Abstract
Aims
In the older population, acute heart failure is a frequent, life‐threatening complication of COVID‐19 that requires urgent specific care. We aimed to explore the impact of point‐of‐care chest ultrasound (CUS) use in older bedridden inpatients during the COVID‐19 pandemic as a tool to distinguish between cardiogenic pulmonary oedema and isolated viral pneumonia‐related dyspnoea.
Methods and results
This prospective series included 16 patients aged 75 or older, hospitalized for acute dyspnoea in an acute geriatric unit of a university hospital and testing positive for a SARS‐Cov2 infection. We collected demographic characteristics, medical history, biological screening, clinical symptoms, CUS findings (n = 16) and chest CT‐scan conclusions (n = 14). Mean age was 89 years (77–97). All patients presented asthenia and dyspnoea, 56% complained of coughing and diarrhoea, and 50% had fever. Acute heart failure was clinically suspected in seven patients. At CUS, evidence of heart failure was confirmed in three patients (including one without clinical suspicion); interstitial syndrome was confirmed in 12 patients on CUS vs. 9 patients with CT.
Conclusions
In older patients with COVID‐19 and acute dyspnoea, the use of point‐of‐care CUS allowed the clinician to quickly rule out heart failure in nearly half of suspected cases while easily identifying virus‐related interstitial syndrome. The use of CUS appears to be suitable for the rapid bedside investigation of dyspnoea in older patients, particularly in the context of the COVID‐19 pandemic.
Keywords: COVID‐19, Dyspnoea, Heart failure, Aged, Pneumonia, Point‐of‐care ultrasound
Background
The severe acute respiratory syndrome coronavirus 2 (SARS‐Cov2) pandemic is a global public health crisis with more than 15 million people testing positive and more than 600 000 deaths so far. The most recent studies indicate that, in all affected regions, the death rate is higher in the older population. 1 , 2
In older individuals, although the onset of COVID‐19 can include fever, cough, dyspnoea, asthenia, diarrhoea, or acute respiratory distress in serious forms, it is often diagnosed at the occurrence of acute heart failure (HF). 3 , 4 In any case, a major clinical deterioration can occur extremely fast, and recognizing HF is fundamental for providing effective treatment. It is therefore crucial to identify the bedside device that would best allow clinicians to rapidly distinguish non‐cardiogenic dyspnoea from associated cardiogenic and infectious dyspnoea in order to optimize patient management.
Aims
This case series aimed to investigate the value of bedside chest ultrasound (CUS) when it is used by a non‐sonographer geriatrician to identify whether dyspnoea has a cardiac origin in lung infection due to COVID‐19.
Methods
Participants
We report a prospective, descriptive and observational case series of 16 patients hospitalized in the acute geriatric unit of a university hospital in April 2020. The inclusion criteria were as follows: individuals hospitalized in the acute geriatric unit who tested positive for SARS‐Cov2 (confirmed by a polymerase chain reaction either on nasopharyngeal swab or sputum), aged 75 years or older and exhibiting dyspnoea or polypnea. Patients were excluded if they were receiving palliative care, if the CUS would have a foreseeable lack of impact of on patient management, or if the patient refused CUS.
This study was conducted using data collected for clinical purposes, all of which were made anonymous in accordance with the requirements of the local ethics committee (Comité de Protection des Personnes) and with the Declaration of Helsinki.
Data collected
Patient data included: (1) demographic data including age and gender, (2) medical history, (3) clinical symptoms (dyspnoea, coughing, chest pain, abdominal pain, diarrhoea, anosmia, dysgeusia, ageusia, heart rate, respiratory rate, temperature, blood pressure, oxygen saturation, abnormality at lung auscultation, clinical signs of HF), (4) biological screening: leucocyte, lymphocyte, platelets, C‐reactive protein (CRP), procalcitonin, troponin level, N terminal pro brain natriuretic peptide (NT‐proBNP), serum creatinine, uraemia, (5) ultrasound findings, (6) chest CT scan findings, and (7) final physicians diagnosis at patient discharge.
Bedside CUS were performed by two senior geriatricians using a VIVID iq Version R1.x.x. (General Electric Healthcare, Boston, USA) with a GE 3Sc‐RS Probe (General Electric Healthcare). Both operators had over 5 years of experience in bedside CUS. Six or more anterior and lateral thoracic regions (three on each hemithorax) were examined according to current guidelines. 5 Transthoracic echocardiography was also performed to screen for left ventricular ejection fraction as well as left and right atrial pressure. This examination did not intend to provide standardized images of all segments but only a rapid point of care ultrasonography, performed and interpreted by the clinician at the bedside. 6 The total examination was completed in less than 10 min.
The presence of multiple B lines is a strong indicator of lung interstitial syndrome. 7 However, B lines were expected for both left ventricular HF and in virus‐related interstitial pneumonia, and were therefore not considered a distinctive marker for HF.
On CUS, acute HF was defined by the presence of three or more B lines in two or more intercostal spaces bilaterally, symmetrical and with a base‐to‐apex gradient, in association with one of the following criteria of volume overload 7 :
Evidence of elevated left atrial pressure (i.e. E/A ratio >1, E deceleration time <150 ms or E/e′ >12).
Evidence of elevated right atrial pressure (i.e. inferior vena cava collapsibility <50%).
Virus‐related dyspnoea was suspected if multiple B lines or sub‐pleural consolidation were spotted without evidence of volume overload (Figure 1 ).
FIGURE 1.
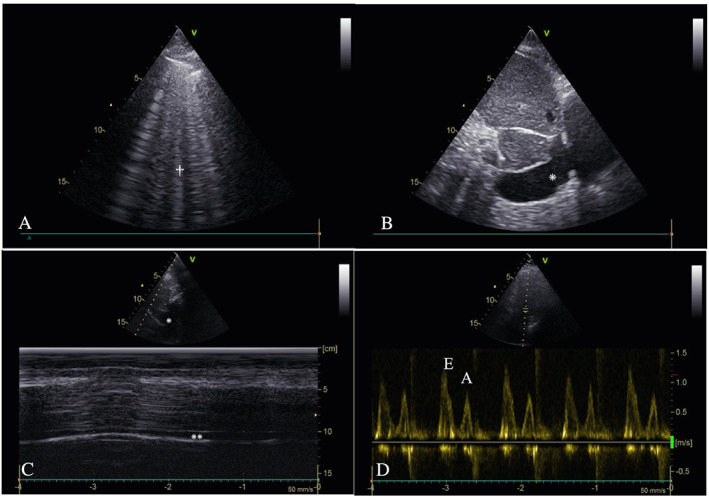
Example of chest ultrasound views in COVID‐19. (A) Right anterior superior lung ultrasound B lines† in an 86‐year‐old male patient at 8 days from the onset of COVID‐19. (B) Enlarged inferior vena cava* measuring >2 cm in a 75‐year‐old female patient at 3 days from the onset of COVID‐19. (C) Normal motion mode profile of an inferior vena cava* with collapsibility >50%**, in an 89‐year‐old female patient 1 day from the onset of COVID‐19. (D) Increased E/A ratio > 1, 4‐apical chamber view, with EE wave higher than AA wave in a 98‐year‐old male patient at the onset of COVID‐19.
Chest CT scan data including the existence of sub‐pleural consolidation, interstitial syndrome, ‘crazy‐paving’ lesions, ground glass opacities, or enlargement of cardiac chambers were also collected.
Patients with a final diagnosis of acute HF were compared with patients without acute HF.
Continuous variables were expressed as median (interquartile range). The Mann–Whitney test (two comparison groups) was used to compare continuous variables. The χ 2 test or Fishers test was used to compare dichotomous data.
Results
Patient characteristics
Mean patient age was 89 ± 7.0 years, and 10/16 were female.
Medical history and clinical and biological presentation at admission are presented in Table 1 .
TABLE 1.
Characteristics of COVID‐19 patients with or without diagnosis of acute heart failure (n (%) or median [IQR])
| Heart failure n = 3 | No heart failure n = 13 | P | ||
|---|---|---|---|---|
| Demographic characteristics | Female | 1 (33%) | 9 (69%) | 0.5 |
| Age | 86 [81–92] | 89 [83–92] | 0.6 | |
| Clinical data | Cough | 2 (66%) | 9 (69%) | 1 |
| Dyspnoea | 3 (100%) | 13 (100%) | 1 | |
| Chest pain | 0 (0%) | 0 (0%) | 1 | |
| Anosmia, ageusia, dysgeusia | 0 (0%) | 2 (15%) | 1 | |
| Asthenia | 3 (100%) | 13 (100%) | 1 | |
| Abdominal pain | 1 (33%) | 4 (30%) | 1 | |
| Diarrhoea | 2 (66%) | 7 (54%) | 1 | |
| Temperature (°C) | 38 [38–38] | 38 [37.8–38.4] | 0.3 | |
| Respiratory rate (/min) | 20 [20–22] | 24 [24–30] | 0.03 | |
| Oxygen saturation (%) | 94 [93–96] | 93 [93–95] | 0.7 | |
| Heart rate (/min) | 75 [75–90] | 78 [70–83] | 0.3 | |
| Systolic blood pressure (mmHg) | 131 [129–133] | 124 [110–141] | 0.8 | |
| Diastolic blood pressure (mmHg) | 74 [66–76] | 65 [59–81] | 0.7 | |
| Pulmonary condensation syndrome | 1 (33%) | 3 (23%) | 1 | |
| Right heart failure | 2 (66%) | 5 (38%) | 0.6 | |
| Left heart failure | 2 (66%) | 0 (0%) | 0.02 | |
| Biological data | Leucocytes (cells/μL) | 9,400 [9,250‐10,550] | 5,700 [4,500‐9,400] | 0.6 |
| Lymphocytes (cells/μL) | 790 [680–1,245] | 1,030 [620–1,390] | 0.8 | |
| Platelets (1000 cells/μL) | 248 [202–292] | 166 [123–184] | 0.2 | |
| C‐reactive protein (mg/dL) | 167 [102–177] | 49 [21–91] | 0.2 | |
| Procalcitonin rate (μg/L) | 0.28 [0.22–0.34] | 0.25 [0.17–0.53] | 0.3 | |
| Troponin I (μg/L) | 0.52 [0.37–0.67] | 0.04 [0.02–0.08] | 0.4 | |
| NT‐proBNP (pmol/L) | 5,609 [4,319‐6,900] | 677 [427–1,800] | 0.3 | |
| Creatinine (μmol/L) | 94 [79–88] | 75 [59–145] | 0.4 | |
| Uraemia (mmol/L) | 13 [11–18] | 10 [8.6–13.9] | 0.4 | |
| Ultrasound | LEVF >50% | 2 (66%) | 4 (31%) | 0.5 |
| E/A >1 | 1 (33%) | 0 (0%) | 0.18 | |
| Enlarged IVC | 3 (100%) | 0 (0%) | 0.002 | |
| Right auricle enlargement | 1 (33%) | 0 (8%) | 0.2 | |
| Sub‐pleural consolidation | 0 (0%) | 4 (31%) | 0.5 | |
| Pleural effusion | 1 (33%) | 3 (23%) | 1 | |
| B Lines | 3 (100%) | 9 (69%) | 0.5 | |
| Heart failure diagnosis | 3 (100%) | 0 (0%) | 0.002 | |
| CT‐Scan | Sub‐pleural consolidation | 2 (66%) | 6 (46%) | 1 |
| Pleural effusion | 0 (0%) | 2 (15%) | 1 | |
| Fibrosis | 0 (0%) | 2 (15%) | 1 | |
| Crazy paving | 1 (33%) | 3 (23%) | 1 | |
| Grounding glass opacity | 2 (66%) | 6 (46%) | 1 | |
| Heart chambers enlargement | 1 (33%) | 2 (15%) | 0.5 | |
| Outcome | Death rate | 1 (33%) | 3 (23%) | 1 |
| Hospitalization length (days) | 15 [9–22] | 10 [6–15] | 0.6 |
LEVF, left ventricular ejection fraction; NT‐ProBNP, N terminal pro brain natriuretic peptide.
The clinical examination concluded that seven patients had acute HF.
Interstitial syndrome (i.e. multiple B lines) was observed on CUS in 12 patients and volume overload was observed in four patients (E/A ratio >1 for one patient, inferior vena cava enlargement for three patients). Left ventricular ejection fraction (LVEF) was >50% in nine patients. Sub‐pleural consolidation and pleural effusion were each found in four patients. Finally, CUS revealed three cases of cardiac‐related dyspnoea and nine cases of non‐cardiac‐related dyspnoea. Four CUS examinations were considered normal by the operating clinician.
Assessment of the chest CT scans found eight cases each of sub‐pleural consolidations and ground glass opacity, four cases of ‘crazy paving’ and two cases of pleural effusion. In addition, three patients had enlarged heart chambers indicating volume overload.
Comparison between clinical examination, CUS, and chest CT scan
Clinically, seven patients were suspected of acute HF in addition to COVID‐19. CUS confirmed two cases and ruled out five. Among the nine patients who were not suspected of acute HF, this assertion was correlated by CUS in all cases except one. Though the patients were all clinically suspected of pneumonia‐related dyspnoea, CUS was normal for four patients and the chest CT scan for five patients (Table 1 ).
Conclusions
To our knowledge, this is the first case series to investigate the use of point‐of‐care CUS for the diagnosis of cardiac‐related dyspnoea during SARS‐Cov2 infection in older bedridden patients. The CUS assessed in this series were performed by non‐specialist sonographers as a complement to the clinical examination in the day‐to‐day activity of an acute geriatric unit. Even if ultrasound is increasingly highlighted as a promising device in the COVID‐19 pandemic, 8 , 9 , 10 , 11 this is to our knowledge the first report of CUS implementation in an acute geriatric setting.
In our series, the clinical presentation of SARS‐Cov2 infection was unspecific, and despite the dyspnoea, pulmonary auscultation failed to detect specific abnormalities. However, whether HF is a component of dyspnoea in COVID‐19 patients is a frequent yet critical issue, as HF dramatically worsen global prognosis and requires specific urgent care. The coexistence of HF and pneumonia is particularly frequent in elderly individuals, and these causes of dyspnoea are not mutually exclusive. In the patients clinically suspected of acute HF associated with COVID‐19, which was almost half of our population, CUS made it possible to quickly confirm or rule out HF and to immediately begin individualized treatment, including diuretic medications. As previously reported, ultrasound was the most suitable device for diagnosing HF in these frail older patients. 12 , 13
Lung ultrasound has also already proven to be a valuable tool for diagnosing interstitial syndrome, 14 and studies show that it is largely superior to chest X‐ray for this purpose. 7 In our series as in others, 9 , 10 , 15 CUS and chest CT scan yielded similar results for interstitial syndrome. Even though a focal sonographic pattern of interstitial syndrome (infectious cause) could theoretically be differentiated from diffuse interstitial syndrome (HF), 7 it was difficult in practice and thus not considered in this report.
It is worth noting that bedside CUS was performed with ease for two patients for whom transport was unsuitable (acute respiratory distress and patient refusal). These situations are particularly frequent in geriatric settings, especially in units where the imaging equipment is not on‐site.
Overall, we found that the use of point‐of‐care CUS in frail, bedridden patients allows the clinician to screen more precisely for associated HF in infection‐related dyspnoea than with the clinical examination alone, even when CUS is performed by a non‐specialist sonographer. Moreover, it appears that CUS has similar results to chest CT scan for the diagnosis of COVID‐19 pneumonia. Point‐of‐care ultrasound is accessible, fast, and does not cause patient discomfort due to transport. In addition, it has already shown to be both feasible and valuable for older patients in acute geriatric wards. 16 By delivering real‐time information during the consultation, CUS can bridge the gap between physical assessment, data acquisition, and clinical decision making. For all of these reasons, we believe that CUS should be the preferred examination when managing acute dyspnoea in older inpatients with COVID‐19.
This study has limits. First, it was performed on a small scale, in a single unit, and for descriptive purposes. Second, the imaging was not reviewed by another examiner, but this situation is consistent with everyday practice. Third, we did not assess the clinical impact of CUS in this series. Further studies should be conducted to evaluate whether CUS use is associated with significant improvements in clinical practice. Fourth, echocardiographic parameters of HF are debatable: diastolic dysfunction is very common in elderly patients even without acute HF. Moreover, the inferior vena cava is a pressure index and not necessarily a volume index when dilated. Similarly, even with a positive COVID‐19 test, focal interstitial syndrome cannot be considered as specific of COVID‐19‐pneumonia, especially in an older comorbid population. Thus, we believe that CUS should be considered as a bedside orientation tool that can be used to complete initial clinical and paraclinical findings more than a definitive diagnostic tool.
In older patients with dyspnoea and SARS‐Cov2 infection, the clinical examination is frequently non‐specific. As an easy‐to‐use clinician‐driven examination, bedside CUS appears to be a promising diagnostic tool for older inpatients with COVID‐19 infection. When a clinician is faced with suspected HF as a cause of acute dyspnoea, CUS is an accessible tool that can be used to provide a rapid diagnosis and to support clinical decision making in an urgent setting.
Conflict of interest
None declared.
Funding
This research received no external funding.
Acknowledgements
The authors thank Suzanne Rankin, a native English speaker, for providing English language review of the manuscript.
Hacquin, A. , Putot, S. , Barben, J. , Chagué, F. , Zeller, M. , Cottin, Y. , Manckoundia, P. , and Putot, A. (2020) Bedside chest ultrasound to distinguish heart failure from pneumonia‐related dyspnoea in older COVID‐19 patients. ESC Heart Failure, 7: 4424–4428. 10.1002/ehf2.13017.
References
- 1. Wang L, He W, Yu X, Hu D, Bao M, Liu H, Zhou J, Jiang H. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4‐week follow‐up. J Infect 2020; 80: 639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shahid Z, Kalayanamitra R, McClafferty B, Kepko D, Ramgobin D, Patel R, Aggarwal CS, Vunnam R, Sahu N, Bhatt D, Jones K, Golamari R, Jain R. COVID‐19 and Older Adults: What We Know. J Am Geriatr Soc 2020; 68: 926–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Matsushita K, Marchandot B, Jesel L, Ohlmann P, Morel O. Impact of COVID‐19 on the cardiovascular system: a review. JCM 2020; 9: 1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Du Y, Tu L, Zhu P, Mu M, Wang R, Yang P, Wang X, Hu C, Ping R, Hu P, Li T, Cao F, Chang C, Hu Q, Jin Y, Xu G. Clinical features of 85 fatal cases of COVID‐19 from Wuhan: a retrospective observational study. Am J Respir Crit Care Med 2020; 201: 1372–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Price S, Platz E, Cullen L, Tavazzi G, Christ M, Cowie MR, Maisel AS, Masip J, Miro O, McMurray JJ, Peacock WF, Martin‐Sanchez FJ, Di Somma S, Bueno H, Zeymer U, Mueller C. Acute Heart Failure Study Group of the European Society of Cardiology Acute Cardiovascular Care Association. Expert consensus document: echocardiography and lung ultrasonography for the assessment and management of acute heart failure. Nat Rev Cardiol 2017; 14: 427–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moore CL. Point‐of‐Care Ultrasonography. N Engl J Med 2011; 9. [DOI] [PubMed] [Google Scholar]
- 7. Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, Melniker L, Gargani L, Noble VE, Via G, Dean A, Tsung JW, Soldati G, Copetti R, Bouhemad B, Reissig A, Agricola E, Rouby J‐J, Arbelot C, Liteplo A, Sargsyan A, Silva F, Hoppmann R, Breitkreutz R, Seibel A, Neri L, Storti E, Petrovic T. International Liaison Committee on Lung Ultrasound (ILC‐LUS) for International Consensus Conference on Lung Ultrasound (ICC‐LUS). International evidence‐based recommendations for point‐of‐care lung ultrasound. Intensive Care Med 2012; 38: 577–591. [DOI] [PubMed] [Google Scholar]
- 8. Piscaglia F, Stefanini F, Cantisani V, Sidhu PS, Barr R, Berzigotti A, Chammas MC, Correas J‐M, Dietrich CF, Feinstein S, Huang P, Jenssen C, Kono Y, Kudo M, Liang P, Lyshchik A, Nolsøe C, Xie X, Tovoli F. Benefits, open questions and challenges of the use of ultrasound in the COVID‐19 pandemic era. The views of a panel of worldwide international experts. Ultraschall Med 2020; 41: 228–236. [DOI] [PubMed] [Google Scholar]
- 9. Ji L, Cao C, Lv Q, Li Y, Xie M. Serial bedside lung ultrasonography in a critically ill COVID‐19 patient. QJM 2020; 113: 491–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buonsenso D, Pata D, Chiaretti A. COVID‐19 outbreak: less stethoscope, more ultrasound. Lancet Respir Med 2020; 8: e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nouvenne A, Ticinesi A, Parise A, Prati B, Esposito M, Cocchi V, Crisafulli E, Volpi A, Rossi S, Bignami EG, Baciarello M, Brianti E, Fabi M, Meschi T. Point‐of‐care chest ultrasonography as a diagnostic resource for COVID‐19 outbreak in nursing homes. J Am Med Dir Assoc 2020; 21: 919–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Manzano L, Escobar C, Cleland JGF, Flather M. Diagnosis of elderly patients with heart failure. Eur J Heart Fail 2012; 14: 1097–1103. [DOI] [PubMed] [Google Scholar]
- 13. From AM, Lam CSP, Pitta SR, Kumar PV, Balbissi KA, Booker JD, Singh IM, Sorajja P, Reeder GS, Borlaug BA. Bedside assessment of cardiac hemodynamics: the impact of noninvasive testing and examiner experience. Am J Med 2011; 124: 1051–1057. [DOI] [PubMed] [Google Scholar]
- 14. Lo Giudice V, Bruni A, Corcioni E, Corcioni B. Ultrasound in the evaluation of interstitial pneumonia. J Ultrasound 2008; 11: 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fiala MJ. Ultrasound in COVID‐19: a timeline of ultrasound findings in relation to CT. Clin Radiol 2020; 75: 553–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ticinesi A, Lauretani F, Nouvenne A, Mori G, Chiussi G, Maggio M, Meschi T. Lung ultrasound and chest X‐ray for detecting pneumonia in an acute geriatric ward. Medicine 2016; 95: e4153. [DOI] [PMC free article] [PubMed] [Google Scholar]


