate. 50 μL serial 2‐fold diluted sera from 1:10 to 1:2560 from each serum sample were prepared, and equal volumes of SARS‐CoV‐2 pseudovirus were added, and the plates were pre‐incubated at 37°C for 1 hour. 24 hours before infection, 100 μL of 104 Vero E6 cells were added into each well of a 96‐well plate. After washed and added 100 μL fresh culture medium, cells were incubated with 100 μL of sera‐pseudovirus mixture for 48 hours. The cells were collected with 200 μL of digestion solution and used to determine the number of eGFP‐expressing cells by FACS. The positive rate of eGFP‐expressing cells (PRG) was calculated after collected 1000 cells. Experiments were repeated twice. The neutralization rate (%) for different dilutions was calculated as following:
The titer of neutralization antibody for each serum sample was expressed as the half‐maximal neutralizing titer (NT50). NT50 of each serum sample was determined as the highest dilution ratio of serum with 50% neutralization rate.
2.8. Statistical analysis
All diagram and statistical analyses were carried out using Prism 8, SPSS, or R software when applicable used. Cluster analysis of IgM and IgG profiles was performed with pheatmap package of R, respectively. NT50 was determined using nonlinear regression by SPSS, and a loess 489 regression model was used to establish the kinetics of neutralizing antibody. The analysis of variance (ANOVA), post hoc test (SNK), Student's t test, and Mann‐Whitney U test were used to compare difference among different groups when required. A statistical significant was considered when P < .05.
3. RESULTS
3.1. The workflow of screening participants
To understand the humoral immunity against SARS‐CoV‐2 in asymptomatic infections, we actively screened by RT‐qPCR from 11 766 personnel returning to work, and close contacts with the confirmed cases in different communities of Wuhan by investigation of clusters and tracing infectious sources. Among them, only 12 asymptomatic individuals with positive NAT results from them were found out. We further conducted a serological survey with the serum samples collected from all participants, using a validated assay for IgM and IgG antibodies against recombinant both N and S proteins of SARS‐CoV‐2. Another 51 asymptomatic infections were further discovered because they had positive results for IgG alone, or both IgG and IgM. Sixty‐three healthy individuals with negative results for both NAT and antibodies were selected as negative controls. Fifty‐one mild patients without any preexisting conditions were also screened from 1056 patients during hospitalization in Tongji Hospital as positive controls. A total of 177 participants were enrolled in this study, and serial serum samples (n = 213) were collected (Figure 1). Clear exposure history or days after symptoms onset were obtained from 48 healthy controls, 36 asymptomatic infections, and 51 mild patients. The screening was conducted between February 17, 2020, and April 28, 2020. Serum IgM and IgG profiles of 177 participants were further probed using a SARS‐CoV‐2 proteome microarray. Neutralizing antibody responses in these population were detected by a pseudotyped virus neutralization assay system. The dynamics of IgM and IgG antibodies and neutralizing antibodies were analyzed with exposure time or symptoms onset. The experiments and data analysis about serum proteome microarray and neutralizing antibody were performed from April 2020 to June 2020.
FIGURE 1.
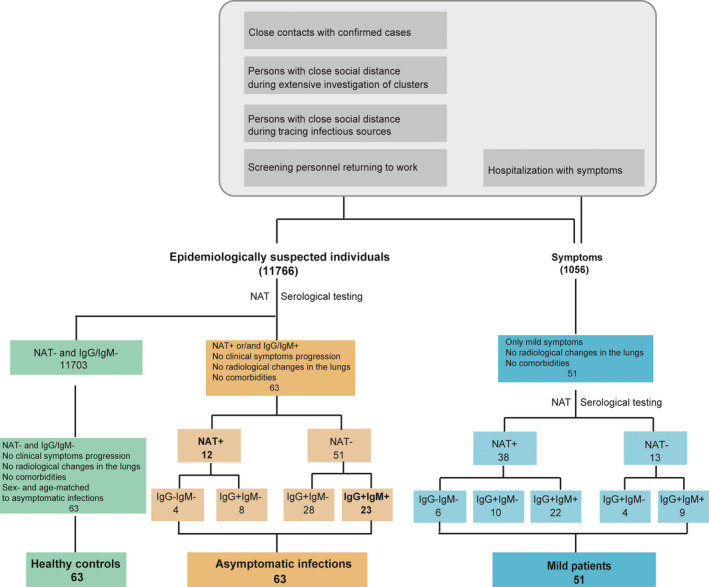
The workflow of screening participants
3.2. The detection sensitivity of NAT alone significantly improved by adjunctive serological testing
Currently, NAT is still the gold standard to diagnose COVID‐19 patients. To explore the role of serological testing for early identifying asymptomatics, we first analyzed the characteristics of study population based on the results of both NAT and commercial serological test (Table 1). We found that 63 asymptomatics were actually classified into four subgroups. Interestingly, 81% (51/63) asymptomatic infections had negative NAT results, but either IgG alone positive (28/63) or 36.5% (23/63) positive for both IgG and IgM responses. Only 19% were NAT‐positive, composed of 6.3% (4/63) NAT alone positive and 12.7% (8/63) positive for both NAT and IgG responses (Table 1). Mild patients were also analyzed in the same way and followed into five subgroups. 25.4% (13/51) mild patients were NAT negative but either IgG alone positive (4/51) or 17.6% (9/51) positive for both IgG and IgM. 60.3% reported positive results for NAT, and also positive for either IgG alone (10/51) or both IgG and IgM responses (22/51). Importantly, only 11.3% (6/51) mild cases belonged to NAT alone positive (Table 1). We further analyzed the diagnostic value of commercial serological kit by using the results of NAT as the gold standard (Table S2). The sensitivity and specificity of commercial serological kit for diagnosing asymptomatic infections were 66.7% and 99.5%, respectively. Taken together, our results suggest that the combination of serological testing and NAT significantly raises the detection sensitivity when compared to the NAT alone, and thus, serological testing might be used as an adjunctive tool of NAT for early identifying infectious asymptomatics.
TABLE 1.
Characteristics of study population
| All | Healthy controls | Asymptomatic(n = 63) | Mild(n = 51) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aa(NAT + IgG−IgM−) | Ab(NAT + IgG + IgM−) | Ac(NAT−IgG + IgM−) | Ad(NAT−IgG + IgM+) | Ma(NAT + IgG−IgM−) | Mb(NAT + IgG + IgM−) | Mc(NAT + IgG + IgM+) | Md(NAT−IgG + IgM−) | Me(NAT−IgG + IgM+) | |||
| N | 177 | 63 | 4 | 8 | 28 | 23 | 6 | 10 | 22 | 4 | 9 |
| Age, years | |||||||||||
| Mean(SD) | 44.6(19.25) | 43.2(19.85) | 42.8(8.62) | 40.6(10.85) | 46.5(18.05) | 44.7(17.89) | 39.7(25.02) | 39.7(23.77) | 47.6(22.46) | 45.0(31.12) | 54.0(7.40) |
| Median(IQR) | 46(30‐59) | 46(27‐60) | 41(36‐50) | 38(31‐52) | 50(29‐58) | 40(29‐55) | 38(29‐46) | 32(20‐58) | 51(30‐64) | 56(25‐66) | 55(48‐59) |
| Sex, n(%) | |||||||||||
| Male | 85(48.0) | 33(52.4) | 0(0.0) | 2(25.0) | 13(46.4) | 10(43.5) | 3(50.0) | 6(60.0) | 11(50.0) | 3(75.0) | 4(44.4) |
| Female | 92(52.0) | 30(47.6) | 4(100.0) | 6(75.0) | 15(53.6) | 13(56.5) | 3(50.0) | 4(40.0) | 11(50.0) | 1(25.0) | 5(55.6) |
Abbreviations: IQR, interquartile range; SD, standard deviation.
3.3. The overall IgM and IgG profiles by SARS‐CoV‐2 proteome microarray
To better understand humoral immune responses against SARS‐CoV‐2, IgM (red) or IgG (green) antibody responses to 20 out of 28 predicted proteins of SARS‐CoV‐2 were further detected in parallel using a proteome microarray (Figure S1A). Three representative microarray pictures probed with sera collected from a healthy contact; an asymptomatic infection and a mild patient were shown as Figure S1B. Serum IgM (red) or IgG (green) antibodies against different proteins were captured and then detected by secondary antibodies labeled with different fluorescent dyes. Based on microarray pictures, asymptomatics and mild individuals showed strong fluorescent signals especially against S1, N, N‐Nter, and N‐Cter proteins, when compared with healthy controls. For the quantitative comparison, the difference among them needs further analysis after fluorescent intensity extraction, data filtering, and normalization. Finally, each protein‐specific IgM and IgG data for different serum samples were built separately. Overall visualizations of IgM (Figure S2) or IgG (Figure S3) profiles in 177 participants were performed by clustering analysis to generate heatmaps. Overall, the serum samples for all three groups, that is, healthy controls, asymptomatic infections, and mild cases, were clustered together, especially for IgG antibodies, although some samples were not correctly grouped which labeled with spots in different colors (Figure S2 and Figure S3). Notably, clinically diagnosed healthy controls, asymptomatics, and mild cases were not divided into three complete independent groups, especially based on microarray‐constructed IgM profiles (Figure S2), which indicate that we cannot distinguish these groups based on antibody detection alone and also confirm the presence of serological testing negative asymptomatic infections and mild cases. In addition, three healthy controls were not correctly clustered as asymptomatic infections based on the IgM profiles (Figure S2), which highlight the significance of keeping social distance and repeated tests.
Based on the analysis of quantitative data, both asymptomatics and mild patients induced stronger IgM (Figure 2A) and IgG responses (Figure 2B), especially against S1, N, N‐Nter, and N‐Cter out of 20 proteins than healthy controls, respectively. Although asymptomatic infections and mild cases had similar IgM profiles against 20 proteins of SARS‐CoV‐2 by clustering analysis (Figure S2), the levels of IgM responses to S1, N, N‐Nter, N‐Cter, and ORF7b were significantly higher in mild patients than asymptomatics (Figure 2A). Mild patients also tended to induce stronger IgG responses against S1, N, N‐Nter, and N‐Cter proteins than asymptomatic infections (Figure 2B). And then, we compared the levels of antibodies in different subgroups of asymptomatics and mild patients with that of healthy controls. Except NAT alone positive asymptomatic individuals, other subgroups of asymptomatics and mild patients elicited higher levels of S1‐, N‐, N‐Nter‐, and N‐Cter‐specific IgM or IgG antibodies than healthy controls (Figure S4A), although these antibody responses could not differentiate the same subgroup between asymptomatics and mild cases (Figure S4B). Taken together, our results demonstrated that IgM and IgG responses to only S1 and N from the 20 proteins of SARS‐CoV‐2 might differentiate both asymptomatics and mild patients from healthy controls.
FIGURE 2.
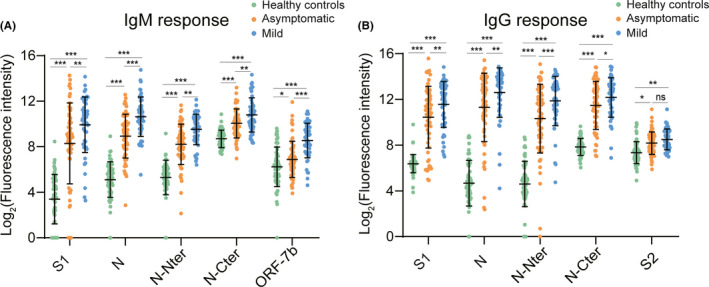
Antibody responses to different proteins of SARS‐CoV‐2. Serum proteome microarray was used to probe IgM or IgG antibody against 20 proteins of SARS‐CoV‐2 in all samples collected from 63 healthy controls, 63 asymptomatic individuals, and 51 mild patients. The results were expressed as mean {log2 (Fluorescence intensity)} ± SD in different groups. A, Comparison of IgM responses to five proteins among three groups. B, Comparison of IgG responses to five proteins among three groups. Both analysis of variance (ANOVA) and post hoc test (SNK) were conducted to test difference in means among healthy controls, asymptomatics, and mild patients. ***P < .001, **P < .01, *P < .05, and ns indicating no significance
3.4. The dynamic changes of S1‐ and N‐specific IgM and IgG responses
To help establish serological tests, we further compared the dynamic changes of S1‐, N‐, N − Nter‐, and N‐Cter‐specific IgM and IgG antibodies in 48 healthy controls, 36 asymptomatic individuals, and 51 mild patients, who had either clear exposure history or serial samples after symptoms onset (Figure 3 and Figure S5). Early to the seventh day after exposure, S1‐ and N‐specific IgM and IgG responses were induced in asymptomatic individuals and peaked on days from 17 days to 25 days and then began to decline. Except N‐specific IgG response, other antibodies in asymptomatics could not be detectable 2 months after exposure (Figure 3 and Figure S5). Compared to asymptomatics, mild patients had distinct dynamic changes of these antibodies. Early to 1 day after symptoms onset, IgM antibody against the N protein rapidly evolved and persisted at a high level, while S1‐specific IgM responses were induced in mild patients and persistently increased until 29 days after symptoms onset. In addition, S1‐ or N‐specific IgM and IgG responses in mild patients maintained for at least 65 days (Figure 3).
FIGURE 3.
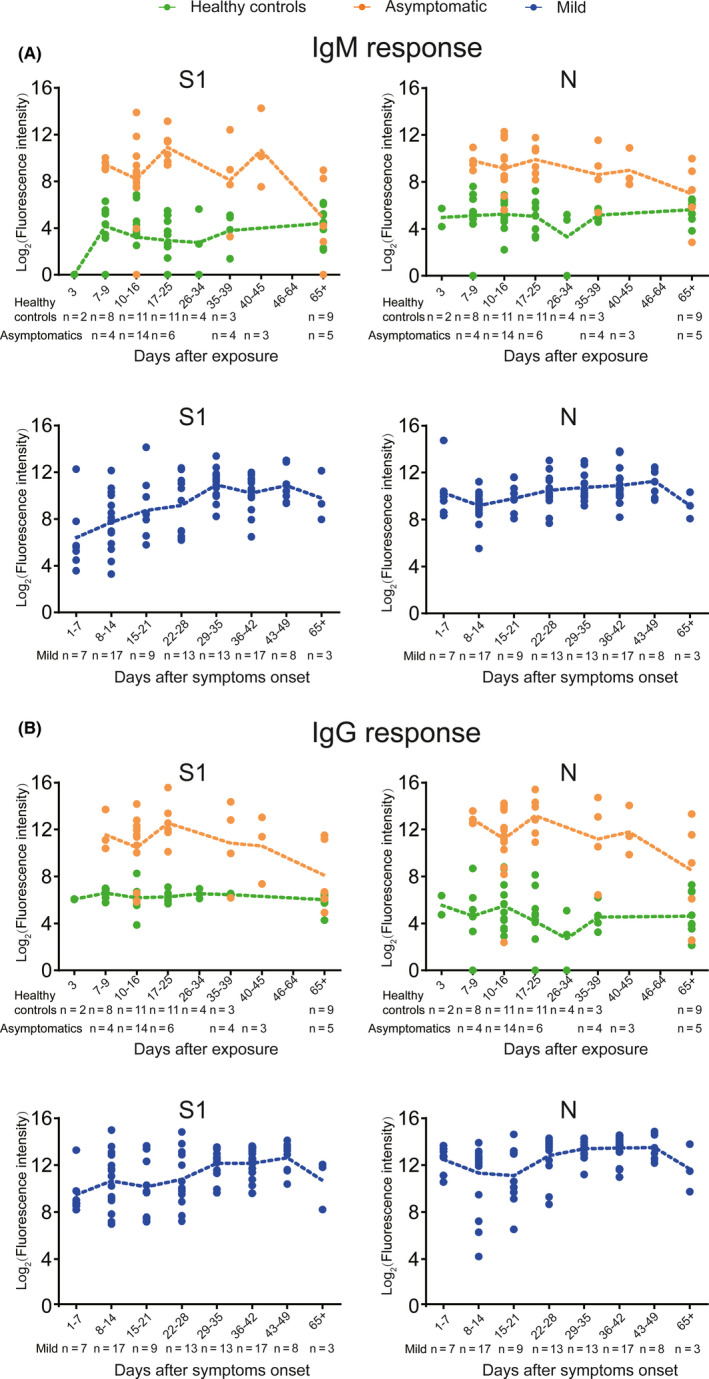
Dynamic changes of S1‐ and N‐specific IgM and IgG responses. Serum proteome microarray was used to probe antibody responses in the samples collected from 48 healthy individuals, 36 asymptomatic individuals, and 51 mild patients. The result of each serum sample was expressed as log2 (fluorescence intensity). 48 healthy controls and 36 asymptomatic infections having clear exposure history were plotted in sections according to the exposure time. 51 mild COVID‐19 patients with serial sera samples (n = 87) were segmented according to days after symptoms onset. The yellow, green and blue line showed the mean level of antibody responses in healthy controls, asymptomatic infections and mild patients, respectively. A, Dynamic changes of S1‐ and N‐specific IgM responses. B, Dynamic changes of S1‐ and N‐specific IgG responses
3.5. The dynamic changes of neutralizing antibody
To better understand the role of humoral immunity against infection, we analyzed the dynamic changes of neutralizing antibody responses using pseudotyped virus‐based neutralization assay platforms (Figure 4). Interestingly, we found that 38.1% (24/63) asymptomatic individuals, mainly NAT‐positive (8/12), did not produce neutralizing antibody. 61.9% (39/63) of asymptomatic infections and 19% of (12/63) healthy controls only produced low titers of neutralizing antibody, with the geometric mean NT50 of 1:24 and 1:13, respectively (Figure 4A). Among three groups, mild patients stimulated the highest levels of neutralizing antibody with the geometric mean NT50 of 1:269, whereas 11.8% (6/51) mild patients, mainly NAT alone positive (4/6), did not elicit neutralizing antibody (Figure 4B). In order to investigate the duration of neutralizing antibody, the dynamics of neutralizing antibody response were also established for three groups. As early as 7 days after exposure, neutralizing antibody rapidly evolved in asymptomatics individuals, peaked on days from 10 days to 25 days, then decayed rapidly within our observed period. (Figure 4C) As early as 1 day post symptom onset, mild patients also produced low level of neutralizing antibody, and then, the titer rose persistently until 22 days and maintain high levels for at least 65 days (Figure 4D).
FIGURE 4.
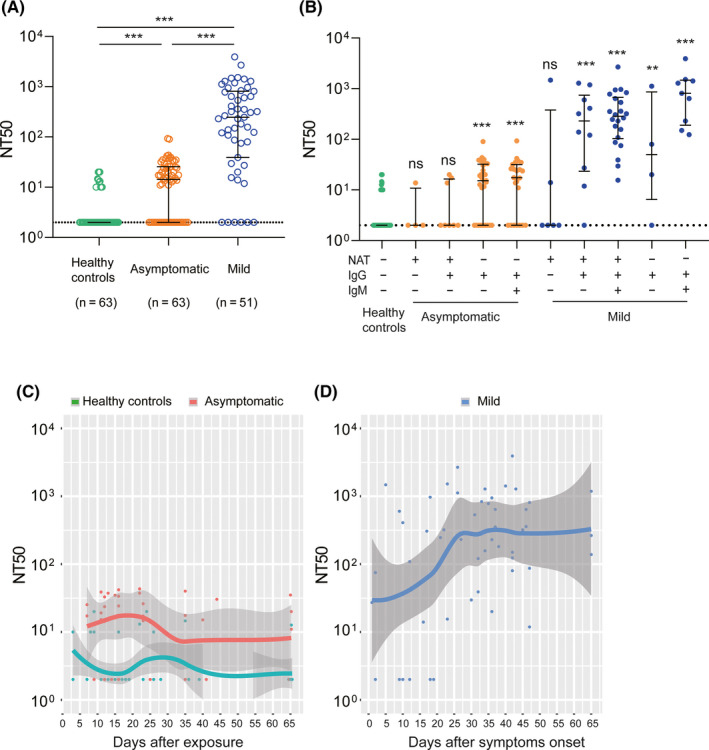
Neutralizing antibody responses and dynamics. The titer of neutralization antibody for each serum sample was expressed as the half‐maximal neutralizing titer (NT50), which was calculated by using nonlinear regression of SPSS. The results were shown as the medians of NT50 and interquartile ranges (IQRs) in different groups. A, Comparison of NT50 among healthy controls, asymptomatic infections and mild patients. B, Comparison of NT50 among different subgroups with that of healthy controls. C, Dynamic changes of NT50 for 48 healthy controls and 36 asymptomatic individuals over exposure time. D, Dynamic changes of NT50 for 51 mild COVID‐19 patients with the day after symptom onset. A loess 489 regression model was used to established the kinetics of neutralizing antibody by R. The lines show the mean value expected from a Loess 489 regression model, and the ribbons indicate the 95% confidence interval. Serum samples with NT50 below 1:10 are plotted at NT50 = 2
4. DISCUSSION
It remains very difficult to early and actively find out all asymptomatic COVID‐19 infections from healthy population, based on the current control strategy. In this study, five epidemiological strategies were adopted as recommended by the NHCC to discover these 63 asymptomatics from more than 10 000 epidemiologically suspected individuals, which provide us opportunities to establish and verify laboratory tests for early and actively screening asymptomatic infections. Firstly, we demonstrated that the current used NAT alone has a very low sensitivity (only 19%) to screen asymptomatic infections, which was supported by the following reports. For instance, the strategy of nucleic acid screening for all citizen had been performed in Wuhan after lift of the lockdown, and there were only another 300 NAT‐positive asymptomatics out of about 10 million citizens. 22 The latest seroprevalence studies also revealed that the proportion of asymptomatic infections might be much higher than the incidence rate reported in China 5 , 6 , 13 , 14 Combining with the results of seroprevalence investigations in different countries, 15 , 16 , 23 we can make a conclusion that substantial missing asymptomatic infections based on the NAT alone screening cannot be found out in time, which also confirm experimentally the view of the prediction model with 87% undiscovered infections. 24 Because of speed, accessibility, and low cost of serological testing, the joint screening strategy based on both NAT and serological testing should be performed to find out as many infectious SARS‐CoV‐2 infected individuals as possible. In our study, NAT in conjunction with serological testing for IgM discovered 55.5% of the total asymptomatic infections, except an additional 17.6% IgM‐positive mild patients. Therefore, the joint screening strategy will significantly attribute to early identifying and actively discovering infectious sources, and repeated tests may further improve the detection sensitivity. 25 Under these context, we provide a new perspective that asymptomatic COVID‐19 infection should be defined as a person has positive NAT or/and IgM antibody response, yet without clinical symptoms and radiological changes of the lung. This definition will give an additional 36.5% IgM seropositivity of asymptomatics, which is more reasonable and practical than the current used.
Furthermore, both humoral immune responses to the antigens of SARS‐CoV‐2 and antibody dynamics in asymptomatics determined by our serum proteome microarray analysis provide scientific foundation for the development and application of serological tests. Our study found that asymptomatics mainly evolved IgM and IgG antibodies against S1 and N proteins out of 20 proteins of SARS‐CoV‐2, supported by the facts that both S and N proteins also are the major targets in commercial or homemade serological tests. 26 , 27 Although asymptomatic individuals have a long duration of viral shedding, 18 the timing of serological tests is also very significant for assisting diagnosis. 28 , 29 As demonstrated in this study, S1‐specific IgM antibody responses were induced in asymptomatics as early as 1 week after exposure and disappeared within two months, which coincides within the duration of viral shedding of SARS‐CoV‐2. Because of rapid emergence and disappearance, S1‐specific IgM antibody response might be meaningful to assist NAT for earlier identification of infectious individuals. By contrast, stronger N‐specific IgM and IgG responses persisted in symptomatic COVID‐19 patients for a longer time than asymptomatics as demonstrated in our study and another report, 30 which might be more suitable for serological survey and recovery monitor. Therefore, serological testing might better be performed based on S‐ or N‐specific antibody responses for different purposes. Further investigation to define more accurate serological biomarkers such as profiling B cells epitopes from S1 antigen should be encouraged. In addition, antibody measurements, especially IgA in sputum or tears, may further improve the accuracy of identifying infectious asymptomatic infections. 31
Although a combination of NAT and antibody testing is a promising strategy for early identifying and actively discovering infectious sources during epidemiological investigation, approximately 50% of asymptomatic individuals remain undetected as demonstrated in this study. There is an urgent need to implement more rational control strategies; otherwise, asymptomatic transmission of SARS‐CoV‐2 would become the Achilles’ heel of controlling COVID‐19 pandemic. 32 We found that 63.5% of asymptomatic individuals only elicited low levels of neutralizing, and the rest did not produce neutralizing antibodies at all. In addition, 11.8% mild patients also did not produce neutralizing antibody, in line with other reports. 18 , 33 , 34 In particular, we demonstrated that neutralizing antibody in asymptomatic individuals decreased rapidly and disappeared in a short time, which indicate that the effectiveness of antibody‐mediated immunity could not be used to guarantee the accuracy of an “immunity passport” or “risk‐free certificate.” Our findings might suggest the risks of “shield immunity” and notably, that asymptomatic individuals might still need immunization with vaccines. Strict public health strategies including lockdown of city, tracing infectious sources and quarantine, keeping social distance, isolate protection of healthy individuals such as wearing mask and washing hands were performed during the phase of high prevalence in Wuhan, so these undetected asymptomatics might not play important roles in disease transmission. Therefore, complying with strict public health measures remains the most important strategy to control the pandemic of COVID.
CONFLICT OF INTEREST
The authors declare no conflicts of interest. Dr Lei has nothing to disclose. Dr Li has nothing to disclose. Dr Hou has nothing to disclose. Dr Wang has nothing to disclose. Dr Ouyang has nothing to disclose. Dr Zhang has nothing to disclose. Dr Lai has nothing to disclose. Dr Banga Ndzouboukou has nothing to disclose. Dr Xu has nothing to disclose. Dr Zhang has nothing to disclose. Dr Chen has nothing to disclose. Dr Xue has nothing to disclose. Dr Lin has nothing to disclose. Dr Zheng has nothing to disclose. Dr Yao has nothing to disclose. Dr Wang has nothing to disclose. Dr Yu has nothing to disclose. Dr Jiang has nothing to disclose. Dr Zhang has nothing to disclose. Dr Qi has nothing to disclose. Dr Guo has nothing to disclose. Dr Huang has nothing to disclose. Dr Sun has nothing to disclose. Dr Tao has nothing to disclose. Dr Fan has nothing to disclose.
AUTHOR CONTRIBUTIONS
X‐LF, S‐CT, and Z‐YS performed experiments and designed the study. Q.L, Y.L, and H‐Y.H performed the experiments. F.W and B.Z collect specimens. ZQ‐OY, Y‐D.Z, JL‐BN, Z‐J.Y, and X‐S.L analyzed the data. D‐Y.L, Z‐W.X, H.C, J‐B.X H‐W.J, H‐N.Z, H.Q, S‐J.G and prepared the reagents. X‐L.F and Q.L wrote the manuscript with suggestions from other authors.
Supporting information
Fig S1
Fig S2
Fig S3
Fig S4
Fig S5
Supplementary Material
ACKNOWLEDGMENTS
We thank Prof. H. Eric Xu (Shanghai Institute of Materia Medica) for providing RdRp protein. We also thank Healthcode Co., Ltd., Hangzhou Bioeast biotech Co., Ltd. and Vacure Biotechnology Co.,Ltd. for providing the proteins. This work was supported by grants from the National Mega‐Projects of Science Research for the 13th Five‐year Plan of China (No. 2018ZX10302302002‐001), the Natural Science Foundation of China (No. 81971909), and the Fundamental Research Funds for the Central Universities (HUST COVID‐19 Rapid Response Call No. 2020kfyXGYJ040) to X‐L Fan. This work was also partially supported by National Key Research and Development Program of China Grant (No. 2016YFA0500600), Interdisciplinary Program of Shanghai Jiao Tong University (No. YG2020YQ10), National Natural Science Foundation of China (No. 31900112, 21907065, 31970130 and 31670831) to S‐C Tao.
Lei Q, Li Y, Hou H‐Y, et al. Antibody dynamics to SARS‐CoV‐2 in asymptomatic COVID‐19 infections. Allergy.2021;76:551–561. 10.1111/all.14622
Lei, Li and Hou these authors contributed equally to this paper.
Contributor Information
Zi‐yong Sun, Email: zysun@tjh.tjmu.edu.cn.
Sheng‐ce Tao, Email: taosc@sjtu.edu.cn.
Xiong‐lin Fan, Email: xlfan@hust.edu.cn, Email: taosc@sjtu.edu.cn, Email: zysun@tjh.tjmu.edu.cn.
REFERENCES
- 1. Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. China Daily . WHO declares COVID‐19 a pandemic. Available at: http://www.nytimes.com/2020/03/11/world/coronavirus‐news.htm#link‐682e5b06. Accessed 21 June, 2020.
- 3. WHO . Coronavirus disease (COVID‐2019) situation reports. Available at: https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/situation‐reports. Accessed 20 June, 2020.
- 4. National Health Commission of the People's Republic of China . COVID‐19 Prevention and Control Plan, 3th edition. Available at: http://www.gov.cn/zhengce/zhengceku/2020‐01/29/content_5472893.htm. Accessed June 22, 2020. [DOI] [PMC free article] [PubMed]
- 5. Wu Z, McGoogan J. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID‐19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239 ‐ 1242. [DOI] [PubMed] [Google Scholar]
- 6. Government of the People's Republic of China . Transcript of press conference on April 15, 2020. Available at: http://www.nhc.gov.cn/xcs/s3574/202004/f2b50e681e7042f8bef2abf2029ffa13.shtml. Accessed June 18, 2020.
- 7. Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019‐nCoV Infection from an Asymptomatic Contact in Germany. N Engl J Med. 2020;382:970‐971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chan J, Yuan S, Kok K, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster. Lancet (London, England). 2020;395:514‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hu Z, Song C, Xu C, et al. Clinical characteristics of 24 asymptomatic infections with COVID‐19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020;63:706‐711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang J, Tian S, Lou J, Chen Y. Familial cluster of COVID‐19 infection from an asymptomatic. Crit Care. 2020;24:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li R, Pei S. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS‐CoV‐2). Science. 2020;368:489‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ai T, Yang Z. Correlation of Chest CT and RT‐PCR Testing for Coronavirus Disease 2019 (COVID‐19) in China: A Report of 1014 Cases. Radiology. 2020;296:E32‐E40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chang L, Hou W, Zhao L, et al. The prevalence of antibodies to SARS‐CoV‐2 among blood donors in China. medRxiv. 2020:2020.2007.2013.20153106 [DOI] [PMC free article] [PubMed]
- 14. Xu X, Sun J. Seroprevalence of immunoglobulin M and G antibodies against SARS‐CoV‐2 in China. Nat Med. 2020;26:1193–1195. [DOI] [PubMed] [Google Scholar]
- 15. Pollán M, Pérez‐Gómez B, Pastor‐Barriuso R, et al. Prevalence of SARS‐CoV‐2 in Spain (ENE‐COVID): a nationwide, population‐based seroepidemiological study. Lancet. 2020;396:535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tanne JH. Covid‐19: US cases are greatly underestimated, seroprevalence studies suggest. BMJ. 2020;370:m2988. [DOI] [PubMed] [Google Scholar]
- 17. Long QX, Liu BZ, Deng HJ. Antibody responses to SARS‐CoV‐2 in patients with COVID‐19. Nat Med. 2020;26:845‐848. [DOI] [PubMed] [Google Scholar]
- 18. Long QX, Tang XJ, Shi QL, Li Q, Deng HJ. Clinical and immunological assessment of asymptomatic SARS‐CoV‐2 infections. Nat Med. 2020;26:1200–1204. [DOI] [PubMed] [Google Scholar]
- 19. National Health Commission of the People's Republic of China . COVID‐19 Prevention and Control Plan, 6th edition. Available at: http://www.nhc.gov.cn/jkj/s3577/202003/4856d5b0458141fa9f376853224d41d7.shtml. Accessed June 22, 2020. [DOI] [PMC free article] [PubMed]
- 20. Jiang HW, Li Y, Zhang HN, et al. SARS‐CoV‐2 proteome microarray for global profiling of COVID‐19 specific IgG and IgM responses. Nat Commun. 2020;11:3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yin W, Mao C, Luan X. Structural basis for inhibition of the RNA‐dependent RNA polymerase from SARS‐CoV‐2 by remdesivir. Science. 2020;368:1499‐1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xinhuanet . Nearly 9.9 million nucleic acid tests in Wuhan have detected 300 asymptomatic infections. Available at: http://www.xinhuanet.com/2020‐06/03/c_1126066386.htm. Accessed 7 Sep, 2020.
- 23. Pallett SJC, Rayment M, Patel A, et al. Point‐of‐care serological assays for delayed SARS‐CoV‐2 case identification among health‐care workers in the UK: a prospective multicentre cohort study. Lancet Respir Med. 2020;8:885–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hao X, Cheng S, Wu D, Wu T. Reconstruction of the full transmission dynamics of COVID‐19 in Wuhan. Nature. 2020;584:420–424. [DOI] [PubMed] [Google Scholar]
- 25. Zhang JJ, Cao YY, Dong X. Distinct characteristics of COVID‐19 patients with initial rRT‐PCR‐positive and rRT‐PCR‐negative results for SARS‐CoV‐2. Allergy. 2020;75:1809‐1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheng MP, Yansouni CP. Serodiagnostics for Severe Acute Respiratory Syndrome‐Related Coronavirus‐2: A Narrative Review. Ann Intern Med. 2020;173:450–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu W, Liu L, Kou G, et al. Evaluation of Nucleocapsid and Spike Protein‐Based Enzyme‐Linked Immunosorbent Assays for Detecting Antibodies against SARS‐CoV‐2. J Clin Microbiol. 2020;58:e00461–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Deeks JJ, Dinnes J, Takwoingi Y, et al. Antibody tests for identification of current and past infection with SARS‐CoV‐2. Cochrane Database Syst Rev. 2020;6:Cd013652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Whitman JD, Hiatt J, Mowery CT, et al. Test performance evaluation of SARS‐CoV‐2 serological assays. medRxiv. 2020:2020.2004.2025.20074856
- 30. Wellinghausen N, Plonné D, Voss M, et al. SARS‐CoV‐2‐IgG response is different in COVID‐19 outpatients and asymptomatic contact persons. J Clin Virol. 2020;130:104542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cervia C, Nilsson J, Zurbuchen Y, et al. Systemic and mucosal antibody secretion specific to SARS‐CoV‐2 during mild versus severe COVID‐19. bioRxiv. 2020:2020.2005.2021.108308 [DOI] [PMC free article] [PubMed]
- 32. Gandhi M, Yokoe DS, Havlir DV. Asymptomatic Transmission, the Achilles' Heel of Current Strategies to Control Covid‐19. N Engl J Med. 2020;382:2158‐2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gattinger P, Borochova K, Dorofeeva Y, et al. Antibodies in serum of convalescent patients following mild COVID‐19 do not always prevent virus‐receptor binding. Allergy. 2021. 10.1111/all.14523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ko JH, Joo EJ, Park SJ, Baek JY. Neutralizing Antibody Production in Asymptomatic and Mild COVID‐19 Patients, in Comparison with Pneumonic COVID‐19 Patients. J Clin Med. 2020;9:2268–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Fig S4
Fig S5
Supplementary Material


