Abstract
Coronavirus disease 2019 (COVID‐19) is an ongoing global pandemic affecting all levels of health systems. This includes the care of patients with noncommunicable diseases (NCDs) who bear a disproportionate burden of both COVID‐19 itself and the public health measures enacted to combat it. In this review, we summarize major COVID‐19‐related considerations for NCD patients and their care providers, focusing on cardiovascular, pulmonary, renal, haematologic, oncologic, traumatic, obstetric/gynaecologic, operative, psychiatric, rheumatologic/immunologic, neurologic, gastrointestinal, ophthalmologic and endocrine disorders. Additionally, we offer a general framework for categorizing the pandemic’s disruptions by disease‐specific factors, direct health system factors and indirect health system factors. We also provide references to major NCD medical specialty professional society statements and guidelines on COVID‐19. COVID‐19 and its control policies have already resulted in major disruptions to the screening, treatment and surveillance of NCD patients. In addition, it differentially impacts those with pre‐existing NCDs and may lead to de novo NCD sequelae. Likely, there will be long‐term effects from this pandemic that will continue to affect practitioners and patients in this field for years to come.
Keywords: COVID‐19, chronic diseases, noncommunicable diseases, public health
Introduction
COVID‐19, a novel infection caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has spread across the globe in a matter of months, leading it to be declared a pandemic by the World Health Organization (WHO) [1, 2]. Due to its high direct mortality for such a contagious disease, COVID‐19 has placed extreme pressures on healthcare systems [3]. Direct stresses include the rapid consumption of inpatient medicine, isolation and critical care services. Indirectly, public health control measures, such as social distancing, shelter‐in‐place laws and personal protective equipment (PPE) requirements, have created new challenges for both patients and healthcare providers (Fig. 1). These difficulties have profound implications for patients living with noncommunicable diseases (NCDs), many of which are chronic, highly technically specialized in screening and treatment requirements, and reliant on multidisciplinary team care for cure and control. Recognizing this threat, most specialty professional societies have released practice guidelines and suggestions for adapting clinical activities during the COVID‐19 pandemic (Table 1). Readers are encouraged to consult these latest guidelines, which will doubtlessly evolve with emerging data. In the present manuscript, we review key ways in which COVID‐19 has impacted the management of NCDs, focusing on both direct effects of the infection on those with pre‐existing chronic diseases as well as unique health system disruptions affecting their care.
Fig. 1.
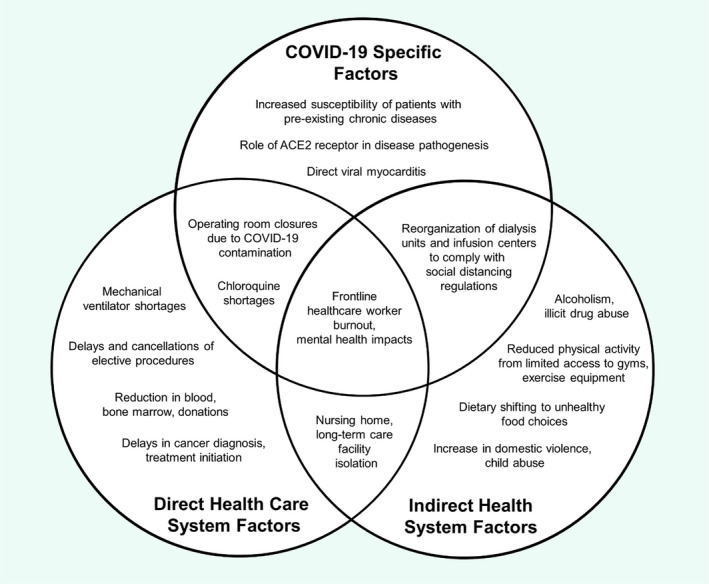
Example impacts of COVID‐19 on NCDs.
Table 1.
Statements and practice guidelines by select major professional societies regarding NCD Care during COVID‐19 Pandemic
Methods: search strategy and selection criteria
Medical professional society guidelines were first queried in the fields of cardiology, pulmonology, nephrology, haematology/oncology, surgery, obstetrics/gynaecology, psychiatry, rheumatology, neurology, endocrinology, gastroenterology/hepatology, otolaryngology and ophthalmology with referenced works further explored (Table 1). PubMed and Google Scholar searches using MeSH and text search terms of “COVID”, “Novel Coronavirus”, “Noncommunicable Disease”, “NCD”, and the above‐mentioned specialty fields were used to review the published literature. Given the ever‐changing nature of the pandemic and the lag between patient/health system developments and peer‐reviewed publication, the lay press, white papers, and preliminary reports were also examined for the purposes of informing further formal research in this field. Sections are organized by the US Centers for Disease Control and Prevention (CDC) list of high‐risk populations [4, 5].
Impact of COVID‐19 on NCDS by field
Cardiovascular disease
Patients with pre‐existing cardiovascular disease (CVD) are amongst those with the highest risk of adverse outcomes from COVID‐19. In its initial outbreak report, the Chinese Center for Disease Control noted the case fatality rate of patients with pre‐existing CVD was 10.5%, higher than those with chronic respiratory disease or cancer [6, 7, 8, 9, 10]. US data also suggest that those living with CVD are amongst the three highest risk groups for COVID‐19 infection [4, 5, 11]. COVID‐19 can cause a fulminant myocarditis associated with acute heart failure and cardiogenic shock as well as asymptomatic myocardial inflammation [12, 13, 14, 15, 16]. COVID‐19 patients also frequently develop elevated serum troponin levels, although it is unclear if this is due to direct myocardial injury by the virus or demand ischaemia from critical illness – nevertheless, this finding is associated with increased mortality [17, 18, 19]. Furthermore, the first wave of COVID‐19 was associated with decreases and delays in acute care presentations for MIs in the United States and Europe, which will likely have implications for increased disease severity, mortality and postinfarct complications such as heart failure [20, 21].
Particularly challenging for frontline practitioners, coronavirus cardiomyopathy can masquerade as ST‐elevation myocardial infarction (STEMI), presenting with chest pain, dyspnoea and ST‐segment elevations on electrocardiogram (ECG) [12, 13]. This has presented cardiologists with a dilemma in activating the catheterization laboratory – on one hand, STEMIs are life‐threatening and should ideally be addressed within 90 min with invasive angiography with intent of percutaneous coronary intervention (PCI). On the other hand, activating the STEMI treatment cascade risks exposing cath laboratory staff and equipment to the virus. Due to this conflict, many Chinese facilities have treated patients with suspected COVID‐19 and STEMI pattern on ECG with pharmaceutical thrombolytics rather than procedural cath laboratory management. Though PCI has been shown to be superior to thrombolytics for STEMI management, these providers have erred towards a conservative approach in the trade‐off between waiting for SARS‐CoV‐2 test results and delaying revascularization of a potentially 100% acutely occluded coronary artery [22, 23]. Indeed, hospitals in Hong Kong which have continued to use PCI first line for STEMIs have reported that door to balloon time, a key quality metric associated with increased MI mortality, has been lengthened since the outbreak of COVID‐19 [24]. The additional time delay, facility sterilization protocols and PPE requirements have significantly disrupted cath laboratory activities for both emergent and urgent procedures, an issue which is only magnified for small/low‐volume centres [25]. The disruption extends to elective but life‐saving cath laboratory procedures such as transcatheter aortic valve replacements, which have been rescheduled or cancelled [26]. Though cardiology professional societies have since published guidelines for triage and prioritization of such procedures, the imminent advent of influenza season may stress these systems once more [25, 27].
In addition to myocardial injury, SARS‐CoV‐2 may be associated with cardiac arrhythmias. Up to 16.7% of hospitalized COVID‐19 patients were noted in one study to have a heart rhythm abnormality [28]. This concern has been magnified by the off‐label use of hydroxychloroquine and azithromycin as a possible antiviral regimen (despite the lack of data to support its efficacy) since they can increase the risk of ventricular tachyarrhythmias via QT‐interval prolongation [29, 30, 31, 32, 33].
Researchers are also exploring the role of angiotensin‐converting enzyme inhibitors (ACEi) and angiotensin‐II receptor blockers (ARB) in the SARS‐CoV‐2 infection pathway. Coronaviruses utilize ACE2 as a coreceptor to invade pulmonary alveolar cells [34]. It was noted early in the pandemic that patients with CVD, hypertension and diabetes (all conditions frequently treated with ACE inhibitors and ARBs) were particularly susceptible both to SARS‐CoV‐2 infection and to poor outcomes from the disease [4, 5, 7, 9, 11, 14, 35]. Presently, it is unclear if these medications upregulate ACE2, increasing susceptibility to SARS‐CoV‐2 or if they act as competitive agonists, thereby exerting a protective effect [34, 36, 37]. Mounting observational evidence suggests that they neither increase risk nor improve outcomes, but further investigation is needed to clarify this effect [38, 39]. Given the widespread use and mortality‐reducing effects of ACEi/ARBs in the above diseases, major US cardiology societies have issued a joint statement to avoid pre‐emptively discontinuing their usage in patients already taking them [40].
Pulmonology
Chronic respiratory diseases are amongst the greatest potentiators of SARS‐CoV‐2 infection and mortality [4, 5, 6, 7, 9, 11]. Cigarette smoking has been noted in multiple studies to be an independent risk factor for COVID‐19 susceptibility, with higher case fatality rate amongst men than women possibly due to the higher rates of smoking amongst men [7, 9, 41]. This finding may extend to e‐cigarettes and vaping products as well [42]. Furthermore, the physical act of sharing cigarettes and hookahs, cultural practices common in some cultures, could present avenues for SARS‐CoV‐2 transmission [43].
As for care system issues, bronchoscopy (used for confirmatory diagnoses in suspected COVID‐19 cases with negative nasopharyngeal tests) represents a particularly high‐risk intervention for operators given the proximity to the airway [44]. Furthermore, there has been direct competition for mechanical ventilators between patients with critical COVID‐19‐related respiratory failure and patients with severe exacerbations of chronic lung disease without COVID‐19 infection. This problem is compounded by the fact that SARS‐CoV‐2 is readily aerosolized by high‐flow nasal cannula, bag‐valve masks and noninvasive positive‐pressure ventilation (e.g. Bi‐level Positive Airway Pressure), necessitating earlier elective intubation and ventilation to protect healthcare workers [45].
Although most patients who survive acute COVID‐19 infection make a full clinical recovery, there have also been reports of lasting lung injury, dubbed post‐COVID‐19 fibrosis [46, 47]. This potential syndrome could cause permanent pulmonary degradation in those already living with chronic lung disease whilst creating new patients with interstitial lung disease.
Nephrology
For the nearly 750 000 end‐stage renal disease (ESRD) patients in the United States, the need for current or imminent haemodialysis (HD) raises the risk of COVID‐19 exposure [48]. Long‐term self‐isolation is not an option for those on HD, as they require regular treatment at dialysis centres. Furthermore, many living with ESRD are reliant on others to transport them to and from dialysis centres, bringing them in contact with potential community virus carriers [49]. Chronic kidney disease (CKD) is itself an immunocompromising state associated with reduced resistance to infections [4, 5, 11, 50]. As noted above, many patients with CKD also take ACE inhibitors and ARBs, which may modulate their susceptibility to SARS‐CoV‐2 [35].
The US CDC recommends rearranging dialysis chairs to comply with minimum six‐foot social distancing regulations [51]. Though some centres are now providing masks for patients and screening them at check in for COVID‐19 symptoms and turning them away if positive, this diverts these individuals to emergency rooms where they are at increased risk of acquiring the disease if they were initially negative [52]. Dialysis centres are subject to the same PPE shortages as hospitals as well.
Peritoneal dialysis (PD) could represent a venue for reduced social interaction, but only a small fraction of patients (roughly 12 000 in the United States) currently undergo PD [48]. Those looking to switch from HD to PD will require surgical dialysis catheter placement, which is not particularly feasible at present whilst surgical capacity, anaesthesiology and intensive care resources remain at a premium.
Haematology/Oncology
Sufferers of haemoglobinopathies such as sickle cell disease and thalassaemias are at heightened risk of negative outcomes from COVID‐19 [4, 5]. Patients receiving chemotherapy or bone marrow transplants are also immunocompromised due to the myelosuppressive effects of many of these treatments. Indeed, the Chinese COVID‐19 experience suggests that cancer survivors, in addition to patients with active malignancy, are at higher risk of death or mechanical ventilation from SARS‐CoV‐2 [53]. On the other hand, those on chemotherapy or immunosuppressive therapies could exhibit a blunted immune response, thereby decreasing their risk for cytokine storm, a feared COVID‐19 complication [54].
In addition, SARS‐CoV‐2 has been noted to be associated with a potent coagulopathic state, manifesting as thromboses in multiple vascular territories including the brain, lung and heart [55]. Chronic sequelae of these events remain to be seen.
For oncologists and cancer patients, COVID‐19 poses multiple major systematic challenges as well. Primary cancer screenings (e.g. low‐dose chest CT, colonoscopies) cannot be performed without significant risk and may lead to delays in diagnosis. Given provider reassignment, social distancing requirements and reduced operating room capacity, it is likely that chemotherapy, radiation therapy and surgical excision of tumours may be delayed as well during outbreaks [56]. Infusion centres, where most chemotherapy is administered, have reorganized to maintain six‐foot social distancing protocols [57]. Clinical trial enrolment and participation is particularly difficult during this time, with many trials suspended or cancelled outright [58]. Cancer survivor surveillance visits and secondary screening intervals may also become lengthened [57].
Blood banks across the world have been impacted severely by the pandemic. Due to widespread fear of health facility‐associated coronavirus transmission, many organizations have reported dramatic decreases in blood donations, leading the American Red Cross and US Food and Drug Administration (FDA) to release public calls for assistance [59, 60, 61]. This setback likely extends to bone marrow and solid organ donations as well [62, 63].
Neurology
Those living with neurologic disorders shoulder an excess burden of COVID‐19’s pathophysiologic and societal impacts [4, 5, 11]. For one, there are increasing reports of COVID‐19 infection‐associated strokes and transient ischaemic attacks, many of them possibly thrombotic in origin [55, 64]. Patients with chronic illnesses specifically weakening the respiratory muscles (e.g. amyotrophic lateral sclerosis, myasthenia gravis (MG)) are at high risk for respiratory decompensation from SARS‐CoV‐2 infection [65]. Those living with autoimmune neurologic diseases (e.g. multiple sclerosis, MG) are also high risk, because many are on long‐term immunosuppressive therapies including corticosteroids and biologics. Many receive treatment at infusion centres and may experience delays in initiation of therapy if newly diagnosed [66].
Movement disorders, conditions associated with cognitive impairment, and developmental syndromes can limit the ability of patients to independently shop and care for themselves in the era of social distancing, leading to businesses and governments to issue preferential treatment ordinances for these groups [67].
Nursing homes and long‐term care facilities, which house a disproportionate number of the nation’s dementia patients, have been ground zero for initial disease outbreaks in the United States, with tenants experiencing extraordinarily high rates of critical illness and mortality [68]. Advanced age is one of the strongest predictors of poor outcome from COVID‐19 [69]. As such, nursing facilities have limited access to residents’ family and friends [70]. Though critical for limiting disease transmission, these measures can both accelerate the dementia disease course and increase the incidence of psychiatric comorbidities such as depression. Indeed, social distancing places the elderly, even in their own homes, at greater risk for depression and anxiety [71].
Psychiatry and behavioural medicine
The COVID‐19 pandemic and its social sequelae, both the fear of mortality from the disease itself and the effects of public health control measures such as social distancing and loss of employment, have caused both de novo psychiatric events as well as exacerbations of pre‐existing mental health conditions [72, 73, 74, 75]. Social isolation can lead to dangerous self‐medicating behaviours such as excessive alcohol, tobacco and illicit drug abuse [76, 77]. Prolonged quarantine with family can lead to domestic conflict – some regions have reported increased divorce filings following lifting of lockdowns [78]. Economic recessions have been noted to be associated with substantial increases in suicides [79]. Indeed, the Well Being Trust estimates that there will be as many as 75 000 excess ‘deaths of despair’ from substance misuse and suicides from the pandemic in the United States [80]. Furthermore, many patients at highest risk of adverse outcomes, like those with psychotic illnesses such as schizophrenia or bipolar disorder, may be lost to acute care.
Those suffering from substance abuse and addiction represent a particularly vulnerable population during the COVID‐19 crisis. Opiate dependence is known to be a risk factor for poor outcome with respiratory disease, whilst chronic methamphetamine use can led to pulmonary hypertension and heart failure, associated with worsened outcomes from COVID‐19 [41, 81, 82, 83]. There is high collinearity between substance abuse and severe psychiatric disease, and those with either condition are more likely to experience homelessness or incarceration, which increases risk of poor COVID‐19 outcomes [41].
The homeless are an exceedingly high‐risk group who have reduced access to healthcare resources and an inability to social distance effectively, leading some experts to call for temporary housing measures to treat them successfully and protect others [84, 85]. Institutionalized populations in general, including nursing home residents, legally held decompensated mentally ill patients, the imprisoned and undocumented immigrants in detection centres, are also at exceptionally high danger of contracting and sustaining complications from COVID‐19 [86].
In addition to caring for the well‐being of the public at large, mental health providers will likely need to deal with the psychiatric sequelae experienced by frontline workers during the pandemic. Already, Chinese health personnel have reported high rates of adverse emotional symptoms, including depression, anxiety and insomnia [72].
Rheumatology/Immunology
Patients with autoimmune disorders, inflammatory diseases and organ transplants are particularly susceptible to SARS‐CoV‐2, as many are on immunosuppressive drugs including corticosteroids and biologics such as TNF⍺ inhibitors [4, 5, 66]. Individuals receiving intravenous therapies are at risk of being exposed to SARS‐CoV‐2 at infusion centres [87]. The outbreak of COVID‐19 may cause delays in initiation of IV therapies for the newly diagnosed [66].
Preliminary studies implying that hydroxychloroquine may improve COVID‐19 outcomes has led to widespread shortages of the drug, which is important for the treatment of those with autoimmune disorders such as systemic lupus erythematosus [30, 31, 88]. Nonsteroidal anti‐inflammatory drugs (NSAIDs), often used in rheumatologic disorders, have been suggested by the French government to portend a worse prognosis in SARS‐CoV‐2, though the FDA and WHO warn that this claim is unsubstantiated [89, 90, 91].
Particularly concerning for paediatric practitioners, COVID‐19 has also been associated with a rare, Kawasaki‐like multisystem inflammatory syndrome (MIS) in children, which may leave affected patients with permanent cardiac or cerebrovascular injuries [92].
Endocrinology, nutrition and preventative medicine
Diabetics have had some of the highest COVID‐19‐related case fatality and complication rates, possibly due to uncontrolled diabetes mellitus being an immunocompromised state [4, 5, 6, 7, 11, 93]. As described above, many diabetics are also prescribed ACE inhibitors and ARBs for nephroprotection, which may affect their susceptibility to the disease [36, 40].
Obesity, which is comorbid with metabolic syndrome, is also a potent risk factor for poor outcomes from SARS‐CoV‐2 infection [4, 5, 94]. This finding is critical because the COVID‐19 pandemic and its public health response has had a major influence on food systems, limiting people’s access to healthy foods and magnifying the effects of food insecurity on vulnerable people (e.g. the elderly, urban populations) [95]. Both supply side (reduction in labour force, livestock access, transportation restrictions, production/processing limitations, stocking workforce) and demand side challenges (e.g. hoarding behaviours, increased reliance on takeout) stress traditional grocery, restaurant and food service systems [96]. Social distancing has incentivized quarantined individuals to switch to cheaper, shelf‐stable foods, which may lead to less nutritious dietary choices [97]. The closure of schools around the globe presents a shock for the world’s 320 million schoolchildren, many of whom are from low‐income families that depend on school lunches for a large percentage their nutritious daily calories [98]. Impacts to food systems disproportionately affect the poor, as loss of employment and rising food prices are most likely to harm them [99]. In addition to dietary disruptions, physical inactivity will increase in regions of the world placed under social distancing directives. It is unclear how long these measures will last, but if prolonged, they may negatively impact the cardiovascular and metabolic health of those without access to gyms or home exercise equipment [100].
Obstetrics/Gynaecology
Emerging data suggest that pregnancy may constitute a risk factor for worsened outcomes from SARS‐CoV‐2 [4, 5, 101]. Nevertheless, the American College of Obstetricians and Gynecologists (ACOG) does not recommend changes in timing or method of childbirth based on COVID‐19 [102, 103]. Data on the potential rate of vertical transmission of SARS‐CoV‐2 are evolving [104, 105, 106, 107]. Initially, the World Food Program suggested continuation of breastfeeding through COVID‐19, albeit with careful handwashing and face masks [108]. Meanwhile, ACOG has recommended that neonates born to patients with COVID‐19 be separated from their mothers [102]. Elective gynaecologic procedures such as tubal ligation, pelvic prolapse repair and in vitro fertilization have had to be postponed to conserve anaesthesiology/critical care resources. Though currently legally contested, a number of US states, including Arkansas, Iowa, Alabama, Oklahoma, Louisiana, Mississippi, Tennessee, West Virginia, Alaska, Ohio and Texas, have attempted to limited access to abortions by classifying them as elective procedures, increasing the difficulty in obtaining medical care for some women [109, 110].
Gastroenterology/Hepatology, otolaryngology
Interestingly, a large percentage of patients presenting for COVID‐19‐related medical care in some studies reported gastrointestinal symptoms such as diarrhoea, vomiting, and abdominal pain [111]. Esophagogastroduodenoscopy, colonoscopy and ear nose and throat procedures place operators at high risk of SARS‐CoV‐2 exposure given the close proximity to the respiratory tract, oral secretions and stool, which represent avenues for viral spread [112, 113]. Systematically, there have been limitations on endoscopic procedures, as many endoscopy suites have been converted into overflow ICU beds at some institutions [3].
Patients with inflammatory bowel disease (IBD) are a particularly vulnerable group, as many are on immunosuppressive therapies including corticosteroids and biologics. Some experts have suggested that IBD patients on infusion anti‐TNF biologics (such as infliximab) be switched to home injection forms such as adalimumab to reduce SARS‐CoV‐2 exposure at infusion centres [114]. Patients with chronic liver disease may also be at higher risk for poor COVID‐19 outcomes [4, 5, 11]. In particular, liver transplants pose a mode of viral transmission based on the experience of prior novel coronavirus outbreaks [115].
Traumatology
Traumas, including both surgical and medical acute injuries (e.g. car accidents, drownings, intoxications) pose a particular risk for emergency room providers, anaesthesiologists and surgeons, since comatose patients may not be able to relay a history of COVID‐19 symptoms at time of initial encounter [116]. Severe traumas requiring intubation and/or operative management further stress intensive care resources, putting them in direct competition with critically ill COVID‐19 patients. As noted previously, blood donation shortages further complicate resuscitation efforts [59, 60, 61].
Unfortunately, shelter‐in‐place regulations and the closure of schools can increase contact between perpetrators of domestic assault, rape and child abuse and their victims, potentially leading to higher rates of these events. Some domestic violence call centres have noted increases in the number of calls, whilst experts worry that this is a underestimation of the true volume, as the close proximity of abusers and victims may disincentivize calls for help [117].
Elective surgery
Most hospitals and surgical professional societies have recommended the rescheduling or reduction of elective surgeries to conserve anaesthesiology staff, equipment and ICU beds for the COVID‐19 response effort [116, 118]. Such conservation policies have become more critical given blood product shortages [59, 60, 61]. Concerningly, there are anecdotal reports of patients hiding COVID‐19 symptoms due to fear of providers not offering them operative management [119]. If true, this could have ramifications on PPE use for operating room staff and equipment, even for asymptomatic cases.
Ophthalmology
Case reports suggest that SARS‐CoV‐2 can present with viral conjunctivitis [9, 120]. This could represent a threat to ophthalmologists and optometrists, who come into close contact with patient’s faces and tears. Of special note, hydroxychloroquine and chloroquine, which have seen off‐label use as possible antiviral agents, are associated with an irreversible retinopathy, usually with prolonged use [30, 31, 121]. The long‐term ocular effects of its usage for COVID‐19 are unclear.
Conclusions
The COVID‐19 pandemic has powerfully affected all areas of the healthcare system, not just infectious disease and critical care. Here, we have provided a broad review of both biologically mediated and systems‐level impacts that the COVID‐19 crisis has levied on patients and practitioners in NCD fields. Given that the peak of the pandemic has not yet passed in many countries, whilst others are experiencing new outbreaks of the infection, the effects are sure to continue evolving in the coming months due to the chronic nature of many NCDs. The scope and severity of these impacts will continue to unfold long after successful control of the present pandemic.
Conflict of interest
All authors report no competing commercial/financial relationships with any associations or organizations that might have an interest in the submitted work; no other relationships or activities that could appear to have influenced or benefited from the submitted work.
Author Contribution
Mark R. Cullen: Project administration (supporting); Supervision (supporting); Writing‐review & editing (supporting). Robert A. Harrington: Project administration (supporting); Supervision (supporting); Writing‐review & editing (supporting). Michele Barry: Conceptualization (lead); Methodology (equal); Project administration (supporting); Writing‐original draft (supporting); Writing‐review & editing (supporting).
Chang AY, Cullen MR, Harrington RA, Barry M (Stanford University, Stanford, CA, USA). The impact of novel coronavirus COVID‐19 on noncommunicable disease patients and health systems: a review (Review). J Intern Med,2021; 289: 450–462. 10.1111/joim.13184
References
- 1. Huang C, Wang Y, Li X et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . WHO Director‐General’s opening remarks at the media briefing on COVID‐19 ‐ 11 March 2020. 2020. [Google Scholar]
- 3. Toner E, Waldhorn R. What US Hospitals Should Do Now to Prepare for a COVID‐19 Pandemic. Baltimore, MD, USA: Johns Hopkins Center for Health Security; 2020. [Google Scholar]
- 4. CDC . CDC COVID‐19 Portal: People Who Are at Increased Risk for Severe Illness. 2020. [Google Scholar]
- 5. CDC . Evidence used to update the list of underlying medical conditions that increase a person’s risk of severe illness from COVID‐19. 2020. [PubMed] [Google Scholar]
- 6. Novel Coronavirus Pneumonia Emergency Response Epidemiology Team . The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID‐19) in China. Zhonghua Liu Xing Bing Xue Za Zhi 2020; 41: 145–51.32064853 [Google Scholar]
- 7. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA 2020; 323: 1239–42. [DOI] [PubMed] [Google Scholar]
- 8. WHO‐China Joint Mission on Coronavirus Disease 2019 . Report of the WHO‐China Joint Mission on Coronavirus Disease 2019 (COVID‐19). 2020. [Google Scholar]
- 9. Guan W, Ni Z, Hu Y et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mullen B. ACC Clinical Bulletin: COVID‐19 Clinical Guidance For the Cardiovascular Care Team. 2020. [Google Scholar]
- 11. CDC COVID‐19 Response Team , CDC COVID‐19 Response Team , Chow N et al. Preliminary Estimates of the Prevalence of Selected Underlying Health Conditions Among Patients with Coronavirus Disease 2019 — United States, February 12–March 28, 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 382–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J 2020; ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Inciardi RM, Lupi L, Zaccone G et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID‐19). JAMA Cardiol 2020; 5: 819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou F, Yu T, Du R et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lindner D, Fitzek A, Bräuninger H et al. Association of cardiac infection with SARS‐CoV‐2 in confirmed COVID‐19 autopsy cases. JAMA Cardiol 2020; e203551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Puntmann VO, Carerj ML, Wieters I et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID‐19). JAMA Cardiol 2020; e203557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shi S, Qin M, Shen B et al. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol 2020; 5: 802–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lippi G, Lavie CJ, Sanchis‐Gomar F, Cardiac troponin I in patients with coronavirus disease 2019 (COVID‐19): Evidence from a meta‐analysis. Prog Cardiovasc Dis 2019; 63: 390–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lala A, Johnson KW, Januzzi JL et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID‐19 infection. J Am College Cardiol 2020; 76: 533–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bhatt AS, Moscone A, McElrath EE et al. Declines in hospitalizations for acute cardiovascular conditions during the COVID‐19 pandemic: a multicenter tertiary care experience. J Am College Cardiol 2020; 76: 280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Garcia S, Albaghdadi MS, Meraj PM et al. Reduction in ST‐segment elevation cardiac catheterization laboratory activations in the United States during COVID‐19 pandemic. J Am College Cardiol 2020; 75: 2871–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zeng J, Huang J, Pan L. How to balance acute myocardial infarction and COVID‐19: the protocols from Sichuan Provincial People’s Hospital. Intensive Care Med 2020; 46: 1111–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hammad TA, Parikh M, Tashtish N et al. Impact of COVID‐19 pandemic on ST‐elevation myocardial infarction in a non‐COVID‐19 epicenter. Catheter Cardiovasc Interv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tam C‐CF, Cheung K‐S, Lam S et al. Impact of coronavirus disease 2019 (COVID‐19) outbreak on ST‐segment–elevation myocardial infarction care in Hong Kong, China. Circ: Cardiovasc Quality Outcomes 2020; 13: e006631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Welt FGP, Shah PB, Aronow HD et al. Catheterization laboratory considerations during the coronavirus (COVID‐19) pandemic: from ACC’s interventional council and SCAI. J Am College Cardiol 2020; 75: 2372–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stefanini GG, Azzolini E, Condorelli G. Critical organizational issues for cardiologists in the COVID‐19 outbreak: a frontline experience from Milan, Italy. Circulation 2020; 141: 1597–99. [DOI] [PubMed] [Google Scholar]
- 27. Mahmud E, Dauerman HL, Welt FGP et al. Management of acute myocardial infarction during the covid ‐19 pandemic: A Consensus Statement from the Society for Cardiovascular Angiography and Interventions (SCAI), the American College of Cardiology (ACC), and the American College of Emergency Physicians (ACEP). Catheter Cardiovasc Interv 2020; 96: 336–45. [DOI] [PubMed] [Google Scholar]
- 28. Wang D, Hu B, Hu C et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Simpson TF, Kovacs RJ, Stecker EC. Ventricular Arrhythmia Risk Due to Hydroxychloroquine‐Azithromycin Treatment for COVID‐19. Manalapan, NJ, USA: American College of Cardiology; 2020. [Google Scholar]
- 30. Gautret P, Lagier J‐C, Parola P et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. Int J Antimicrobial Agents 2020; 56: 105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31. Liu J, Cao R, Xu M et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS‐CoV‐2 infection in vitro. Cell Discov 2020; 6: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mercuro NJ, Yen CF, Shim DJ et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID‐19). JAMA Cardiol 2020; 5: 1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bessière F, Roccia H, Delinière A et al. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID‐19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit. JAMA Cardiol 2020; 5: 1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin–angiotensin–aldosterone system inhibitors in patients with COVID‐19. N Engl J Med 2020; 382: 1653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID‐19 infection? Lancet Respiratory Med 2020; 8: e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kuba K, Imai Y, Rao S et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus‐induced lung injury. Nat Med 2005; 11: 875–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Imai Y, Kuba K, Rao S et al. Angiotensin‐converting enzyme 2 protects from severe acute lung failure. Nature 2005; 436: 112–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mackey K, King VJ, Gurley S et al. Risks and impact of angiotensin‐converting enzyme inhibitors or angiotensin‐receptor blockers on SARS‐CoV‐2 infection in adults: a living systematic review. Annals Internal Med 2020; 173: 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fosbol EL, Holmes DN, Piccini JP et al. Provider specialty and atrial fibrillation treatment strategies in United States community practice: findings from the ORBIT‐AF registry. J Am Heart Assoc 2013; 2: e000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bozkurt B, Kovacs R, Harrington B. HFSA/ACC/AHA statement addresses concerns re: using RAAS antagonists in COVID‐19. J Card Fail 2020; 26: 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Volkow N. COVID‐19: Potential Implications for Individuals with Substance Use Disorders. Bethesda, MD, USA: National Institute on Drug Abuse; 2020. [Google Scholar]
- 42. Gaiha SM, Cheng J, Halpern‐Felsher B. Association between youth smoking, electronic cigarette use, and coronavirus disease 2019. J Adolescent Health 2020; 67: 519–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. World Health Organization . Information Note ‐ COVID‐19 and NCDs. 2020. [Google Scholar]
- 44. Ost DE. Bronchoscopy in the age of COVID‐19. J Bronchol Interv Pulmonol 2020; 27: 160–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cheung JC‐H, Ho LT, Cheng JV, Cham EYK, Lam KN. Staff safety during emergency airway management for COVID‐19 in Hong Kong. Lancet Respir Med 2020; 8: e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Spagnolo P, Balestro E, Aliberti S et al. Pulmonary fibrosis secondary to COVID‐19: a call to arms? Lancet Respir Med 2020; 8: 750–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Grillo F, Barisione E, Ball L, Mastracci L, Fiocca R. Lung fibrosis: an undervalued finding in COVID‐19 pathological series. Lancet Infect Dis 2020; 10.1016/S1473-3099(20)30582-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Saran R, Robinson B, Abbott KC et al. US renal data system 2019 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis 2020; 75: A6–7. [DOI] [PubMed] [Google Scholar]
- 49. Bernstein L, Rowland C, Hamburger T et al. Dialysis patients are at high risk during COVID‐19 outbreak. Washington Post. https://www.washingtonpost.com/health/dialysis‐patients‐are‐at‐high‐risk‐during‐covid‐19‐outbreak/2020/03/24/6e69f908‐6aa7‐11ea‐b313‐df458622c2cc_story.html. Accessed March 29, 2020. [Google Scholar]
- 50. Kato S, Chmielewski M, Honda H et al. Aspects of immune dysfunction in end‐stage renal disease. Clin J Am Soc Nephrol 2008; 3: 1526–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. CDC . Coronavirus Disease 2019 (COVID‐19): Dialysis Facilities. Atlanta, GA, USA: Centers for Disease Control and Prevention. 2020. [Google Scholar]
- 52. Livingston S. Dialysis centers face significant challenges in protecting patients from COVID‐19. Modern Healthcare. https://www.modernhealthcare.com/patient‐care/dialysis‐centers‐face‐significant‐challenges‐protecting‐patients‐covid‐19. Published March 26, 2020. Accessed March 30, 2020. [Google Scholar]
- 53. Liang W, Guan W, Chen R et al. Cancer patients in SARS‐CoV‐2 infection: a nationwide analysis in China. Lancet Oncol 2020; 21: 335–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xia Y, Jin R, Zhao J, Li W, Shen H. Risk of COVID‐19 for cancer patients. Lancet Oncol 2020; 21: e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Connors JM, Levy JH. COVID‐19 and its implications for thrombosis and anticoagulation. Blood 2020; 135: 2033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Willan J, King AJ, Hayes S, Collins GP, Peniket A. Care of haematology patients in a COVID‐19 epidemic. Br J Haematol 2020; 189: 241–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. American Society of Clinical Oncology . ASCO COVID‐19 Provider & Practice Information. ASCO. 2020. [Google Scholar]
- 58. Ledford H. Coronavirus shuts down trials of drugs for multiple other diseases. Nature 2020; 580: 15–6. [DOI] [PubMed] [Google Scholar]
- 59. American Red Cross . Red Cross Media Statement on Coronavirus Disease 2019. 2020. [Google Scholar]
- 60. The American Red Cross . What to know about the Coronavirus and Blood Donation. Washington, DC, USA: American Red Cross, 2020. [Google Scholar]
- 61. Marks P. Coronavirus (COVID‐19) Update: Blood Donations. Silver Spring, MD, USA: Food and Drug Administration; 2020. [Google Scholar]
- 62. Burki TK. Cancer care in the time of COVID‐19. Lancet Oncol 2020; 21: 628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Szer J, Weisdorf D, Querol S, Foeken L, Madrigal A. The impact of COVID‐19 on the provision of donor hematopoietic stem cell products worldwide: collateral damage. Bone Marrow Transplant 2020; 55: 2043–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Varatharaj A, Thomas N, Ellul MA et al. Neurological and neuropsychiatric complications of COVID‐19 in 153 patients: a UK‐wide surveillance study. Lancet Psychiatry 2020; 7: 875–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. ABN Executive . Association of British Neurologists Guidance on COVID‐19 for people with neurological conditions, their doctors and carers. 2020. [Google Scholar]
- 66. Ferro F, Elefante E, Baldini C et al. COVID‐19: the new challenge for rheumatologists. Clin Exp Rheumatol 2020; 38: 175–80. [PubMed] [Google Scholar]
- 67. Beachum L. Experts debate whether ‘senior‐only’ shopping hours protect the elderly from getting COVID‐19. Washington Post. https://www.washingtonpost.com/business/2020/03/17/senior‐only‐shopping‐coronavirus/. Accessed March 29, 2020. [Google Scholar]
- 68. Barnett ML, Grabowski DC. Nursing homes are ground zero for COVID‐19 pandemic. JAMA Health Forum 2020; 1: e200369. [DOI] [PubMed] [Google Scholar]
- 69. Richardson S, Hirsch JS, Narasimhan M et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City Area. JAMA 2020; 323: 2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Centers for Disease Control . Preparing for COVID‐19: Long‐term Care Facilities, Nursing Homes. Atlanta, GA, USA: Centers for Disease Control; 2020. [Google Scholar]
- 71. Atabakhsh V. Social distancing: 6 ways to help older adults change their routines. The Conversation. [Google Scholar]
- 72. Lai J, Ma S, Wang Y et al. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw Open 2020; 3: e203976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Talev M. Axios‐Ipsos Coronavirus Index: Americans hit by stress, job losses. Axios 2020;1. https://www.axios.com/axios‐ipsos‐coronavirus‐index‐stress‐72c409d2‐0a66‐48f1‐b736‐30d07cfce95b.html. [Google Scholar]
- 74. Inter‐Agency Standing Committee . Interim Briefing Note Addressing Mental Health and Psychosocial Aspects of COVID‐19 Outbreak (developed by the IASC’s Reference Group on Mental Health and Psychosocial Support). Geneva, CH; New York, NY, USA: IASC, 2020. [Google Scholar]
- 75. American Psychiatric Association . COVID‐19 Mental Health Impacts: Resources for Psychiatrists. Washington, DC, USA: American Psychiatric Association; 2020. [Google Scholar]
- 76. Brooks SK, Webster RK, Smith LE et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet 2020; 395: 912–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wu P, Liu X, Fang Y et al. Alcohol abuse/dependence symptoms among hospital employees exposed to a SARS Outbreak: table 1. Alcalc 2008; 43: 706–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Prasso S. Divorce Rate After Coronavirus Quarantine in China Is Warning. Bloomberg. https://www.bloomberg.com/news/articles/2020‐03‐31/divorces‐spike‐in‐china‐after‐coronavirus‐quarantines. Published March 31, 2020. Accessed April 1, 2020. [Google Scholar]
- 79. Reeves A, McKee M, Stuckler D. Economic suicides in the Great Recession in Europe and North America. Br J Psychiatry 2014; 205: 246–7. [DOI] [PubMed] [Google Scholar]
- 80. Petterson S, Westfall JM, Miller BF. Projected Deaths of Despair from COVID‐19. Oakland, CA, USA: Well Being Trust, Robert Graham Center; 2020. https://wellbeingtrust.org/wp‐content/uploads/2020/05/WBT_Deaths‐of‐Despair_COVID‐19‐FINAL‐FINAL.pdf. [Google Scholar]
- 81. Leece P, Cavacuiti C, Macdonald EM et al. Predictors of opioid‐related death during methadone therapy. J Substance Abuse Treat 2015; 57: 30–5. [DOI] [PubMed] [Google Scholar]
- 82. Ramirez RL, Perez VDJ, Zamanian RT. Methamphetamine and the risk of pulmonary arterial hypertension. Curr Opin Pulm Med 2018; 24: 416–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Schwarzbach V, Lenk K, Laufs U. Methamphetamine‐related cardiovascular diseases. ESC Heart Failure 2020; 7: 407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tsai J, Wilson M. COVID‐19: a potential public health problem for homeless populations. Lancet Public Health 2020; 5: e186–e187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kushel M. UCSF Benioff Homelessness and Housing Initiative. State of California COVID‐19 Recommended Protocol for People Experiencing Homelessness. San Francisco, CA, USA: UCSF Benioff Homelessness and Housing Initiative; 2020. https://endhomelessness.org/wp‐content/uploads/2020/04/flowchart‐COVID19‐homelessness‐4.3.‐BHHI.pdf. [Google Scholar]
- 86. Page KR, Venkataramani M, Beyrer C, Polk S, Undocumented US. Immigrants and Covid‐19. N Engl J Med 2020; 382: e62. [DOI] [PubMed] [Google Scholar]
- 87. American College of Rheumatology . ACR Infusion Guidance During COVID‐19 Crisis. Atlanta, GA, USA: American College of Rheumatology; 2020. https://www.rheumatology.org/Portals/0/Files/ACR‐Infusion‐Guidance‐COVID‐19.pdf. [Google Scholar]
- 88. Lockshin MD. COVID‐19 Coronavirus Causes Plaquenil & Chloroquine Shortage. New York, NY, USA: Hospital for Special Surgery; 2020. https://www.hss.edu/conditions_hydroxychloroquine‐plaquenil‐chloroquine‐shortage‐covid‐19‐coronavirus.asp. [Google Scholar]
- 89. FitzGerald GA. Misguided drug advice for COVID‐19. Sills J, ed. Science 2020; 367: 1434.1–1434. [DOI] [PubMed] [Google Scholar]
- 90. US Food and Drug Administration . FDA advises patients on use of non‐steroidal anti‐inflammatory drugs (NSAIDs) for COVID‐19. Silver Spring, MD, USA: US Food and Drug Administration; 2020. https://www.fda.gov/drugs/drug‐safety‐and‐availability/fda‐advises‐patients‐use‐non‐steroidal‐anti‐inflammatory‐drugs‐nsaids‐covid‐19. [Google Scholar]
- 91. World Health Organization . Could ibuprofen worsen disease for people with COVID‐19?. Geneva, CH: World Health Organization; 2020. [Google Scholar]
- 92. Shulman ST. Pediatric COVID‐associated Multi‐system Inflammatory Syndrome (PMIS). J Pediatric Infect Dis Soc 2020; 9: 285–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Alves C, Casqueiro J, Casqueiro J. Infections in patients with diabetes mellitus: A review of pathogenesis. Indian J Endocr Metab 2012; 16: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Klang E, Kassim G, Soffer S, Freeman R, Levin MA, Reich DL. Morbid obesity as an independent risk factor for COVID‐19 mortality in hospitalized patients younger than 50. Obesity 2020; 28: 1595–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. United Nations System Standing Committee on Nutrition . The COVID‐19 Pandemic is Disrupting People’s Food Environments: A Resource List on Food Systems and Nutrition Responses. Rome, IT: UNSCN. 2020. https://www.unscn.org/en/news‐events/recent‐news?idnews=2039. [Google Scholar]
- 96. Cullen MT. Coronavirus: Food Supply Chain Under Strain. What to do? Food and Agriculture Organization of the United Nations, 2020: 39. [Google Scholar]
- 97. Tiensin T. Impact of COVID‐19 on Food Security and Nutrition (FSN). Rome, IT: Food and Agriculture Organization of the United Nations; 2020: 1–8. http://www.fao.org/fileadmin/templates/cfs/Docs1920/Chair/HLPE_English.pdf. [Google Scholar]
- 98. United Nations World Food Programme . World Food Programme gears up to support children left without meals due to COVID‐19 school closures. Rome, IT: World Food Programme. 2020. https://www.wfp.org/news/world‐food‐programme‐gears‐support‐children‐left‐without‐meals‐due‐covid‐19‐school‐closures. [Google Scholar]
- 99. World Food Programme, VAM Food Security Analysis . Economic and food security implications of the COVID‐19 outbreak. Rome, IT: World Food Programme; 2020: 1–7. https://docs.wfp.org/api/documents/WFP‐0000113485/download/. [Google Scholar]
- 100. Nagata JM, Abdel Magid HS, Gabriel KP. Screen time for children and adolescents during the COVID‐19 pandemic. Obesity (Silver Spring) 2020; 28: 1582–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ellington S, Strid P, Tong VT et al. Characteristics of women of reproductive age with laboratory‐confirmed SARS‐CoV‐2 infection by pregnancy status — United States, January 22–June 7, 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 769–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. American College of Obstetricians and Gynecologists . ACOG Novel Coronavirus 2019 (COVID‐19) Practice Advisory. Washington, DC, USA: American College of Obstetricians and Gynecologists; 2020. https://www.acog.org/clinical/clinical‐guidance/practice‐advisory/articles/2020/03/novel‐coronavirus‐2019. [Google Scholar]
- 103. Rasmussen SA, Jamieson DJ. Coronavirus disease 2019 (COVID‐19) and pregnancy: responding to a rapidly evolving situation. Obstetrics Gynecol 2020; 135: 999–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kimberlin DW, Stagno S. Can SARS‐CoV‐2 infection be acquired in utero?: More definitive evidence is needed. JAMA 2020; 323: 1788–9. [DOI] [PubMed] [Google Scholar]
- 105. Dong L, Tian J, He S et al. Possible vertical transmission of SARS‐CoV‐2 from an infected mother to her newborn. JAMA 2020; 323: 1846–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Zeng H, Xu C, Fan J et al. Antibodies in infants born to mothers with COVID‐19 Pneumonia. JAMA 2020; 323: 1848–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zeng L, Xia S, Yuan W et al. Neonatal early‐onset infection with SARS‐CoV‐2 in 33 neonates born to mothers with COVID‐19 in Wuhan, China. JAMA Pediatr 2020; 174: 722–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. World Food Programme . COVID‐19 and Breastfeeding. Geneva, CH: World Health Organization; 2020. https://www.who.int/news‐room/commentaries/detail/breastfeeding‐and‐covid‐19. [Google Scholar]
- 109. Bazelon E. The Coronavirus Becomes an Excuse to Restrict Abortions. The New York Times. https://www.nytimes.com/2020/03/26/opinion/covid‐abortion‐ohio‐texas.html. Published March 26, 2020. Accessed March 29, 2020. [Google Scholar]
- 110. Sobel L, Ramaswamy A, Frederiksen B, Salganicoff A. State Action to Limit Abortion Access During the COVID‐19 Pandemic. San Francisco, CA, USA: Kaiser Family Foundation; 2020. https://www.kff.org/coronavirus‐covid‐19/issue‐brief/state‐action‐to‐limit‐abortion‐access‐during‐the‐covid‐19‐pandemic/. [Google Scholar]
- 111. Pan L, Mu M, Yang P et al. Clinical characteristics of COVID‐19 patients with digestive symptoms in Hubei, China: a descriptive, cross‐sectional, multicenter study. Am J Gastroenterol 2020; 115: 766–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Repici A, Maselli R, Colombo M et al. Coronavirus (COVID‐19) outbreak: what the department of endoscopy should know. Gastrointest Endosc 2020; 92: 192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Gu J, Han B, Wang J. COVID‐19: Gastrointestinal manifestations and potential fecal‐oral transmission. Gastroenterology 2020; 158: 1518–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Mao R, Liang J, Shen J et al. Implications of COVID‐19 for patients with pre‐existing digestive diseases. Lancet Gastroenterol Hepatol 2020; 5: 425–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kumar D, Tellier R, Draker R, Levy G, Humar A. Severe Acute Respiratory Syndrome (SARS) in a liver transplant recipient and guidelines for donor SARS screening. Am J Transplant 2003; 3: 977–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. American College of Surgeons Committee on Trauma . Maintaining Trauma Center Access & Care during the COVID‐19 Pandemic: Guidance Document for Trauma Medical Directors. Chicago, IL, USA: American College of Surgeons; 2020. . https://www.facs.org/quality‐programs/trauma/maintaining‐access. [Google Scholar]
- 117. Selvaratnam T. Where Can Domestic Violence Victims Turn During COVID‐19? The New York Times. https://www.nytimes.com/2020/03/23/opinion/covid‐domestic‐violence.html. Published March 23, 2020. Accessed March 31, 2020. [Google Scholar]
- 118. American College of Surgeons . COVID‐19 and Surgery: Clinical Issues and Guidance. Chicago, IL, USA: American College of Surgeons; 2020. https://www.facs.org/covid‐19/clinical‐guidance. [Google Scholar]
- 119. Impelli M. Italian man with coronavirus who hid symptoms to get rhinoplasty facing 12 years in prison. Newsweek 2020. [Google Scholar]
- 120. Xia J, Tong J, Liu M, Shen Y, Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS‐CoV‐2 infection. J Med Virol 2020; 92: 589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Yusuf IH, Sharma S, Luqmani R, Downes SM. Hydroxychloroquine retinopathy. Eye 2017; 31: 828–45. [DOI] [PMC free article] [PubMed] [Google Scholar]


