Abstract
Immunosuppressed organ-transplanted patients are considered at risk for severe forms of COVID-19. Moreover, exaggerated innate and adaptive immune responses might be involved in severe progression of the disease. However, no data on the immune response to SARS-CoV-2 in transplanted patients are currently available. Here, we report the first assessment of antibody and T cell responses to SARS-CoV-2 in 11 kidney-transplanted patients recovered from RT-PCR–confirmed (n = 5) or initially suspected (n = 6) COVID-19. After reduction of immunosuppressive therapy, RT-PCR–confirmed COVID-19 transplant patients were able to mount vigorous antiviral T cell and antibody responses, as efficiently as two nontherapeutically immunosuppressed COVID-19 patients on hemodialysis. By contrast, six RT-PCR–negative patients displayed no antibody response. Among them, three showed very low numbers of SARS-CoV-2–reactive T cells, whereas no T cell response was detected in the other three, potentially ruling out COVID-19 diagnosis. Low levels of T cell reactivity to SARS-CoV-2 were also detected in seronegative healthy controls without known exposure to the virus. These results suggest that during COVID-19, monitoring both T cell and serological immunity might be helpful for the differential diagnosis of COVID-19 but are also needed to evaluate a potential role of antiviral T cells in the development of severe forms of the disease.
KEYWORDS: immunobiology, infection and infectious agents - viral, kidney transplantation / nephrology, monitoring: immune, translational research / science
Abbreviations: ALBIA, multiplex addressable laser bead immunoassay; COVID-19, coronavirus disease 2019; ELISPOT, enzyme-linked immunorsorbent spot assay; ICU, intensive care unit; RT-PCR, reverse transcription–polymerase chain reaction; SD, standard deviation; SFC, spot forming cell
1. INTRODUCTION
During the course of coronavirus disease 2019 (COVID-19), both the humoral and cellular arms of the adaptive immune system are required for viral clearance and resolution of the infection, as well as possibly for protection against a second SARS-CoV-2 infection.1 It has been suggested that exaggerated innate and adaptive immune responses might be involved in the severe progression of the disease occurring in 15% of cases, leading to severe distress respiratory syndrome and/or multiple organ failure.2 , 3 Immunosuppressed patients such as transplanted patients have been considered at risk for severe forms of the disease.4 Here, we report the first assessment of the cellular and humoral immune response to SARS-CoV-2 in 11 kidney-transplanted patients and two patients on hemodialysis awaiting a kidney transplant, recovering or recovered from a SARS-CoV-2 reverse transcription–polymerase chain reaction (RT-PCR)–confirmed (n = 5) or initially suspected (n = 6) COVID-19 infection. We show that after tapering of therapeutic immunosuppression, confirmed COVID-19 transplant patients were able to mount vigorous antiviral-specific T cell and antibody responses, as efficiently as patients on hemodialysis. By contrast, SARS-CoV-2–PCR-negative patients displayed no antibody response and no or very few specific T cells. Finally, low levels of T cell reactivity to SARS-CoV-2 antigens were detected in seronegative healthy controls with no known exposure to the virus during the study period.
2. METHODS
2.1. Subjects
All subjects were recruited between April 14, 2020 and May 28, 2020. Eleven kidney-transplanted patients were included in the study, including five patients diagnosed with SARS-CoV-2 RT-PCR–confirmed COVID-19, six patients suspected of COVID-19 based on suggestive symptomatology (n = 5) or typical pulmonary radiological imaging (n = 1), and two patients on hemodialysis awaiting a kidney transplant and diagnosed with RT-PCR–confirmed COVID-19. Blood samplings were performed close to or after their recovery, except in one patient still hospitalized for post-COVID-19 pulmonary functional impairment. In addition, 31 healthy donors were included in the study during the same period. None of them had a known exposure to SARS-CoV-2 during the epidemic and were not RT-PCR tested. All subjects provided informed and written consent.
2.2. SARS-CoV-2 serology
A multiplex addressable laser bead immunoassays (ALBIA) was designed for the detection of IgG and IgM targeting the S1 subunit of S protein as well as IgG specific for the N protein. Sensitivity of these assays was, respectively, >97%, 75%, and 100% at >13 days post–symptom onset (Drouot et al, manuscript in preparation). Specificity was ≥98% for all three parameters.
2.3. IFNγ enzyme-linked immunospot assay (ELISPOT)
Peripheral blood mononuclear cells were isolated by density gradient centrifugation of blood samples and used immediately. PBMCs (in concentrations adjusted to 2x105 CD3+ T cells per well) were plated in anti-IFNγ–coated Elispot 96-well plate in presence of overlapping 15-mer peptide pools spanning the sequence of SARS-CoV-2 structural and nonstructural proteins: S (pool S1 spanning the N-terminal part of the protein including the S1-subunit, and pool S2 spanning the C-terminal part), N, M, E, NS3A, NS7A, NS8, NS9B (JPT, Strassberg, Germany). Negative and positive control stimulation, medium only, and phytohemagglutinin, respectively, were included in the assay. After an overnight culture, cells were washed and captured IFNγ was revealed using a colorimetric assay (UCytech, Utrecht, The Netherlands). Spots were counted with an automated ELISPOT reader (AID, Strassberg, Germany). For each stimulation condition, the average spot number observed in wells without antigen was subtracted. Results were expressed as spot forming cells (SFC) per 106 CD3+ T cells. For each assay, a specific response was considered positive if SFC number was superior to 3 standard deviations of spot numbers observed in wells without antigens (ranging between 9 and 20 SFC/106 CD3+ T cells).
3. RESULTS
Between February and April 2020, six kidney-transplanted patients were diagnosed with COVID-19 based on a positive SARS-CoV-2 RT-PCR on a nasopharyngeal swab (n = 5, KTX1-5) or typical pulmonary radiological imaging (n = 1, KTX6; Table 1). All of them were hospitalized. Four (KTX1, 2, 3, and 5) experienced a severe course of the disease, requiring intensive care with mechanical ventilation in one case ( Table 2). Five of them presented with lymphopenia ranging 190 to 730 lymphocytes/mm3 ( Table 3). Immunosuppressive therapy was reduced in all six cases, with withdrawal of mycophenolate acid (n = 6), calcineurin inhibitors (n = 4), or belatacept (n = 1) (Table 2). Steroids were maintained at 20 mg per day in all patients including KTX6. All patients but one recovered and were discharged from hospital on 26 ± 12 (mean±standard deviation [SD]) days after symptom onset with reintroduction of the previous immunosuppressive regimen shortly before or on the day of hospital discharge (Table 2). A single patient (KTX3) was still hospitalized in intensive care unit (ICU) more than 80 days after symptom onset, due to post-COVID-19 pulmonary function impairment, without reintroduction of immunosuppressive therapy and no impact on kidney graft function to date. Five additional transplanted patients (KTX7-11) reported mild symptoms compatible with COVID-19. SARS-CoV-2 RT-PCR testing was negative in all of them. Only one displayed significant lymphopenia (KTX9). The patients who remained suspected of COVID-19 were cared for as confined outpatients. Their immunosuppressive therapy was unchanged and they recovered rapidly without complications. Two patients on hemodialysis awaiting a kidney transplant and followed in our center were also diagnosed with COVID-19 based on PCR testing. One of them was hospitalized for dyspnea that retroceded rapidly allowing for hospital discharge a week later.
TABLE 1.
Clinical characteristics of patients
| Patient | Age | Sex | Time from transplant | Comorbiditiesa | Symptoms at onsetb | Radiological pneumoniac | SARS-CoV–2 RT-PCR | Disease severity at diagnosisd |
|---|---|---|---|---|---|---|---|---|
| Kidney-transplanted patients | ||||||||
| KTX1 | 76 | M | 2013 | HBP | Dy, Co, Fe | + | + | Moderate |
| KTX2 | 32 | M | 2009 | HBP | Fe, Di, An/Ag | + | + | Moderate |
| KTX3 | 62 | F | 2017 | Diab, Card, Isch | Dy, Co, Fe | + | + | Severe |
| KTX4 | 36 | M | 2016 | HBP | Dy, Co, Fe | + | + | Moderate |
| KTX5 | 76 | M | 2016 | HBP, Card, Isch | Co, Fe | + | + | Severe |
| KTX6 | 62 | F | 2001 | Diab, HBP | Dy, Fe, | + | - | Moderate |
| KTX7 | 26 | F | 2018 | HBP | Co, Fe | nd | - | Mild |
| KTX8 | 36 | M | 2012 | HBP | An/Ag, Fe | nd | - | Mild |
| KTX9 | 57 | M | 2006 | HBP, Diab | Di, Fe | nd | - | Mild |
| KTX10 | 30 | F | 2016 | — | Fe, Di | nd | - | Mild |
| KTX11 | 70 | F | 1993 | HBP, Card, Isch | Fe, Dy, Co | nd | - | Mild |
| Patients on hemodialysis | ||||||||
| HD1 | 77 | M | - | HBP | Fe, Di | nd | + | Moderate |
| HD2 | 60 | M | - | HBP | Dy, Co, Fe, Di | + | + | Severe |
Abbreviations: HBP, high blood pressure; Diab, diabetes; Card, cardiopathy; Isch, previous ischemic episode; Dy, dyspnea; Co, cough; Fe, fever; Di, diarrhea; An/Ag, anosmia/ageusia; nd, not done.
Mild: no O2 therapy required; moderate: O2<5 L/mn; severe: O2≥5 L/mn.
TABLE 2.
Clinical management of patients
| Patient | Type of carea | Day of hospitalization post–symptom onset | Day of ICU transfer post–symptom onset | Type of O2 therapyb | Immuno suppression at onsetc | Immuno suppression level at diagnosisd | Immuno suppression taperinge | Immuno suppressants during COVID–19f | Day of immunosuppressive regimen resumptiong | Time of recovery post–symptom onset (days) | Sampling days post–symptom onseth |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kidney transplanted patients | |||||||||||
| KTX1 | ICU | 4 | 5 | HFNC | Tac, MPA, Cs | Tac <2.2i MPA 500 mg x2 |
Tac↕, MPA↕ | Tac <2.2 Cs 20 mg |
24 (Tac MPA Cs 7.5 mg) | 24 | 19E–38S–60E,S |
| KTX2 | ICU | 10 | 16 | HFNC | Tac, MPA, Cs | Tac 8 MPA 750 mg x2 |
Tac↕, MPA↕ | dy 13 Tac 4.4 dy 16 Tac<2.2 Cs 20 mg |
37 (Tac MPA Cs 10 mg) | 37 | 31E–53E,S |
| KTX3 | ICU | 10 | 10 | MV | Bel, MPA, Cs | Bel day –27 MPA 500 mg x2 |
Bel↕, MPA↕ | Cs 20 mg | - (Cs 20 mg) | >80j | 26E,S |
| KTX4 | Hosp | 7 | — | Conv | Tac, MPA, Cs | Tac 4.7 MPA 750 mg x 2 |
MPA↕ | Tac 4 Cs 20 mg |
12 (Tac MPA Cs 7.5 mg) | 12 | 42E,S |
| KTX5 | ICU | 14 | 14 | HFNC | Tac, MPA, Cs | Tac 6.1 MPA 500 mg x2 |
MPA↕ | Tac 4 Cs 20 mg |
40 (Tac MPA Cs 7.5 mg) | 40 | 39E,S–56E,S |
| KTX6 | Hosp | 12 | — | Conv | CsA, MPA | CsA 78 MPA 500 mg x 2 |
Csa↕, MPA↕ | Cs 20 mg | 25 (CsA MPA Cs 5 mg) | 29 | 26E,S |
| KTX7 | Outpatient | — | — | — | Tac, MPA, Cs | Tac 6.6 MPA 500 mg x2 |
— | — | — | — | 48E,S |
| KTX8 | Outpatient | — | — | — | Bel, MPA, Cs | Bel day 0 MPA 500 mg x2 |
— | — | — | — | 37E,S |
| KTX9 | Outpatient | — | — | — | Bel, MPA, Cs | Bel day –5 MPA 500 mg x2 |
— | — | — | — | 37E,S |
| KTX10 | Outpatient | — | — | — | Tac, MPA | Tac 9 MPA 750 mg x2 |
— | — | — | — | 40E,S |
| KTX11 | Outpatient | — | — | — | Sir, Cs | Sir 10 | — | — | — | — | 49E,S |
| Patients on hemodialysis | |||||||||||
| HD1 | Outpatient | — | — | — | — | — | – | — | — | — | 21E,S–49S |
| HD2 | Hosp | 4 | — | Conv | — | — | – | — | — | 16 | 17E |
ICU: intensive care unit; Hosp: patient hospitalized in conventional medical unit.
Conv: conventional oxygen therapy; HFNC: high-flow nasal canula; MV: mechanical ventilation.
Tac: tacrolimus; CsA: cyclosporine A; MPA: mycophenolic acid or mycophenolate mofetil; Cs: corticosteroids; Bel: belatacept. Sir: sirolimus.
Trough levels of tacrolimus (Tac), cyclosporine A (CsA), or sirolimus (Sir) in ng/ml, day of the last belatacept (Bel) infusion relative to symptoms onset, and mycophenolic acid or mycophenolate mofetil (MPA) dosage per day.
↕: withdrawal.
Trough levels of tacrolimus on the indicated day relative to symptoms onset and dosage of corticosteroids (Cs).
Immunosuppressive regimen at resumption in brackets. Immunosuppressants were not re-administered in patient KTX3.
The type of immunological assay performed at each sampling date is indicated as E: ELISPOT, S: serology. In bold, sampling done under or after reintroduction of a full immunosuppressive regimen.
Low Tac trough levels maintained prior to and after COVID-19 due to Kaposi sarcoma in this patient.
Patient still hospitalized in ICU due to post-COVID-19 pulmonary function impairment.
TABLE 3.
Lymphocytes and T cell counts at COVID-19 diagnosis and during follow-up
| Patienta | Lymphocyte counts at diagnosis (N: 1200-4000/mm3) | Day of ELISPOT testing post–symptom onsetb | Lymphocytes counts at ELISPOT testing (/mm3) (N: 1200-4000/mm3) | Total T cells at ELISPOT testing |
SARS-CoV–2- reactive T cellsc |
||||
|---|---|---|---|---|---|---|---|---|---|
| %CD3 (N: 56%–84%) | CD3 (N: 1400 –3300/mm3) | CD4 (N: 1000 - 2200/mm3) | CD8 (N: 330 - 920/mm3) | /mm3 | % or ‰ of CD3+ T cells | ||||
| Kidney transplanted patients | |||||||||
| KTX1 | 380 | 19 60 |
960 1743 |
76.7% 86.7% |
736 1512 |
304 577 |
426 1002 |
2.5 3.9 |
0.33% 0.26% |
| KTX2 | 730 | 31 53 |
3672 2977 |
84.8% 84.6% |
3114 2519 |
2014 1595 |
1041 897 |
21.1 7.4 |
0.68% 0.29% |
| KTX3 | 530 | 26 | 601 | 80.2% | 482 | 266 | 203 | 5.9 | 0.25% |
| KTX4 | 1010 | 42 | 1681 | 80.6% | 1355 | 848 | 452 | 1.5 | 0.11% |
| KTX5 | 190 | 39 56 |
549 725 |
79.2% 81.0% |
435 587 |
156 221 |
267 326 |
0.4 0.3 |
0.08% 0.07% |
| KTX6 | 1080 | 50 | 1099 | 61.7% | 678 | 412 | 258 | 0.0 | 0.0% |
| KTX7 | 1300 | 48 | 1297 | 74.3% | 964 | 481 | 358 | 0.07 | 0.08‰ |
| KTX8 | 1310 | 37 | 780 | 91.0% | 710 | 231 | 449 | 0.00 | 0.0% |
| KTX9 | 590 | 37 | 1344 | 66.1% | 888 | 618 | 249 | 0.02 | 0.02‰ |
| KTX10 | 2230 | 40 | 1789 | 67.4% | 1206 | 836 | 353 | 0.03 | 0.03‰ |
| KTX11 | 1040 | 49 | 1488 | 84.9% | 1263 | 499 | 715 | 0.00 | 0.0% |
| Patients on hemodialysis | |||||||||
| HD1 | 260 | 21 | 955 | 71.5% | 683 | 347 | 331 | 3.1 | 0.45% |
| HD2 | 570 | 17 | 777 | 69.6% | 541 | 358 | 167 | 4.5 | 0.83% |
Abbreviation: N, normal values.
Patients with confirmed COVID-19 (RT-PCR positive) are highlighted in bold.
Two values are shown for patients KTX1, KTX2, and KTX5 who were tested twice.
The frequency of SARS-CoV-2–reactive T cells, as detected by ELISPOT assay, is expressed either as counts per mm3 of peripheral blood or as proportions (% or ‰) of total CD3+ T cells.
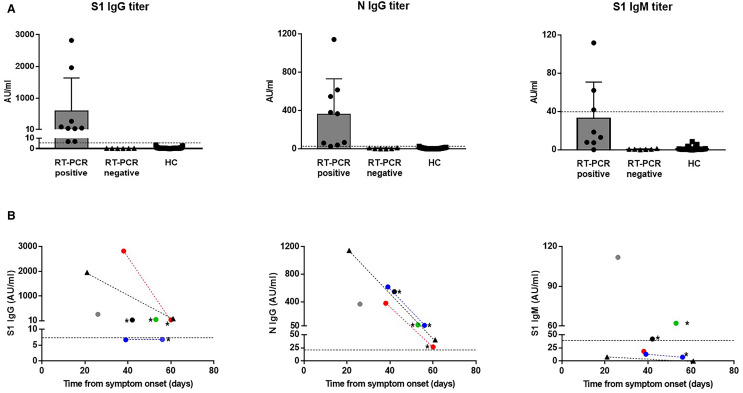
We assessed the immune response to SARS-CoV-2 in these 13 patients (seven RT-PCR positive and six RT-PCR-negative) close to and/or after their recovery, except in one patient (KTX3) who was still in the ICU (Table 2). Using a multiplex ALBIA serological assay, we found that all seven patients with RT-PCR–confirmed COVID-19 displayed SARS-CoV-2 antibodies ( Figure 1A) 21 to 42 days after symptom onset (mean ± SD: 33 ± 8 days): IgG reactive to the N protein was detected in all seven cases; IgG reactive to the S1 subunit was detected in five cases while S1 IgM was found in only three patients. In three cases (KTX1, KTX5, and HD1), testing was repeated 17 to 28 days later showing a decrease in anti-S1 (n = 2) and anti-N (n = 3) IgG titers (Figure 1B). There was a trend toward a negative correlation between N IgG titer and time from symptom onset. In the six transplanted patients with negative RT-PCR, no SARS-CoV-2–specific antibodies could be detected, thus potentially ruling out COVID-19 diagnosis. None of the 31 healthy controls, except one, showed significant titers of SARS-CoV-2 antibodies.
FIGURE 1.
SARS-CoV-2 antibody response in SARS-CoV-2 RT-PCR–positive or RT-PCR–negative patients. A, Titers of S1 IgG, N IgG, and S1 IgM are shown in the first samplings of six patients with RT-PCR–positive COVID-19 (KTX1, 2, 3, 4, 5, and HD1), six RT-PCR–negative patients (KTX6, 7, 8, 9, 10, and 11), and healthy controls (HC). B, Relation between SARS-CoV-2 antibody titers and time of sampling relative to symptom onset in RT-PCR–positive patients. Triangles: patients on hemodialysis (HD1: black); circles: kidney-transplanted patients (KTX1: red, KTX2: green, KTX3: white, KTX4: black, KTX5: blue). Three patients (KTX1, KTX5, and HD1) were tested twice. Samples from the same patient are connected through dotted lines. * Test performed after reintroduction of a full immunosuppressive regimen [Color figure can be viewed at wileyonlinelibrary.com]
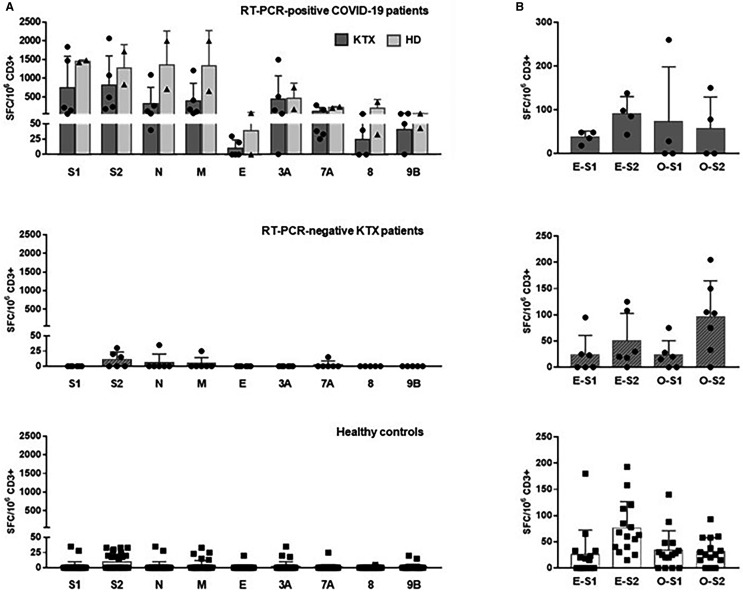
We then evaluated the T cell response to nine structural and nonstructural SARS-CoV-2 peptide pools using an IFNγ ELISPOT assay ( Figure 2A). All seven PCR-confirmed COVID-19 patients, transplanted or on hemodialysis, displayed IFNγ-producing T cells reactive to at least six of the nine peptide pools, 17 to 42 days post–symptom onset (mean ± SD: 28 ± 10 days). Total numbers of SARS-CoV-2–reactive T cells were high (mean ± SD: 3911 ± 2837 SFC/106 CD3+ T cells; Table 3). Responses to S (pool S1 and S2), N, M, and accessory protein ORF3A were dominant (mean ± SD: 948 ± 770; 946 ± 727; 616 ± 725, 663 ± 713; and 449 ± 536 SFC/106 CD3+ T cells for S1, S2, N, M, and ORF3A, respectively). T cells reactive to structural protein E and accessory proteins ORF7A, ORF8, and ORF9B were less numerous (mean ± SD: 18 ± 29; 140 ± 107; 84 ± 142; and 45 ± 23 SFC/106 CD3+ T cells, respectively). Overall, SARS-CoV-2–reactive T cells represented on average 0.4% ± 0.3% of total CD3+ T cells or, taking into account CD3+ T cell counts, moderately low in six of seven patients, 5.0 ± 7.3 specific cells/mm3 (Table 3). The level of responses did not significantly differ between transplanted patients and those on hemodialysis. No correlation between time since symptom onset and number of T cells reactive was evidenced (Figure S1). However, in KTX1, the number of T cells reactive to S1, S2, N, and M decreased significantly (at least 2 fold) when tested 22 days later, after hospital discharge and reintroduction of a full immunosuppressive regimen (Figure S1).
FIGURE 2.
SARS-CoV-2–reactive IFNγ-producing T cells in SARS-CoV-2 RT-PCR–positive or RT-PCR–negative patients. A, Numbers of T cells (expressed as SFC/106 CD3+ T cells) reactive to nine overlapping peptide pools spanning SARS-CoV-2 structural proteins S (pool S1 and S2), N, M, E, and accessory proteins ORF3A, 7A, 8, and 9B in transplanted patients (KTX, dark gray bars) and patients on hemodialysis (HD, light gray bars) with SARS-CoV-2–positive RT-PCR (upper panel), in SARS-CoV-2 RT-PCR–negative transplanted patients (hatched gray bars, middle panel) and 31 healthy controls (white bars, lower panel). In patients with several samplings, only the first ones are plotted. B, Numbers of T cells reactive to S1 and S2 peptide pools representative of the S protein of HCoV-229E (E-S1 and E-S2) and HCoV-OC43 (O-S1 and O-S2) in four RT-PCR–positive transplanted patients, six RT-PCR negative transplanted patients, and 15 healthy controls
By contrast, among patients with negative SARS-CoV-2 RT-PCR (KTX-6-11) suspected of COVID-19, three did not show any SARS-CoV-2–reactive T cells, including patient KTX6 who displayed pulmonary radiological imaging strongly suggestive of SARS-CoV-2 pneumonia. One patient (KTX7) displayed low numbers (≤35 SFC/106 CD3+ T cells) of T cells producing IFNγ in response to S2, N, and M while two others (KTX9 and KTX10) showed a weak response to S2 only (≤30 SFC/106 CD3+ T cells). Similar reactivity profiles were observed among healthy controls with no known exposure to SARS-CoV-2: few T cells (≤35 SFC/106 CD3+ T cells) reactive to S2 only, S2 plus one or two other proteins or a single non-S2 structural or accessory protein were detected in 29.0%, 12.9%, and 29.0% of the cases, respectively. Of note, in the single healthy donor with a low titer of anti-N IgG, no SARS-CoV-2 T cell reactivity was detected. Overall, SARS-CoV-2 RT-PCR–negative patients suspected of COVID-19 and healthy controls showed similar low total number of T cells reactive to three or less SARS-CoV-2 peptide pools (mean ± SD: 21 ± 29 and 21 ± 19 SFC/106 CD3+ T cells, respectively, P > .05). To explore a possible T cell cross-reactivity toward common coronavirus and SARS-CoV-2, we evaluated the response to the S protein of HCoV-229E and HCoV-OC43, two endemic benign coronaviruses, in 15 of the 31 healthy controls and 10 of the 11 transplanted patients. T cells reactive to HCoV-229E and/or HCoV-OC43 were detected in these 15 healthy controls as well as in all transplant recipients with positive (n = 4) or negative (n = 6) SARS-CoV-2 RT-PCR (Figure 2B). Similar numbers of T cells reactive to HCoV-229E or HCoV-OC43 (S1 plus S2 pools) were observed in these three groups of patients (mean ± SD; HCoV-229E: 128 ± 29, 74 ± 80, and 102 ± 70 SFC/106 CD3+ T cells in RT-PCR–positive COVID-19 patients, RT-PCR–negative patients, and healthy controls, respectively; HCoV-OC43: 175 ± 170, 135 ± 71 SFC, and 65 ± 54 SFC/106 CD3+ T cells, respectively). A trend for a positive correlation between numbers of T cells reactive to SARS-CoV-2 and HCoV-OC43 S2 pools was observed in seronegative healthy controls and transplanted patients (n = 21, r = .4394, P = .0463), that was not observed for HCoV-229E (n = 21, r = .3641, P = .1047).
4. DISCUSSION
We present here the first report of assessment of the humoral and cellular immune response to SARS-CoV-2 in kidney transplant recipients recovering or recovered from COVID-19 after reduction of immunosuppressive therapy. In patients with RT-PCR–confirmed COVID-19, transplanted or on hemodialysis, significant titers of anti-S1 and anti-N IgGs were observed from day 26 to day 53 following symptom onset. Anti-N IgGs were detected in all six tested patients and anti-S1 IgGs in five of them. Anti-S1 IgMs were found in three patients only. These results are in line with several reports showing that anti-SARS-CoV-2 IgG antibodies can be first detected between 7 and 14 days after symptom onset and that they persist following virus clearance.5 Surprisingly, we found in two recovered transplanted patients (severe disease course) and one patient on hemodialysis (moderate severity), who could be tested again 2 to 4 weeks after the first sampling, a sharp decrease in anti-S1 or anti-N IgG titers that still remained detectable. Based on the knowledge regarding the kinetics of the immune response to SARS-CoV-1 and MERS-CoV, it is expected that antibody responses to SARS-CoV-2 might wane slowly and persist up to 60 weeks after symptom onset.5, 6, 7 In a recent retrospective study in COVID-19 kidney-transplanted patients in whom immunosuppressive regimen was withdrawn or reduced, IgG antibodies to SARS-CoV-2 N and S proteins were maintained at stable titers up to 59 days following infection, independently of disease severity.8 However, a rapid decay of IgGs targeting the spike receptor-binding domain (RBD) was recently shown in 34 non immunosuppressed patients recovered from mild COVID-19 between 37 days and 86 days after symptoms onset, corresponding to an approximate half-life of 36 days.9 Moreover, such decline in antibody titers was also reported among 65 patients sequentially analyzed up to 90 days after disease onset.10 Interestingly, the severity of the disease impacted the magnitude of the neutralizing antibody response but not its kinetics. Further studies are needed to confirm the fast decrease in anti-SARS-CoV-2 antibody titers found in these three patients and determine if such kinetics are generally observed in immunosuppressed patients and/or dependent upon the severity of the disease.
Patients with RT-PCR–confirmed COVID-19 also displayed high numbers of T cells reactive to at least six SARS-CoV-2 structural and accessory protein-derived peptide pools. S (S1 and S2 peptide pools), N, and M structural proteins were clearly immunodominant, evoking T cell responses in all seven tested patients. T cell reactivity toward ORF3A and ORF7A was also significant. These results, reflecting the reactivity of both CD4+ and CD8+ T cells to 15-mer peptide pools, are in accordance with recent findings in nonimmunosuppressed patients recovered from COVID-19.11, 12, 13, 14 Indeed, Grifoni et al., using a flow cytometry-based T cell receptor-dependent activation marker (AIM) assay in patients with mild disease, described the coimmunodominance of S, M, and N proteins for CD4+ T cells with significant reactivity toward ORF3, ORF7A, and ORF8.13 CD8+ T cells targeted both structural and accessory proteins with no clear dominance. Frequencies of total SARS-CoV-2–reactive T cells were in the same range identified (up to 2% of CD4+ and 1% of CD8+ T cells) as those found in our study (ranging from 0.08% to 0.83% of CD3+ T cells) and those commonly detected for CMV-specific T cells, particularly in transplanted patients.13 , 15 , 16 Importantly, in our study, the five transplanted patients, in whom the immunosuppressive regimen was systematically reduced at diagnosis, showed similar amplitude and reactivity profile of SARS-CoV-2 T cell response than the two patients on hemodialysis. We did not observe a significant correlation between SARS-CoV-2–reactive T cell numbers and antibody titers in patients with confirmed COVID-19 (data not shown). Noteworthily, the two patients (KTX1 and KTX5) in whom a sharp decrease in SARS-CoV-2 antibody titers (S1 and/or N IgGs) was observed between two samplings (day 38-60 and day 39-56, respectively) did not show any significant change in their S- or N-specific T cell numbers. This may reflect differential kinetics of the humoral and cellular responses, with a faster decline in antibody titers9 , 10 as compared to the rate of contraction of T cell numbers that can be expected following viral clearance.
The role of numerous and diverse anti-SARS-CoV-2 T cell responses, such as those evidenced here, in the severity of the disease could not be addressed due to the limited size of our study. Such T cell response may well contribute to severe progression of the disease through cytokine production and tissular immunopathology. A recent study found however no significant difference in the numbers of IFNγ-producing T cells in response to S, M, or N peptide pools between patients convalescent from mild or severe disease.17 In our study, the frequencies of SARS-CoV-2–specific T cells were in fact in the same range as those observed in convalescent patients from predominantly mild13 , 18 or severe COVID-19.11 Interestingly, Kroemer et al. observed higher numbers of IFNγ-producing T cells reactive to N peptide pool in patients with mild illness as compared to patients with severe pneumonia, 21 to 53 days following symptoms onset.19 Such preliminary observations in convalescent individuals suggest that the amplitude of the T cell response may not directly impact disease severity. However, precise kinetics of peripheral and tissular SARS-CoV-2–specific T cell expansion and function over the course of the disease are needed to directly address this important issue.
Six transplanted patients showing symptoms compatible with COVID-19 were also included in the study. All of them were SARS-CoV-2 RT-PCR negative but were suspected of the disease based on the knowledge of an approximate 30% rate of false negativity of PCR testing20 or, in one patient (KTX6), on typical pulmonary radiological imaging. However, all of them displayed a negative SARS-CoV-2 serology and none of them showed significantly higher frequency of SARS-CoV-2 reactive T cells as compared to healthy controls without known exposure to the virus. Thus, COVID-19 can most likely be ruled out at least in the three transplanted patients in whom no SARS-CoV-2 T cells could be detected, including KTX6 who underwent the withdrawal of immunosuppressants. However, in the case of the three patients who showed detectable low numbers of SARS-CoV-2–reactive T cells and remained under full immunosuppressive therapy, we cannot exclude that they might have been exposed to the virus and, despite their inability to mount an antibody response, protected from a severe form of the disease through minimal T cell immunity. Development of antiviral T cells in absence of a concomitant antibody response has indeed been described in other contexts such as subclinical HCV infection21 or even, as recently reported, during intrafamilial exposure to SARS-CoV-2 in subjects reporting mild symptoms or even asymptomatic.17 , 22 However, in the latter situations, SARS-CoV-2–reactive T cells were found generally at higher frequencies17 , 22 than those observed in the seronegative transplanted patients from our center. Nevertheless, our results suggest that assessment of both humoral and cellular immunity may be useful for the differential diagnosis of COVID-19, after resolution of the infection and also possibly early during its course.
Due to the small number of transplanted patients, the lack of COVID-19 nontransplanted patients and the cross-sectional nature of our study, the impact of therapeutic immunosuppression on the amplitude of anti-SARS-CoV-2 T cell responses and the course of the disease could not be evaluated. Noteworthily, four of the five COVID-19 transplanted patients presented with a severe form of the disease, immediately at diagnosis before reduction of immunosuppressive therapy (KTX3 and KTX5), or 1 (KTX1) to 6 days (KTX2) following hospitalization and immunosuppressant withdrawal. Such picture suggests that immunosuppressed transplant recipients are at risk for severe forms of COVID-19, which is in accordance with reports from other transplantation centers.4 , 23 , 24 However, the respective role of comorbidities and therapeutic immunosuppression on disease progression remains to be clarified.
All of the 31 healthy controls with no known exposure to the virus were seronegative except one who showed a low titer of anti-N IgG, close to the positivity threshold of the assay and no specific T cell response. Low levels of T cell responses to SARS-CoV-2 proteins were observed in 71% of the donors, ranging from 0.01‰ to 0.08‰ of total CD3+ T cells, as compared to 0.08% to 0.83% in COVID-19 patients. The C-terminal part of the S protein (S2 peptide pool) was targeted in 41.9% of the cases. These results are in accordance with other reports showing the presence of SARS-CoV-2–reactive T cells in seronegative healthy subjects.11, 12, 13, 14 , 18 Using a cytometry-based assay, low frequencies of CD4+ T cells, responding almost exclusively to the same S2 peptide pool as the one used in our study, were found in 34% of seronegative healthy donors (n = 68).12 Grifoni et al and Weiskopf et al also showed, using similar cytometry-based assays, that CD4+, and CD8+ T cell responses were detected in 40-60% of unexposed subjects before the pandemic.11 , 13 , 18 Cross-reactivity with endemic HCoV (229E, OC43, HKU1, and NL63), estimated to account for 20% of benign upper respiratory tract infections, is currently proposed to explain this SARS-CoV-2 T cell reactivity in unexposed individuals. In fact, some level of homology exists between SARS-CoV-2 and several HCoV proteins, although relatively low within the C-terminal part of the S protein (ie, 34.6% and 39.7% identity between SARS-CoV-2 and HCoV-229E or HCoV-OC43, respectively, in the region spanned by the respective S2 peptide pools).12 Such cross-reactivity was recently demonstrated by Mateus et al, who derived from unexposed donors 42 CD4+ T cell lines, specific for various SARS-CoV-2 epitopes (derived from the C-terminal end of S, or from N and nonstructural proteins) and showed that 24% of them were in fact also reactive to analogous epitopes from HCoVs, that in some cases were better antigens than the corresponding SARS-CoV-2 peptides.25 Such results support the speculation that preexisting cross-reactive T cells to HCoVs could impact infection outcome in case of exposure to SARS-CoV-2, and potentially afford some level of protection as observed for influenza virus H1N1. However, as in RT-PCR–negative transplanted patients, we cannot rule out that in some of our healthy controls who were recruited during the pandemic, low numbers of SARS-CoV-2–specific T cells may have been induced through exposure to SARS-CoV-2 in absence of overt symptomatology and detectable antibody responses, as described in SARS-CoV-2–exposed family members and 2020 blood donors.17
Overall, we show that following reduction of immunosuppressive therapy, kidney-transplanted patients with severe COVID-19 are able to mount vigorous T cell and antibody responses to SARS-CoV-2, that are detectable up to 60 days following symptom onset. The ability of fully immunosuppressed transplanted patients to mount T cell and antibody responses to SARS-CoV-2 remains however to be determined. Assessment of T cell immunity together with humoral immunity may be helpful for differential diagnosis of COVID-19 in case of negative SARS-CoV-2 RT-PCR testing. In addition, low levels of T cell reactivity to SARS-CoV-2 can be observed in seronegative patients and healthy controls, that may arise from T cell cross-reactivity to endemic HCoVs or alternatively from unknown exposure to SARS-CoV-2. Further longitudinal studies will be necessary to determine the early and long-term kinetics of T cell and antibody responses as well as the role of T cells in the progression of the disease.
ACKNOWLEDGMENTS
The authors wish to thank the health-care professionals of the University hospital of Rouen who were involved in the care of the patients, including those from the department of Infectious and Tropical Diseases (Pr Manuel Etienne, Pr François Caron), the department of Virology (Pr Jean-Christophe Plantier) and the Intensive Care Units (Pr Benoit Veber, Pr Fabienne Tamion). The authors also are thankful to Juliane Bigand for her technical help.
DISCLOSURE
The authors of this manuscript have conflicts of interests to disclose as described by the American Journal of Transplantation. The authors (Olivier Boyer and Laurent Drouot) were designated as inventors for the European Patent application EP 20315157.6 filed on April 14, 2020 in the names of Inserm, Université de Rouen and CHU de Rouen and entitled “METHODS FOR DETECTING THE PRESENCE OF CORONAVIRUS-SPECIFIC ANTIBODIES IN A SUBJECT.” The other authors have no conflict of interest to disclose related to the work reported here.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Shi Y, Wang Y, Shao C, et al. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27(5):1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore. JAMA. 2020. [DOI] [PMC free article] [PubMed]
- 4.Coates PT, Wong G, Drueke T, Rovin B, Ronco P. Associate Editors ftEET. Early experience with COVID-19 in kidney transplantation. Kidney Int. 2020;97(6):1074–1075. doi: 10.1016/j.kint.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vabret N, Britton GJ, Gruber C, et al. Immunology of COVID-19: Current State of the Science. Immunity. 2020;52(6):910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu WJ, Zhao M, Liu K, et al. T-cell immunity of SARS-CoV: Implications for vaccine development against MERS-CoV. Antiviral Res. 2017;137:82–92. doi: 10.1016/j.antiviral.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang AT, Garcia-Carreras B, Hitchings MDT, et al. A systematic review of antibody mediated immunity to coronaviruses: antibody kinetics, correlates of protection, and association of antibody responses with severity of disease. medRxiv. 2020. [DOI] [PMC free article] [PubMed]
- 8.Benotmane I, Gautier Vargas G, Wendling MJ, et al. In-depth virological assessment of kidney transplant recipients with COVID-19. Am J Transplant. 2020. [DOI] [PMC free article] [PubMed]
- 9.Ibarrondo FJ, Fulcher JA, Goodman-Meza D, et al. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild Covid-19. N Engl J Med. 2020. [DOI] [PMC free article] [PubMed]
- 10.Seow J, Graham C, Merrick B, et al. Longitudinal evaluation and decline of antibody responses in SARS-CoV-2 infection. medRxiv. 2020:2020.2007.2009.20148429.
- 11.Weiskopf D, Schmitz KS, Raadsen MP, et al. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci Immunol. 2020;5(48) doi: 10.1126/sciimmunol.abd2071. 2020.2004.2011.20062349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braun J, Loyal L, Frentsch M, et al. Presence of SARS-CoV-2 reactive T cells in COVID-19 patients and healthy donors. medRxiv. 2020:2020.2004.2017.20061440.
- 13.Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181(7):1489–1501. doi: 10.1016/j.cell.2020.05.015. e1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ni L, Ye F, Cheng ML, et al. Detection of SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity. 2020;52(6):971–977. doi: 10.1016/j.immuni.2020.04.023. e973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bererhi L, Pallet N, Zuber J, et al. Clinical and immunological features of very long-term survivors with a single renal transplant. Transpl Int. 2012;25(5):545–554. doi: 10.1111/j.1432-2277.2012.01451.x. [DOI] [PubMed] [Google Scholar]
- 16.Lucia M, Crespo E, Melilli E, et al. Preformed frequencies of cytomegalovirus (CMV)-specific memory T and B cells identify protected CMV-sensitized individuals among seronegative kidney transplant recipients. Clin Infect Dis. 2014;59(11):1537–1545. doi: 10.1093/cid/ciu589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sekine T, Perez-Potti A, Rivera-Ballesteros O, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020. [DOI] [PMC free article] [PubMed]
- 18.Le Bert N, Tan AT, Kunasegaran K, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584(7821):457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- 19.Kroemer M, Spehner L, Vettoretti L, et al. COVID-19 patients display distinct SARS-CoV-2 specific T-cell responses according to disease severity. J Infect. 2020. [DOI] [PMC free article] [PubMed]
- 20.Arnaout R, Lee RA, Lee GR, et al. SARS-CoV2 Testing. The Limit of Detection Matters. bioRxiv. 2020.
- 21.Freeman AJ, Ffrench RA, Post JJ, et al. Prevalence of production of virus-specific interferon-gamma among seronegative hepatitis C-resistant subjects reporting injection drug use. J Infect Dis. 2004;190(6):1093–1097. doi: 10.1086/422605. [DOI] [PubMed] [Google Scholar]
- 22.Gallais F, Velay A, Wendling M-J, et al. Intrafamilial exposure to SARS-CoV-2 induces cellular immune response without seroconversion. medRxiv. 2020:2020.2006.2021.20132449. [DOI] [PMC free article] [PubMed]
- 23.Banerjee D, Popoola J, Shah S, Ster IC, Quan V, Phanish M. COVID-19 infection in kidney transplant recipients. Kidney Int. 2020;97(6):1076–1082. doi: 10.1016/j.kint.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant. 2020;20(7):1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mateus J, Grifoni A, Tarke A, et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science. 2020. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.