Abstract
Objectives
Several reports had observed a high risk of pulmonary embolism (PE) in patients with coronavirus disease 2019 (COVID‐19), most of them in the intensive care unit. Reported findings indicate that a direct viral‐mediated hyperinflammatory response leads to local thromboinflammation. According to those findings, the incidence of deep venous thrombosis (DVT) in patients with COVID‐19 and PE should be low. The objective of this study was to evaluate the incidence of DVT in patients with COVID‐19 who developed PE.
Methods
In this prospective observational study, consecutive patients hospitalized in the internal medicine ward with a diagnosis of COVID‐19 who developed PE were screened for DVT in the lower extremities with complete compression ultrasound.
Results
The study comprised 26 patients. Fifteen patients (57.7%) were male. The median age was 60 years (interquartile range, 54–73 years). Compression ultrasound findings were positive for DVT in 2 patients (7.7%; 95% confidence interval, 3.6%–11.7%). Patients with DVT had central and bilateral PE. In both, venous thromboembolism was diagnosed in the emergency department, so they did not receive previous prophylactic therapy with low‐molecular‐weight heparin. Patients without DVT had higher median d‐dimer levels: 25,688 μg/dL (interquartile range, 80,000–1210 μg/dL) versus 5310 μg/dL (P < .05).
Conclusions
Our study showed a low incidence of DVT in a cohort of patients with COVID‐19 and PE. This observation suggests that PE in these patients could be produced mainly by a local thromboinflammatory syndrome induced by severe acute respiratory syndrome coronavirus 2 infection and not by a thromboembolic event.
Keywords: compression ultrasound, coronavirus disease 2019, COVID‐19, deep venous thrombosis, pulmonary embolism, thromboinflammatory syndrome
Abbreviations
- COVID‐19
coronavirus disease 2019
- CUS
compression ultrasound
- DVT
deep venous thrombosis
- PE
pulmonary embolism
- SARS‐CoV
severe acute respiratory syndrome coronavirus
Arterial and venous thrombotic events seem to emerge as important issues in patients with coronavirus disease 2019 (COVID‐19). 1 , 2 Increased levels of d‐dimer, fibrin degradation products, and prothrombin time prolongation are associated with a poor prognosis in several studies of patients with COVID‐19. 3 , 4 , 5 Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infects the epithelial cells by using the angiotensin‐converting enzyme 2 receptor. 6 Consequently, a hyperinflammatory response is initiated, which sets the stage for thrombosis through several mechanisms.
Pulmonary embolism (PE) is the most common thrombotic manifestation of COVID‐19. An increase of PE has been reported in intensive care unit patients with COVID‐19. 7 , 8 , 9 , 10 , 11 , 12 , 13 Previous outbreaks of SARS‐CoV‐1 and Middle Eastern respiratory syndrome have been associated with an increased risk of thrombosis. 14 , 15 Despite previous experience with these coronaviruses, the pathophysiologic characteristics of PE in SARS‐CoV‐2 infection are not well documented. In patients with COVID‐19, it has been hypothesized that the pathophysiologic characteristics of PE are different than in patients without COVID‐19. Proposed hypotheses include a severe inflammatory response that leads to local thromboinflammation through mechanisms such as complement activation, a cytokine storm, endotheliitis, and activation of the coagulation cascade. 6 , 16 In fact, in several autopsies from patients who died of COVID‐19, the lung histologic analysis showed widespread thrombosis with microangiopathy in pulmonary vessels. 17 , 18 , 19 These mechanisms of thromboinflammation triggered by SARS‐CoV‐2 infection could explain the microvascular pulmonary thrombosis. A recent study suggested that microvascular COVID‐19 lung vessel obstructive thromboinflammatory syndrome determines this pulmonary thromboinflammatory mechanism. 20 This local hypercoagulable state in the pulmonary tissue seems to be the pathophysiologic characteristic of PE in patients with COVID‐19 rather than the classic emboli coming from the lower extremities. According to that idea, the incidence of deep venous thrombosis (DVT) in patients with COVID‐19 and PE should be low. The aim of this study was to investigate the incidence of concomitant lower limb DVT using compression ultrasound (CUS) in patients with COVID‐19 and PE admitted to the general ward of an internal medicine department.
Materials and Methods
Study Design and Setting
We conducted a prospective observational study in patients older than 18 years admitted to the internal medicine ward for COVID‐19 and PE at Infanta Leonor University Hospital, a second‐level hospital in Madrid. The COVID‐19 diagnosis was defined by RNA detection of SARS‐CoV‐2 from a nasopharyngeal swab or by the presence of clinical, radiologic, and analytical findings highly suggestive of the disease in patients with reverse transcription polymerase chain reaction–negative results and absence of an alternative diagnosis, according to the World Health Organization guideline. 21 The diagnosis of PE was achieved by pulmonary computed tomographic angiography.
Study Protocol
Patients with COVID‐19 and PE underwent complete CUS examinations of both legs, which included the proximal territory (common femoral vein, saphenofemoral junction, and popliteal vein). As described in previous reports, 22 , 23 3‐point compression means doing CUS scans in 3 regions with higher turbulence and at the greatest risk of developing thrombosis. Ultrasound examinations were performed with a MyLab 2 system (Esaote SpA, Genoa, Italy) using a high‐frequency linear transducer (6–15 MHz). Compression ultrasound examinations were performed by 2 clinically accredited, trained operators. Patients were excluded if they were receiving therapeutic doses of anticoagulation for a previous PE diagnosis. Demographic and clinical data were obtained from the clinical charts. This study was conducted according to the international ethical principles to guide physicians in medical research involving humans in the latest revision of the Declaration of Helsinki. Considering the isolation of patients, written informed consent was obtained by an impartial witness, who was present during the entire consent process. The witness attested to the voluntariness of the patient's consent and the adequacy of the consent process by ensuring that the information was accurately conveyed and that the patient's questions were answered. The study was approved by the Institutional Ethics Committee.
Statistical Analysis
Quantitative variables are presented as the median and interquartile range when they had a non‐normal distribution. Qualitative variables are presented as percentages.
Results
From March 30, 2020, to May 6, 2020, a total of 412 patients were admitted to the internal medicine ward with COVID‐19. Thirty‐nine patients (9.46%) had a diagnosis of acute PE. Among of them, CUS examinations were performed in 26 patients. Three patients were excluded because they were receiving therapeutic doses of anticoagulation (2 for atrial fibrillation and 1 for prior unprovoked venous thromboembolism); 2 patients died before CUS examinations were performed; 1 patient was transferred to another geographic location; in 7 patients, CUS examinations were not performed.
Compression ultrasound findings were positive for DVT in 2 patients (7.7%; 95% confidence interval, 3.6–11.7) with left popliteal vein thrombosis (Figure 1A) and left femoral vein thrombosis (Figure 2A). Basal characteristics, laboratory test results, and CUS findings are summarized in Table 1. Seventeen patients (65.4%) had a diagnosis of COVID‐19 with reverse transcription polymerase chain reaction–positive results, and 9 patients had clinical, laboratory, and radiologic findings suggestive of SARS‐CoV‐2 infection with reverse transcription polymerase chain reaction–negative results. Fifteen patients (57.7%) were male, and the median age of the sample was 60 years (interquartile range, 54–73 years). Median time from PE diagnosis until CUS was 6 days. Five hospitalized patients did not receive thromboprophylaxis with low‐molecular‐weight heparin for a previous PE diagnosis, and in 5 patients, including the 2 patients with DVT, venous thromboembolism was diagnosed in the emergency department. The 2 patients with DVT had central and bilateral PE (Figures 1B and 2B). Patients without DVT had higher median d‐dimer levels: 25,688 μg/dL (interquartile range, 80,000–1210 μg/dL) versus 5310 μg/dL (P < .05). None of the included patients died.
Figure 1.
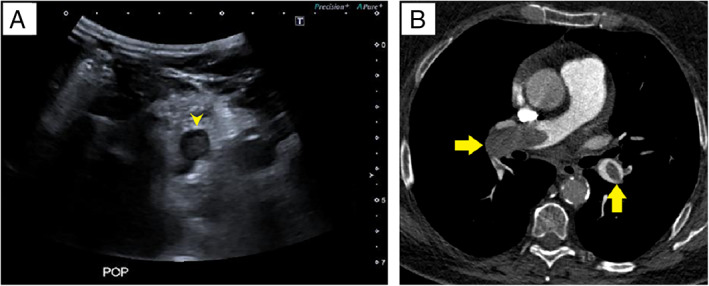
A, Thrombosed popliteal vein. Transverse ultrasound scan shows an echogenic clot in the left popliteal vein (arrowhead). B, Bilateral PE. Computed tomographic pulmonary angiography shows bilateral filling defects in the right pulmonary artery and the left lower lobar artery (arrows).
Figure 2.
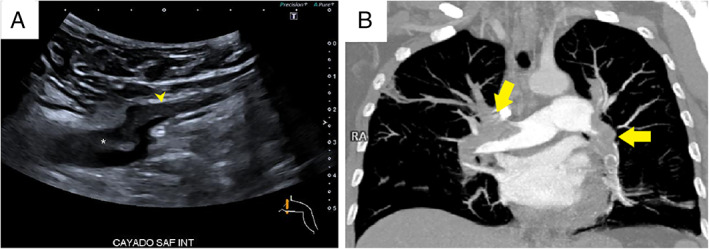
A, Thrombus in the common femoral vein (CFV). Transverse ultrasound scan shows a partial filling defect in the saphenous vein (arrowhead) and the common femoral vein (asterisk) just above the saphenous junction. B, Bilateral pulmonary embolism. Coronal maximum‐intensity projection CT pulmonary angiography shows bilateral filling defects in the two main pulmonary arteries and their lobar branches (arrows).
Table 1.
Basal Characteristics, Laboratory Test Results, and CUS Findings of Hospitalized Patients With COVID‐19 and PE
| Patient | DVT | Sex Age, y | Obesity, BMI >30 kg/m2 | History of VTE | Known Thrombophilia | Active Cancer | Days a | PE Location | Thromboprophylaxis on Admission | Oxygen Therapy | Lymphocytes, ×103/μL | Platelets, ×103/μL | Peak d‐Dimer, μg/dL | LDH, U/L | IL‐6, U/L | Ferritin, ng/dL | CRP, mg/dL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | No |
Male 65 |
35.3 | No | No | No | 28 |
Unilateral peripheral |
Enoxaparin, 60 mg OD |
Nasal cannula | 2,500 | 286 | 24,880 | 277 | 123 | 538 | 0.4 |
| 2 | No |
Male 73 |
30.1 | No | No | No | 21 |
Bilateral peripheral |
Enoxaparin, 40 mg OD |
NIV | 1,300 | 186 | 80,000 | 727 | NA | NA | 1 |
| 3 | No |
Male 75 |
NA | No | No | No | 15 |
Bilateral peripheral |
Enoxaparin, 40 mg OD |
NIV | 1,000 | 482 | 63,590 | 946 | 476 | 1,698 | 2.5 |
| 4 | No |
Male 73 |
27.3 | No | No | No | 1 |
Bilateral central |
No prophylaxis | Nasal cannula | 2,800 | 162 | 8,980 | 266 | NA | 420 | 0.9 |
| 5 | No |
Male 49 |
26.7 | No | No | No | 9 |
Bilateral central |
Enoxaparin, 40 mg OD |
NIV | 1,300 | 349 | 35,200 | 950 | NA | 801 | NA |
| 6 | No |
Male 68 |
27.3 | No | No | No | 15 |
Unilateral peripheral |
Enoxaparin, 60 mg OD |
Nasal cannula | 600 | 322 | 19,510 | 256 | 19.4 | 1,122 | 1.2 |
| 7 | No |
Male 56 |
24.6 | No | No | No | 9 |
Bilateral peripheral |
Enoxaparin, 40 mg OD |
High flow | 500 | 540 | 45,350 | 480 | NA | 810 | 106 |
| 8 | No |
Female 58 |
26.9 | No | No | No | 8 |
Unilateral peripheral |
Enoxaparin, 40 mg OD |
High flow | 1,000 | 370 | 14,940 | 408 | 486.0 | 461 | NA |
| 9 | No |
Female 37 |
27.3 | No | No | No | 12 |
Unilateral peripheral |
No prophylaxis | No oxygen | 1,800 | 240 | 1,400 | 151 | NA | 200 | 1 |
| 10 | No |
Male 58 |
32.4 | No | No | No | 20 |
Bilateral peripheral |
Enoxaparin, 40 mg OD |
Nasal cannula | 1,300 | 455 | 26,160 | 245 | NA | NA | 30.6 |
| 11 | No |
Male 60 |
32.9 | No | No | No | 7 |
Unilateral peripheral |
Enoxaparin, 40 mg OD |
High flow | 1,000 | 288 | 7,470 | 172 | 6.8 | 406 | 3.5 |
| 12 | No |
Female 78 |
26.2 | No | No | No | 9 |
Unilateral peripheral |
No prophylaxis | High flow | 700 | 548 | 5,530 | 319 | 6.1 | 3,565 | 267 |
| 13 | No |
Male 80 |
30.5 | No | No | No | 5 |
Unilateral central |
Enoxaparin, 60 mg OD |
High flow | 4,500 | 234 | 28,740 | 253 | 5.8 | 1,373 | 53 |
| 14 | No |
Male 70 |
31.8 | No | No | No | 2 |
Bilateral central |
Enoxaparin, 60 mg OD |
NIV | 1,000 | 207 | 80,000 | 619 | 93 | 1,389 | 470.7 |
| 15 | No |
Male 43 |
22.9 | No | No | No | 2 |
Bilateral peripheral |
Enoxaparin, 80 mg OD |
No oxygen | 300 | 566 | 10,630 | 228 | 2.8 | 990 | 3.6 |
| 16 | No |
Female 67 |
32.3 | No | No | No | 0 |
Bilateral central |
No prophylaxis | Nasal cannula | 1,800 | 149 | 21,380 | 306 | NA | NA | 32.5 |
| 17 | No |
Female 75 |
19.7 | No | No | No | 12 |
Bilateral peripheral |
Enoxaparin, 80 mg OD |
Nasal cannula | 600 | 149 | 47,970 | 283 | NA | 566 | 238.9 |
| 18 | No |
Female 81 |
33.3 | No | No | No | 3 |
Unilateral peripheral |
No prophylaxis | Nasal cannula | 1,000 | 230 | 5,540 | 248 | NA | NA | 5.3 |
| 19 | No |
Female 58 |
31.5 | No | No | No | 7 |
Bilateral peripheral |
Enoxaparin, 60 mg OD |
Nasal cannula | 2,200 | 429 | 11,520 | 231 | 5.3 | 419 | 16.9 |
| 20 | No |
Male 54 |
24.9 | No | No | No | 0 |
Bilateral central |
No prophylaxis | Nasal cannula | 2,200 | 333 | 36,170 | 164 | 73.8 | 677 | 71 |
| 21 | No |
Male 60 |
25.3 | No | No | No | 0 |
Unilateral peripheral |
No prophylaxis | Nasal cannula | 1,500 | 302 | 1,210 | 139 | 66.4 | 754 | 157 |
| 22 | No |
Male 54 |
27.0 | No | No | No | 1 |
Bilateral peripheral |
Enoxaparin, 60 mg OD |
Nasal cannula | 1,000 | 256 | 18,680 | 224 | 40.0 | 387 | 90.9 |
| 23 | No |
Female 54 |
NA | No | No | No | 15 |
Unilateral peripheral |
Enoxaparin, 80 mg OD |
NIV | 1,000 | 164 | 22,460 | 621 | 1001 | 1,807 | 27.3 |
| 24 | No |
Female 53 |
27.1 | No | No | Yes | 11 |
Unilateral peripheral |
No prophylaxis | Nasal cannula | 1,900 | 182 | 4,210 | 136 | 211 | 202 | 12.1 |
| 25 | Yes |
Female 68 |
33.5 | No | No | No | 0 |
Bilateral central |
No prophylaxis | Nasal cannula | 2,500 | 287 | 3,430 | 163 | 10 | 115 | 11.3 |
| 26 | Yes |
Female 56 |
21.8 | No | No | No | 0 |
Bilateral central |
No prophylaxis | No oxygen | 1,700 | 240 | 7,190 | NA | NA | NA | 126 |
BMI indicates body mass index; CRP, C‐reactive protein; IL‐6, interleukin‐6; LDH, lactate dehydrogenase; NA, not available; NIV, noninvasive ventilation; OD, once daily; and VTE, venous thromboembolism.
Days from admission to hospital to PE diagnosis. .
Discussion
Severe acute respiratory syndrome caused by SARS‐CoV‐2 may predispose patients to thrombotic complications in the arterial and venous circulations because of excessive inflammation, platelet activation, endothelial dysfunction, and stasis. 1 , 2 Several recent studies reported a high incidence of PE in patients with COVID‐19 admitted to the intensive care unit. 7 , 8 , 9 , 10 , 11 , 12 , 13 However, scarce data have been published about the incidence of DVT in patients with SARS‐CoV‐2 infection and PE. In a meta‐analysis, the prevalence of DVT in the general population with PE was estimated as 35% to 45%. 24 Our investigation showed that the incidence of DVT was remarkably lower. Only 2 previous studies investigated the incidence of DVT in patients with COVID‐19 and PE. Although in both the sample sizes were smaller, similar observations were reported. In a study conducted by Poissy et al, 9 the incidence of PE was 20.6% (22 of 107) in patients with severe COVID‐19 admitted to the intensive care unit. It is interesting to note that 3 of these patients (13.6%) had DVT. In a series of 362 patients with COVID‐19 admitted to the intensive care unit and general wards, PE occurred in 10 patients (2.8%). Among of them, DVT was confirmed in 1 patient. 10 Although our study was limited by a small sample size, to our knowledge, this is the largest study reported to date of a cohort of non–critically ill patients with COVID‐19 and a diagnosis of acute PE in whom concomitant DVT was investigated.
Our findings suggest a local hypercoagulable state rather than emboli from lower extremity veins. In close connection with our hypothesis, the studies that have investigated the incidence of asymptomatic DVT in patients with COVID‐19 have shown controversial results. A single‐center study from Wuhan including 48 critically ill patients with COVID‐19 reported an 85.4% rate of asymptomatic DVT. 25 Surprisingly, the incidence of DVT was extremely high. This finding could have been limited by the small sample size. Furthermore, the incidence of venous thromboembolism is higher in patients with severe COVID‐19. Demelo et al 26 observed an incidence rate of 14.7% for asymptomatic DVT in a cohort of patients admitted to medical wards with COVID‐19 pneumonia. However, in an Italian study, none of the 64 tested patients with COVID‐19 admitted to the medical ward developed asymptomatic DVT. 27 A study in Germany that included 12 autopsies of patients who died of COVID‐19 revealed PE as the cause of death in 4 patients, with the thrombi derived from the deep veins of the lower extremities. In another 3 patients, DVT was present in the absence of PE. In all cases with DVT, both legs were involved. 18
Few studies have investigated the location of PE in patients with concomitant DVT. In a retrospective study, Lee et al 28 reported an association between concomitant DVT and a proximal location of PE. According with this data, in our study, in 2 patients with DVT, the lung thrombus was located in the main bilateral pulmonary arteries. In our cohort of patients, there was no relationship between the presence of concomitant DVT and a PE‐related unfavorable outcome or all‐cause mortality.
This study had several limitations: First, the main limitation was the small sample size, which could have limited the significance of our findings. Second, the 3‐point CUS protocol does not evaluate distal DVT; nevertheless, distal DVT is associated with a low rate of embolization.
In conclusion, our observations suggest that PE in SARS‐CoV‐2 infection could be due to pulmonary thromboinflammation syndrome rather than a thromboembolic event.
Members of the Infanta Leonor Thrombosis Research Group: Teresa Sáez‐Vaquero, Fernando Sierra‐Hidalgo, Eva Moya Mateo, María de Carranza, Miriam Akasbi, Eloy Gómez Mariscal, Karen Marín‐Mori, Cristina Figueras, Sonia López, Domingo Díaz, Cristina Mauleón, Juan Martín, Pedro Torres, Carmen Matesanz, Juan Churruca, Jacobo Rogado, Berta Obispo, Rosa María Lorente‐Ramos, Cristina Cortina‐Camarero, Ana Such‐Díaz, and Eva Ruiz‐Velasco.
All of the authors of this article have reported no disclosures.
References
- 1. Kollias A, Kyriakoulis KG, Dimakakos E, Poulakou G, Stergiou GS, Syrigos K. Thromboembolic risk and anticoagulant therapy in COVID‐19 patients: emerging evidence and call for action. Br J Haematol 2020; 189:846–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bikdeli B, Madhavan MV, Jimenez D, et al. COVID‐19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow‐up—JACC state‐of‐the‐art review. J Am Coll Cardiol 2020; 75:2950–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020; 18:844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arachchillage DRJ, Laffan M. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020; 18:1233–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lippi G, Favaloro EJ. d‐Dimer is associated with severity of coronavirus disease 2019: a pooled analysis. Thromb Haemost 2020; 120:876–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burcu F, Sarıalioğlu F. Pulmonary intravascular coagulation in COVID‐19: possible pathogenesis and recommendations on anticoagulant/thrombolytic therapy. J Thromb Thrombolysis 2020; 50:278–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res 2020; 191:145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost 2020; 18:1421–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Poissy J, Goutay J, Caplan M, et al. Pulmonary embolism in COVID‐19 patients: awareness of an increased prevalence. Circulation 2020; 142:184–186. [DOI] [PubMed] [Google Scholar]
- 10. Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID‐19 patients admitted to an academic hospital in Milan, Italy. Thromb Res 2020; 191:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS‐CoV‐2 infection: a multicenter prospective cohort study. Intensive Care Med 2020; 46:1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost 2020; 18:1995–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Llitjos JF, Leclerc M, Chochois C, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID‐19 patients. J Thromb Haemost 2020; 18:1743–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chong PY, Chui P, Ling AE, et al. Analysis of deaths during the severe acute respiratory syndrome (SARS) epidemic in Singapore: challenges in determining a SARS diagnosis. Arch Pathol Lab Med 2004; 128:195–204. [DOI] [PubMed] [Google Scholar]
- 15. Giannis D, Ziogas LA, Gianni P. Coagulation disorders in coronavirus infected patients: COVID‐19, SARS‐CoV‐1, MERS‐CoV and lessons from the past. J Clin Virol 2020; 127:104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abou‐Ismail MY, Diamond A, Kapoor S, Arafah Y, Nayak L. The hypercoagulable state in COVID‐19: incidence, pathophysiology, and management. Thromb Res 2020; 194:101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid‐19. N Engl J Med 2020; 383:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID‐19: a prospective cohort study. Ann Intern Med 2020; 173:268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Menter T, Haslbauer JD, Nienhold R, et al. Postmortem examination of COVID‐19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020; 77:198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ciceri F, Beretta L, Scandroglio AM, et al. Microvascular COVID‐19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc 2020; 22:95–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. World Health Organization . Coronavirus disease 2019. (COVID‐19): situation report 73. World Health Organization website. https://www.who.int/docs/default‐source/coronaviruse/situation‐reports/20200402‐sitrep‐73‐covid‐19.pdf?sfvrsn=5ae25bc7_2. April 2, 2020.
- 22. Zuker‐Herman R, Ayalon Dangur I, Berant R, et al. Comparison between two‐point and three‐point compression ultrasound for the diagnosis of deep vein thrombosis. J Thromb Thrombolysis 2018; 45:99–105. [DOI] [PubMed] [Google Scholar]
- 23. c SC , Zwiebel WJ, Miller FJ. Distribution of acute lower extremity deep venous thrombosis in symptomatic and asymptomatic patients: imaging implications. J Ultrasound Med 1994; 13:243–250. [DOI] [PubMed] [Google Scholar]
- 24. van Rossum AB, van Houwelingen HC, Kieft GJ, Pattynama PM. Prevalence of deep vein thrombosis in suspected and proven pulmonary embolism: a meta‐analysis. Br J Radiol 1998; 71:1260–1265. [DOI] [PubMed] [Google Scholar]
- 25. Ren B, Yan F, Deng Z, et al. extremely high incidence of lower extremity deep venous thrombosis in 48 patients with severe COVID‐19 in Wuhan. Circulation 2020; 142:181–183. [DOI] [PubMed] [Google Scholar]
- 26. Demelo‐Rodríguez P, Cervilla‐Muñoz E, Ordieres‐Ortega L, et al. Incidence of asymptomatic deep vein thrombosis in patients with COVID‐19 pneumonia and elevated d‐dimer levels. Thromb Res 2020; 192:23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cattaneo M, Bertinato EM, Birocchi S, et al. Pulmonary embolism or pulmonary thrombosis in COVID‐19? Is the recommendation to use high‐dose heparin for thromboprophylaxis justified? Thromb Haemost 2020; 120:1230–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee JS, Moon T, Kim TH, et al. Deep vein thrombosis in patients with pulmonary embolism: prevalence, clinical significance and outcome. Vasc Specialist Int 2016; 32:166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]


