Abstract
Liver injury is commonly seen in coronavirus disease 2019 (COVID‐19); however, the mechanism behind liver injury, particularly in patients with severe and critical COVID‐19, remains unclear, and the clinical course is poorly described. We conducted a single‐center retrospective cohort study of consecutive patients hospitalized with severe and critical COVID‐19 with or without liver injury and who underwent immunologic testing (interleukin [IL]‐6, IL‐8, tumor necrosis factor alpha [TNF‐α], and IL‐1β). Liver injury was defined as peak aminotransferases ≥3 times the upper limit of normal (40 U/L) or ≥120 U/L. Patients with liver injury were compared to those who had normal aminotransferases throughout the hospital course. We studied 176 patients: 109 with liver injury and 67 controls. Patients with liver injury were more likely to be men (71.6% vs. 37.3%, P < 0.001). Peak inflammatory markers and IL‐6 were higher in the liver injury group: C‐reactive protein (CRP), 247 vs. 168 mg/L, P < 0.001; lactate dehydrogenase (LDH), 706 vs. 421 U/L; ferritin, 2,973 vs. 751 ng/mL, P < 0.001; IL‐6, 121.0 vs. 71.8 pg/mL, P < 0.001. There was no difference in the levels of IL‐8, TNF‐α, and IL‐1β. The liver injury group had a longer length of stay in the hospital and more severe COVID‐19 despite having less diabetes and chronic kidney disease. Conclusion: An exaggerated hyperinflammatory response (cytokine storm) characterized by significantly elevated CRP, LDH, ferritin, and IL‐6 levels and increasing severity of COVID‐19 appears to be associated with the occurrence of liver injury in patients with severe/critical COVID‐19.
Abbreviations
- AKI
acute kidney injury
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CLD
chronic liver disease
- COVID‐19
coronavirus disease 2019
- CRP
C‐reactive protein
- CRS
cytokine release syndrome
- DILI
drug‐induced liver injury
- ICU
intensive care unit
- IL
interleukin
- INR
international normalized ratio
- IQR
interquartile range
- LDH
lactate dehydrogenase
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- SARS‐CoV
severe acute respiratory syndrome coronavirus
- TNF
tumor necrosis factor
- ULN
upper limit of normal
Coronavirus disease 2019 (COVID‐19), caused by the novel coronavirus severe acute respiratory syndrome coronavirus 2 (SARS‐CoV2), has evolved into a global pandemic since it began in Wuhan, China, in the fall of 2019.( 1 ) COVID‐19 usually presents with fever and/or upper and/or lower respiratory symptoms, which may then develop into respiratory failure, septic shock, and multiorgan failure.( 2 ) Liver enzymes are elevated in 14% to 53% of patients hospitalized with COVID‐19.( 3 ) Although the degree of elevation is usually mild, severe elevations in aminotransferase levels have been reported.( 3 ) Liver enzyme elevation is important because elevations in liver enzymes may be associated with an increased risk of mortality.( 4 )
The exact mechanism driving COVID‐19‐related liver injury remains unclear, and several mechanisms that could co‐occur have been postulated. SARS‐CoV2 may cause liver injury by direct viral toxicity similar to the first SARS‐CoV in 2005.( 5 , 6 , 7 ) A recent case reported that abundant SARS‐CoV2 viral particles were observed in the cytoplasm of hepatocytes, with marked mitochondrial swelling and massive hepatocyte apoptosis in 2 patients with COVID‐19‐related liver injury.( 7 ) Liver injury may also occur as a result of liver ischemia due to hypoxia related to COVID‐19 pneumonia, with microthrombi formation or hypotension necessitating vasoconstrictors.( 8 ) This may be supported by the higher rates of aspartate aminotransferase (AST) over alanine aminotransferase (ALT) elevations seen in COVID‐19‐related liver injury, which is consistent with zone 3 necrosis and ischemic liver injury.( 9 ) A final possibility is that COVID‐19 liver injury may result from the body’s hyperinflammatory response to COVID‐19. This could be in the form of general immune activation caused by the release of circulating cytokines similar to many systemic viral infections in which the liver suffers from “bystander hepatitis.”( 10 , 11 , 12 ) In turn, this response may progress into a phenomenon akin to a “cytokine storm” characterized by an exaggerated and aberrant inflammatory response with severe systemic impact, including extremely high levels of inflammatory markers, such as ferritin and C‐reactive protein (CRP), fevers, and cytopenias, with some patients presenting with secondary hemophagocytic lymphohistiocytosis (sHLH).( 13 , 14 ) Interleukin (IL)‐1β, IL‐2, IL‐6, IL‐8, tumor necrosis factor alpha (TNF‐α), and granulocyte colony‐stimulating factor are among the inflammatory cytokines that are hypothesized to be some of the key drivers of severe COVID‐19 with a cytokine storm.( 15 , 16 ) Pathologic studies, including autopsy evaluation from our own institution, have failed to identify a unifying pathologic diagnosis in patients with COVID‐19 and liver injury based on histologic evaluation of liver specimens from patients with COVID‐19.( 17 ) This lack of clarity may be because of multiple potential mechanisms of liver injury in patients with COVID‐19, such as drug‐induced liver injury (DILI) “muddling” the picture. DILI can be seen with various antiviral/antibiotics that may be commonly given to patients with COVID‐19.( 18 )
In this study, we describe the clinical findings of patients hospitalized with and without COVID‐19‐related liver injury and attempt to clarify the associated findings underlying COVID‐19‐related liver injury in severe or critical COVID‐19. Specifically, we sought to characterize 1) the hyperinflammatory response, 2) the specific inflammatory cytokine profile, and 3) the clinical characteristics of patients hospitalized with COVID‐19‐related liver injury compared to those who never develop liver injury.
Patients and Methods
Study Design and Patient Population
We conducted a retrospective observational study that included consecutive adult patients (>18 years old) diagnosed with severe or critical COVID‐19, with or without evidence of liver injury, who underwent testing for cytokine release syndrome (CRS) blood markers (IL‐6, IL‐8, TNF‐α, and/or IL‐1β) and who were admitted to Mount Sinai Hospital in New York City, NY, from March 20, 2020, through April 22, 2020 (Fig. 1). Testing for CRS markers was done at the discretion of the individual physician.
FIG. 1.
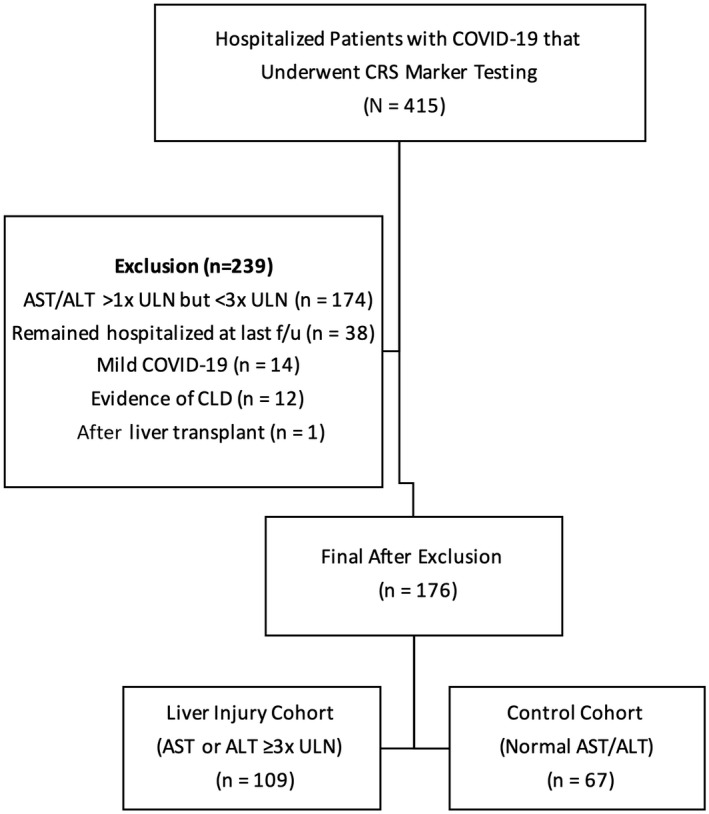
Study flow diagram. Abbreviation: f/u, follow‐up.
Testing for CRS markers was done in accordance to the institutional protocol that recommended that CRS markers be checked routinely on admission in patients with COVID‐19 who are hospitalized. COVID‐19 diagnosis was made using the Roche Cobas SARS‐CoV2 reverse‐transcription polymerase chain reaction assay, with test specimen obtained by nasopharyngeal or oropharyngeal swab in all patients. Testing for CRS markers was performed by the in‐house CRS ELLA Cytokine Storm Panel (Bio‐Techne Corporation), which consists of IL‐6, IL‐8, TNF‐α, and IL‐1β.
Follow‐up for this study ended on May 6, 2020. Exclusion criteria for this study included history of chronic liver disease (CLD), history of liver transplantation, mild peak aminotransferase elevations (>1 times upper limit of normal [ULN] but <3 times ULN), and mild COVID‐19 (no or mild pneumonia without dyspnea). In addition, we excluded patients who remained hospitalized by the last follow‐up given that they may not have reached peak laboratory test values. Patients with mild peak liver enzyme elevations were excluded to limit the confounding effect of undiagnosed underlying liver disease, particularly nonalcoholic fatty liver disease (NAFLD)/nonalcoholic steatohepatitis (NASH) and alcohol‐associated liver disease, on study results and instead to focus on those with COVID‐19‐related liver injury, especially as NAFLD may be an independent predictor of COVID‐19 disease progression.( 19 )
This study was approved by the Institutional Review Board of the Icahn School of Medicine at Mount Sinai (IRB‐20‐03419) and COVID‐19 Study Committee and was exempt from the need for patient informed consent.
Outcome Definitions
Liver injury was defined as ALT and/or AST >3 times ULN (ULN was defined as 40 U/L) at any single time point during the hospitalization.( 20 ) A lack of liver injury was defined as never having an ALT and/or AST >1 times ULN (>40 U/L). Acute kidney injury (AKI) was defined as an increase in creatinine by ≥0.3 mg/dL within 48 hours or an increase ≥1.5 times baseline. Baseline was defined as prepandemic laboratory results (if available); otherwise, laboratory results on admission were used for baseline. The severity of COVID‐19 disease was categorized as severe (dyspnea, respiratory frequency >30/minute, blood oxygen saturation <93%, partial pressure of arterial oxygen to fraction of inspired oxygen ratio <300, and/or lung infiltrate >50% within 24 to 48 hours) or critical (respiratory failure requiring mechanical ventilation, septic shock, and/or multiorgan dysfunction or failure) based on published criteria.( 2 )
Data Collection
Demographic, clinical, laboratory, treatment, and outcome data were extracted from the patients’ electronic medical records and collected and managed using Research Electronic Data Capture, which is an electronic data capture tool hosted at Icahn School of Medicine at Mount Sinai.( 21 , 22 )
The data collected were adapted from the World Health Organization/International Severe Acute Respiratory and Emerging Infection Consortium case record for severe acute respiratory infections.( 23 ) Laboratory findings that were recorded included liver tests (alkaline phosphatase [ALP], ALT, AST, total bilirubin, albumin, prothrombin time, international normalized ratio [INR]), inflammatory markers (lactate dehydrogenase [LDH], CRP, ferritin), and inflammatory cytokine markers (IL‐6, IL‐8, TNF‐α, and IL‐1β).
Treatment data that were documented included the use of antibiotics/antiviral therapy and immunomodulatory agents (i.e., IL‐6 antagonists). Disease outcome data that were collected included length of stay, the highest level of care, administration of escalating concentrations of supplemental oxygen, need for inotropes/vasopressors, development of AKI, need for dialysis, COVID‐19 disease severity, and in‐hospital death.
Statistical Analysis
Categorical variables are reported as counts and percentages, while continuous variables are reported as medians (interquartile range [IQR]). Differences in baseline characteristics, key outcomes, and laboratory parameters between the liver injury cohort and control patients were evaluated. We performed a subgroup analysis on patients with severe COVID‐19 to determine if similar findings were applied within the disease severity class. Chi‐square test and Kruskal‐Wallis test were used to describe differences in categorical and continuous variables, respectively. P < 0.05 was determined as statistically significant. Spearman’s rank correlation analysis was performed to identify relationships between elevated liver enzymes and key inflammatory markers. Statistical analyses were performed using the STATA software package (version 15.1; STATA, College Station, TX).
Results
Baseline Characteristics of Patients With COVID‐19 With And Without Liver Injury
We evaluated 415 hospitalized patients with COVID‐19 who underwent CRS marker testing. Of these, 239 patients were excluded from this study, as outlined in (Fig. 1). In total, 176 patients who met the inclusion/exclusion criteria with severe or critical COVID‐19 with (109 [61.9%] patients) or without (67 [38.1%] patients) liver injury and who underwent CRS marker testing were included in this study (Table 1). Of the 109 patients with liver injury, 74 (67.8%) had abnormal ALT (>1 times ULN), 87 (79.8%) had abnormal AST (>1 times ULN), and only 34 (31.2%) had evidence of liver injury (>3 times ULN) on admission. The remainder of the patients developed liver injury during hospitalization.
TABLE 1.
Admission Clinical and Laboratory Characteristics
| Characteristics | Liver Injury Cohort (AST or ALT ≥3 times ULN) (n = 109) | Control Cohort (Normal AST/ALT) (n = 67) | P |
|---|---|---|---|
| Age, years | 61 (53, 69) | 62 (51, 80) | 0.14 |
| Sex, n (%) | <0.001 | ||
| Male | 78 (71.6%) | 25 (37.3%) | |
| Female | 31 (28.4%) | 42 (62.7%) | |
| Race, n (%) | 0.02 | ||
| White | 24 (22.2%) | 30 (46.2%) | |
| Black | 25 (23.2%) | 10 (15.4%) | |
| Asian | 8 (7.4%) | 2 (3.0%) | |
| Unknown | 51 (47.2%) | 23 (35.4%) | |
| Ethnicity | |||
| Hispanic | 33 (30.3%) | 22 (32.8%) | 0.92 |
| BMI | 28.4 (25.4, 32.9) | 26.9 (24.2, 31.6) | 0.16 |
| Comorbidities, n (%) | |||
| Chronic cardiac disease | 13 (11.9%) | 15 (22.4%) | 0.07 |
| Chronic pulmonary disease | 20 (18.4%) | 16 (23.9%) | 0.38 |
| Chronic kidney disease | 13 (11.9%) | 19 (28.4%) | 0.006 |
| Chronic neurologic disease | 10 (9.2%) | 7 (10.5%) | 0.78 |
| Cancer | 14 (12.8%) | 7 (10.5%) | 0.63 |
| HIV | 1 (0.9%) | 1 (1.5%) | 0.73 |
| DM | 36 (33.0%) | 39 (58.2%) | 0.001 |
| HTN | 48 (44.0%) | 36 (53.7%) | 0.21 |
Values expressed as median (IQR) unless otherwise stated; ULN defined as 40 U/L.
Abbreviations: BMI, body mass index; DM, diabetes mellitus; HIV, human immunodeficiency virus; HTN, hypertension.
There was no significant difference in age or body mass index between the two cohorts. However, patients in the liver injury cohort were significantly more likely to be men (71.6% vs. 37.3%, P < 0.001) (Table 1). Patients who developed liver injury were less likely to have chronic kidney disease (11.9% vs. 28.4%, P = 0.006) and diabetes (33.0% vs. 58.2%, P = 0.001). There were significant differences in the frequency of other comorbidities between the two groups; however, there was a trend toward less chronic heart disease in the liver injury cohort, which did not reach statistical significance (11.9% vs. 22.4%, P = 0.07).
On admission laboratory evaluation, patients who developed liver injury compared to patients without liver injury had a higher white blood cell count and higher total serum bilirubin (Supporting Table S1). There was no difference in absolute lymphocyte count, ALP, albumin, or INR. On admission, LDH and CRP were significantly higher in the liver injury cohort (LDH: median, 520; IQR, 403, 697; vs. median, 345; IQR, 246, 407 U/L; P = 0.0001; CRP: median, 143.8; IQR, 85.7, 223.8; vs. median, 101.9; IQR, 53.2, 170.5 mg/L; P = 0.01).
Peak Laboratory Values and Inflammatory/Cytokine Markers
Peak aminotransferases were about 6‐fold to 9‐fold higher, while peak total bilirubin was about 2‐fold higher in the liver injury cohort (Table 2). Peak ALT correlated with peak AST levels (ρ = 0.87, P < 0.001). In the liver injury group, only 5 of 109 (4.6%) patients had AST or ALT ≥1,000 U/L, and similarly only 4.6% had a peak total bilirubin level >3 mg/dL. However, 36 of 89 (40%) patients had a peak INR ≥1.5 (data not available for all patients on peak INR).
TABLE 2.
Peak Laboratory Values
| Laboratory Findings | Liver Injury Cohort (AST or ALT ≥3 times ULN) (n = 109) | Control Cohort (Normal AST/ALT) (n = 67) | P |
|---|---|---|---|
| Creatinine (mg/dL) | 1.3 (0.8, 4.9) | 1.2 (0.8, 2.2) | 0.13 |
| AST (U/L) | 187 (134, 263) | 30 (25, 33) | <0.001 |
| ALT (U/L) | 180 (140, 258) | 20 (15, 29) | <0.001 |
| Alkaline phosphatase (U/L) | 134 (97, 209) | 84 (66, 117) | <0.001 |
| Total bilirubin (mg/dL) | 1.1 (0.8, 1.7) | 0.6 (0.4, 0.8) | <0.001 |
| Prothrombin time (seconds) | 16.7 (14.8, 18.6)* | 14.9 (13.9, 17.2)* | 0.02 |
| INR | 1.4 (1.2, 1.6)* | 1.2 (1.1, 1.5)* | 0.03 |
| LDH (U/L) | 706 (569, 996) | 421 (329, 518) | <0.001 |
| CRP (mg/L) | 247 (141, 329) | 168 (83, 260) | <0.001 |
| Ferritin (ng/mL) | 2,973 (1,643, 5,368) | 751 (407, 1,984) | <0.001 |
| D‐dimer (µg/mL) | 3.8 (1.6, 14.7) | 2.3 (1.1, 9.7) | 0.08 |
| Creatinine kinase (U/L) | 288 (145, 1,258)* | 88 (37, 242)* | <0.001 |
| Uric acid (mg/dL) | 4.8 (3.9, 8.8)* | 5.9 (4.0, 9.3)* | 0.59 |
| Procalcitonin (ng/mL) | 0.9 (0.3, 5.0) | 0.24 (0.08, 0.94) | <0.001 |
| Cytokine markers | |||
| IL‐6 (nl 0‐5 pg/mL) | 121.0 (66.0, 245.8) | 71.8 (33.8, 123.0) | <0.001 |
| IL‐8 (nl 0‐5 pg/mL) | 50.1 (34.6, 93.5) | 42.7 (29.8, 78.1) | 0.3 |
| TNF‐α (nl 0‐22 pg/mL) | 24.6 (20.1, 34.9) | 26.0 (17.1, 38.3) | 0.69 |
| IL‐1β (nl 0‐5 pg/mL) | 0.6 (0.3, 1.0) | 0.6 (0.3, 0.9) | 0.83 |
Values expressed as median (IQR) unless otherwise stated; ULN defined as 40 U/L.
>10% missing values.
Abbreviation: nl, normal level.
Median peak inflammatory markers were generally higher in the liver injury group (CRP: 247 vs. 168 mg/L, P < 0.001; LDH: 706 vs. 421 U/L, P < 0.001), and ferritin was nearly 4‐fold higher in the liver injury cohort (2,973 vs. 751 ng/mL, P < 0.001) (Fig. 2A,B). Peak ferritin moderately correlated with peak AST levels (ρ = 0.56, P < 0.001). Creatinine kinase and procalcitonin levels were also about 3‐fold higher in the liver injury cohort (288 vs. 88 U/L, P < 0.001 and 0.9 vs. 0.24 ng/mL, P < 0.001, respectively). There was also a trend toward a higher D‐dimer level in the liver injury cohort (median, 3.8; IQR, 1.6, 14.7 µg/mL vs. median, 2.3; IQR, 1.1, 9.7 µg/mL; P = 0.08), although this did not achieve statistical significance.
FIG. 2.
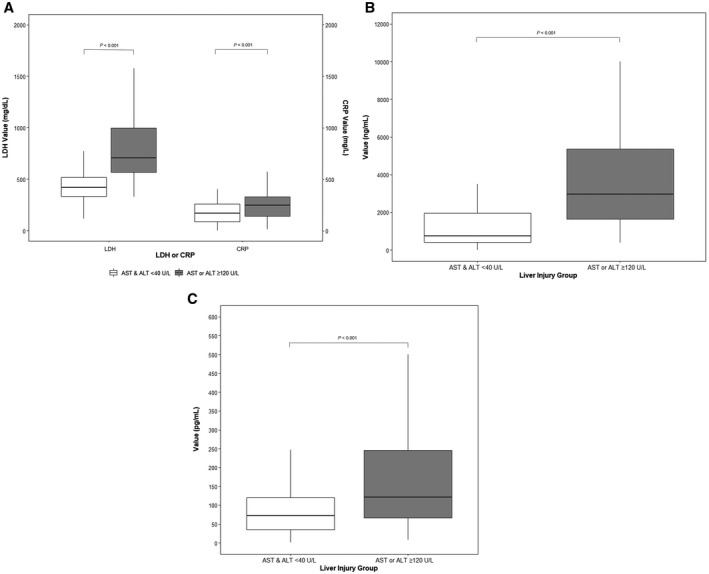
Box plot of inflammatory markers by the presence or absence of liver injury. (A) LDH and CRP levels, (B) ferritin, (C) IL‐6. Bold lines inside the box plot denote median, ends of the box are the upper and lower quartiles, and two lines outside the box extend to the highest and lowest observations.
Among the cytokines measured, IL‐6 levels (normal range, 0‐5 pg/mL) were higher in the liver injury group (median, 121.0; IQR, 66.0, 245.8 pg/mL) compared to the control group (median, 71.8; IQR, 33.8, 123.0 pg/mL; P < 0.001) (Table 2; Fig. 2C). Peak IL‐6 demonstrated a weak correlation to peak AST levels (ρ = 0.31, P < 0.001). Otherwise, there was no significant difference in IL‐8, TNF‐α, and IL‐1β levels between the two cohorts (Table 2).
Comparison of Peak Inflammatory/Cytokine Markers in Severe COVID‐19
We performed a comparison of peak key inflammatory and cytokine markers in only severe COVID‐19. As expected, patients with liver injury with severe COVID‐19 had higher peak AST and ALT compared to patients used as controls (Table 3). Patients with severe COVID‐19 with liver injury still had higher LDH and ferritin levels compared to patients with severe COVID‐19 without liver injury (LDH: median, 600; IQR, 518, 756 vs. median, 380; IQR, 312, 478 U/L; ferritin: median, 2,349; IQR, 1,235, 4,507 vs. median, 631; IQR, 389, 1,341 ng/mL; P < 0.001). Further, IL‐6 levels continued to be higher in the liver injury group (median, 96.5; IQR, 53.3, 142.0 vs. median, 56.0; IQR, 33.8, 97.2 pg/mL; P = 0.015).
TABLE 3.
Comparison of Peak Key Values (Liver Injury vs. Control) in Patients With Severe COVID‐19
| Laboratory Findings | Liver Injury Cohort (AST or ALT ≥3 times ULN) (n = 62) | Control Cohort (Normal AST/ALT) (n = 54) | P |
|---|---|---|---|
| AST (U/L) | 164 (115, 238) | 30 (25, 33) | <0.001 |
| ALT (U/L) | 177 (135, 258) | 20 (15, 29) | <0.001 |
| LDH (U/L) | 600 (518, 756) | 380 (312, 478) | <0.001 |
| CRP (mg/L) | 182 (120, 247) | 159 (80, 248) | 0.2 |
| Ferritin (ng/mL) | 2,349 (1,235, 4,507) | 631 (389, 1,341) | <0.001 |
| D‐dimer (µg/mL) | 1.8 (1.3, 3.8) | 2.1 (0.9, 6.9) | 0.9 |
| Cytokine markers | |||
| IL‐6 (nl 0‐5 pg/mL) | 96.5 (53.3, 142.0) | 56.0 (33.8, 97.2) | 0.015 |
| IL‐8 (nl 0‐5 pg/mL) | 41.8 (27.5, 56.4) | 40.2 (26.3, 68.4) | 0.97 |
| TNF‐α (nl 0‐22 pg/mL) | 23.4 (17.9, 33.8) | 25.1 (16.8, 33.0) | 0.71 |
| IL‐1β (nl 0‐5 pg/mL) | 0.5 (0.3, 0.8) | 0.6 (0.4, 0.9) | 0.39 |
Values expressed as median (IQR) unless otherwise stated; ULN defined as 40 U/L.
Abbreviation: nl, normal level.
COVID‐19 Treatments and Clinical Outcomes
Patients in the liver injury cohort were more likely to be treated with antibiotics (89.0% vs. 59.7%, P < 0.001), therapeutic anticoagulation (68.8% vs. 27.4%, P < 0.001), and corticosteroids (50.5% vs. 26.9%, P = 0.002) compared to the control cohort (Table 4). Less than 10 patients from each cohort were enrolled in a clinical trial with remdesivir, tocilizumab, convalescent plasma, or mesenchymal stem cell therapy.
TABLE 4.
Treatments and Outcomes
| Treatments and Outcomes | Liver Injury Cohort (AST or ALT ≥3 times ULN) (n = 109) | Control Cohort (Normal AST/ALT) (n = 67) | P |
|---|---|---|---|
| COVID‐19 treatments | |||
| Antibiotic therapy | 97 (89.0%) | 40 (59.7%) | <0.001 |
| Therapeutic anticoagulation | 75 (68.8%) | 19 (28.4%) | <0.001 |
| Azithromycin | 86 (78.9%) | 44 (65.7%) | 0.052 |
| Hydroxychloroquine | 102 (93.6%) | 58 (86.6%) | 0.12 |
| Remdesivir | 5 (4.6%) | 1 (1.5%) | 0.27 |
| Tocilizumab | 4 (3.7%) | 0 (0.0%) | 0.11 |
| Convalescent plasma | 7 (6.4%) | 5 (7.5%) | 0.79 |
| Corticosteroid | 55 (50.5%) | 18 (26.9%) | 0.002 |
| Mesenchymal stem cells | 2 (1.8%) | 0 (0.0%) | 0.27 |
| Outcomes | |||
| Length of stay (days) | 14 (10, 20) | 7 (5, 12) | 0.001 |
| Highest level care | <0.001 | ||
| Floor bed | 67 (61.5%) | 61 (91.0%) | |
| ICU | 42 (38.5%) | 6 (9.0%) | |
| Required supplemental oxygen | 108 (99.0%) | 59 (88.1%) | |
| Maximal oxygen requirement | <0.001 | ||
| Nasal cannula | 38 (34.9%) | 34 (50.8%) | |
| Non‐rebreather mask | 18 (16.5%) | 14 (20.9%) | |
| HFNC | 8 (7.3%) | 2 (3.0%) | |
| NIPPV | 1 (0.9%) | 5 (7.5%) | |
| Mechanical ventilation | 43 (39.5%) | 4 (6.0%) | |
| Required inotropes/vasopressors | 35 (32.4%) | 6 (9.0%) | <0.001 |
| Developed AKI | 52 (47.7%) | 20 (29.9%) | 0.019 |
| Required dialysis | 20 (18.4%) | 8 (11.9%) | 0.26 |
| COVID‐19 severity | 0.001 | ||
| Severe | 62 (56.9%) | 54 (80.6%) | |
| Critical | 47 (43.1%) | 13 (19.4%) | |
| Death | 30 (28.3%) | 13 (19.4%) | 0.19 |
Values expressed as median (IQR) unless otherwise stated; ULN defined as 40 U/L.
Abbreviations: HFNC, high‐flow nasal cannula; NIPPV, Noninvasive positive pressure ventilation.
Patients who had liver injury were more likely to have critical COVID‐19 (43.1% vs. 19.4%, P = 0.001) and require an intensive care unit (ICU) stay (38.5% vs. 9.0%, P < 0.001), inotropes/vasopressor support (32.4% vs. 9.0%, P < 0.001), and mechanical ventilation (39.5% vs. 6.0%, P < 0.001). Liver injury was associated with a longer length of stay (median, 14; IQR, 10, 20 vs. median, 7; IQR, 5, 2 days; P < 0.001). There was no difference in in‐house rate of death between the two groups, with 30 out of 109 patients (28.3%) in the liver injury group compared to 13 out of 67 patients (19.4%) in the control group (P = 0.19).
Discussion
In this retrospective observational study of patients hospitalized with severe or critical COVID‐19, we describe several key clinical findings of patients with COVID‐19 who developed liver injury. A hyperinflammatory response (cytokine storm) appears to be more commonly associated with liver injury. We found that most patients with COVID‐19 exhibit a hyperinflammatory response, as demonstrated by elevated peak ferritin, LDH, and CRP levels. Those with liver injury had a considerably more pronounced response compared to patients without liver injury. In particular, the acute‐phase reactant ferritin is the single most distinguishing inflammatory marker, with levels greater than 4 times higher in those with liver injury compared to those without. These findings may suggest that among patients with severe/critical COVID‐19, there may be an inflammatory “threshold” after which liver injury tends to occur more often.
We also evaluated the peak levels of several inflammatory cytokines, including IL‐6, IL‐8, TNF‐α, and IL‐1β. Surprisingly, the only difference in the levels of the different inflammatory cytokines between the two groups was in IL‐6. In COVID‐19, it is hypothesized that SARS‐CoV2 activates the innate and adaptive immune system, which results in the release of IL‐6.( 24 ) IL‐6 is produced by a variety of cells, including monocytes/macrophages and T cells, and is involved in a large number of processes, including neutrophil trafficking, acute phase response, B‐cell differentiation, and autoantibody production.( 25 ) In COVID‐19, IL‐6 surges during illness and declines during recovery, and levels correlate with disease severity.( 26 , 27 ) Our findings that IL‐6 is the main cytokine that is associated with higher liver enzymes in COVID‐19 may be related to the accuracy of IL‐6 in detecting inflammatory response, which results in liver injury. However, the role of IL‐6 in the pathophysiology of COVID‐19 and liver enzyme elevation remains unclear. Whether IL‐6 functions as a deleterious or protective mediator is unknown.( 28 ) Although higher IL‐6 levels were seen in patients with liver injury, this does not in itself prove a causal relationship between IL‐6 and liver enzyme elevation. Meanwhile, the lack of difference in IL‐8, TNF‐α, and IL‐1β levels between the two groups suggests that these cytokines most likely are not directly involved in liver injury, even though higher levels of TNF‐α were recently reported in severe COVID‐19 compared to moderately severe COVID‐19 infection.( 15 )
Our findings have several important clinical implications. Patients with severe to critical COVID‐19, who have very high levels of inflammatory markers, such as ferritin and IL‐6, may be at a higher risk for development of aminotransferase elevations beyond 3 times ULN. Therefore, monitoring of liver enzymes and liver function tests in these patients is advisable. Another clinical implication is that the role of IL‐6 inhibition on the trend of liver enzymes should be evaluated in patients with severe COVID‐19 in future clinical studies. Tocilizumab, a recombinant humanized monoclonal antibody against IL‐6, was recently shown to prevent the need for mechanical intubation or death in patients with COVID‐19; however, its role in liver involvement of patients with COVID has not been studied.( 29 , 30 , 31 ) Therapeutic strategies targeting this overactive cytokine response need to be balanced with maintaining an adequate inflammatory response to clear the SARS‐CoV2 virus.( 16 ) For example, IL‐6 has a protective effect in viral infections, such as hepatitis B and influenza, and is essential for viral control.( 32 , 33 )
The COVID‐19 cytokine storm is closely related to COVID‐19 disease severity. This occurs likely due to increased pulmonary injury, T‐cell depletion, and cluster of differentiation (CD)4+ T‐cell dysfunction.( 34 ) We found that patients who experienced a hyperinflammatory response and liver injury also required higher rates of ICU stay, needed inotropes/vasopressors and/or mechanical ventilation more often, and also had higher rates of multiorgan failure. However, our study suggests that COVID‐19‐related liver injury and cytokine storm are not entirely dependent on COVID‐19 disease severity. In comparing patients within the severity class of COVID‐19 (i.e., only those with severe COVID‐19) with and without liver injury, patients with liver injury continued to have significantly higher levels of LDH, ferritin, and IL‐6. This suggests that there may be a link between presence of liver injury and higher inflammatory markers that is independent of COVID‐19 infection severity.
As mentioned earlier, although we noticed an association between higher IL‐6 and liver injury, our study was not designed to prove causality, which remains unknown. It is important to note that there are various other contributory factors that may play a role in liver injury in patients with COVID‐19, including hyperinflammatory response (cytokine storm), direct virus effect on the liver, ischemia due to hypotension or microthrombi, hypoxemia, DILI, and vascular injury. Patients with more severe forms of COVID‐19 infection are often started on multiple medications, such as antibiotics at the time of presentation/admission. However, DILI often occurs weeks to months after the initial start of medications, and we noticed in our study that most patients in the liver injury cohort already had elevations of ALT and/or AST at the time of presentation; all of which makes DILI less likely to be the main cause of liver injury. Nevertheless, we cannot rule this out as a contributory factor. A recent study evaluating liver autopsies of patients with COVID‐19 found microthrombi and hemophagocytes in the liver, suggesting ischemia and HLH as other potential causes of liver injury.( 7 , 17 )
Despite the notably worse overall disease severity of patients with COVID‐19 with liver injury in our study, this was not associated with increased in‐hospital mortality for several possible reasons. First, our study design focused on patients with severe disease. Thus, our entire cohort was considerably sicker than cohorts described in previous retrospective studies. This could have dampened any significant differences in mortality rate between groups. In addition, worse liver injury and severity of COVID‐19 may have been additional triggers for physicians to initiate therapy, including anticoagulation to treat microvascular and macrovascular thrombi formation, which may improve survival in severe COVID‐19.( 35 , 36 ) This may have improved survival in the liver injury group. Finally, given the retrospective nature, relatively short duration of follow‐up, and study size, our study was not designed and powered enough to detect differences in survival between the two groups.
Our study has several limitations. First, we focused on severe to critical COVID‐19 disease, and our findings may not generally be applicable to patients with COVID‐19 with mild disease and liver injury.( 37 ) We also excluded patients with known CLD and liver transplant recipients, which limits our findings.( 38 ) Different pathogenic mechanisms may predominate in patients with COVID‐19 with CLD or after liver transplantation who develop liver injury. In addition, we did not have data regarding underlying NAFLD/NASH in our patient cohorts because the majority of patients did not have liver ultrasound, liver elastography, or liver biopsy. We did find higher rates of diabetes in the cohort without liver injury, which may suggest higher rates of NAFLD/NASH in this group. This was a surprise finding that may have been related to selection bias. At the present time, it is unclear if underlying NAFLD/NASH will predispose patients with COVID‐19 to higher rates or more severe forms of liver injury. Due to the study design and limited follow‐up, we also did not have data on the trend of the liver enzymes relevant to the clinical course during and after hospitalization. Another limitation is that testing for CRS markers was not performed for all admitted patients despite recommended institutional protocol and longitudinal testing was typically not performed. Theoretically, a protocol testing for CRS markers at set increments likely would have shown a higher correlation between IL‐6 and liver injury. Finally, we did not record data on patients and their characteristics who did not undergo CRS marker testing and thus were not included in this study.
Our study has several unique strengths. First, we evaluated a large number of patients who were treated in accordance with the protocols of one institution. Second, the patients in our cohort all underwent immunologic testing, which allows comparison of cytokine markers and basic inflammatory markers between groups. Third, we eliminated patients with mild enzyme elevations, had a stringent criterion for liver injury compared to prior studies, and only included patients who had been discharged. These study conditions were implemented with the goal of solely studying patients with COVID‐19 with virus‐related liver injury as opposed to preexisting liver disease or other etiologies.
In summary, significant liver injury in COVID‐19 is associated with the severity of the hyperinflammatory response and COVID‐19 disease. The hyperinflammatory response is characterized by elevated inflammatory markers (i.e., LDH, ferritin, and CRP) and increased IL‐6. The occurrence of liver injury in patients with COVID‐19 should prompt the investigation of basic inflammatory markers and IL‐6 levels. Other inflammatory cytokines, such as IL‐8, TNF‐α, and IL‐1β, are not associated with COVID‐19‐related liver injury. Our findings do not prove causality, and the exact mechanism of liver injury in patients with COVID‐19 remains unknown. The role of IL‐6 inhibition and severe inflammatory response on the course of COVID‐19 disease and liver injury is important and requires further investigation in future studies.
Supporting information
Supplementary Material
Potential conflict of interest: Nothing to report.
References
Author names in bold designate shared co‐first authorship.
- 1. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239‐1242. [DOI] [PubMed] [Google Scholar]
- 3. Zhang C, Shi L, Wang FS. Liver injury in COVID‐19: management and challenges. Lancet. Gastroenterol Hepatol 2020;5:428‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, et al. Longitudinal association between markers of liver injury and mortality in COVID‐19 in China. Hepatology 2020;72:389‐398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tan YJ, Fielding BC, Goh PY, Shen S, Tan TH, Lim SG, et al. Overexpression of 7a, a protein specifically encoded by the severe acute respiratory syndrome coronavirus, induces apoptosis via a caspase‐dependent pathway. J Virol 2004;78:14043‐14047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chau TN, Lee KC, Yao H, Tsang TY, Chow TC, Yeung YC, et al. SARS‐associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology 2004;39:302‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, et al. SARS‐CoV‐2 infection of the liver directly contributes to hepatic impairment in patients with COVID‐19. J Hepatol 2020;73:807‐816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Poor HD, Ventetuolo CE, Tolbert T, Chun G, Serrao G, Zeidman A, et al. COVID‐19 critical illness pathophysiology driven by diffuse pulmonary thrombi and pulmonary endothelial dysfunction responsive to thrombolysis. medRxiv 2020; 10.1101/2020.04.17.20057125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ciobanu AO, Gherasim L. Ischemic hepatitis ‐ intercorrelated pathology. Maedica (Bucur) 2018;13:5‐11. [PMC free article] [PubMed] [Google Scholar]
- 10. Boettler T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Cornberg M, et al. Care of patients with liver disease during the COVID‐19 pandemic: EASL‐ESCMID position paper. JHEP Rep 2020;2:100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Seretis C, Lagoudianakis E, Salemis N, Pappas A, Gemenetzis G, Seretis F, et al. Liver biochemistry during the course of influenza A/H1N1 infection. Gastroenterology Res 2013;6:103‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Papic N, Pangercic A, Vargovic M, Barsic B, Vince A, Kuzman I. Liver involvement during influenza infection: perspective on the 2009 influenza pandemic. Influenza Other Respir Viruses 2012;6:e2‐e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xu XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, Ma CL, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS‐Cov‐2) outside of Wuhan, China: retrospective case series. BMJ 2020;368:m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration, UK. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen P, Zhou B. Clinical characteristics of COVID‐19 patients with abnormal liver tests. J Hepatol 2020;73:712‐713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jose RJ, Manuel A. COVID‐19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med 2020;8:e46‐e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bryce C, Grimes Z, Pujadas E, Ahuja S, Beasley MB, Albrecht R, et al. Pathophysiology of SARS‐CoV‐2: targeting of endothelial cells renders a complex disease with thrombotic microangiopathy and aberrant immune response. The Mount Sinai COVID‐19 autopsy experience. medRxiv 2020; 10.1101/2020.05.18.20099960. [DOI] [Google Scholar]
- 18. Olry A, Meunier L, Delire B, Larrey D, Horsmans Y, Le Louet H. Drug‐induced liver injury and COVID‐19 infection: the rules remain the same. Drug Saf 2020;43:615‐617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, et al. Non‐alcoholic fatty liver diseases in patients with COVID‐19: a retrospective study. J Hepatol 2020;73:451‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kwo PY, Cohen SM, Lim JK. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol 2017;112:18‐35. [DOI] [PubMed] [Google Scholar]
- 21. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, et al.; REDCap Consortium . The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. World Health Organization, International Repository for Information Sharing . Global COVID‐19: clinical platform: novel coronavius (COVID‐19): rapid version. https://apps.who.int/iris/handle/10665/331768. Published 2020. Accessed May 1, 2020. [Google Scholar]
- 24. di Mauro G, Scavone C, Rafaniello C, Rossi F, Capuano A. SARS‐Cov‐2 infection: response of human immune system and possible implications for the rapid test and treatment. Int Immunopharmacol 2020;84:106519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M. IL‐6/IL‐6 receptor system and its role in physiological and pathological conditions. Clin Sci (Lond) 2012;122:143‐159. [DOI] [PubMed] [Google Scholar]
- 26. Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID‐19). Front Immunol 2020;11:827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID‐19 in New York City: a prospective cohort study. medRxiv 2020; 10.1101/2020.04.15.20067157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tanaka T, Narazaki M, Kishimoto T. IL‐6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 2014;6:a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell‐engaging therapies. Cancer J 2014;20:119‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xu X, Han M, Li T, Sun W, Wang D, Fu B, et al. Effective treatment of severe COVID‐19 patients with tocilizumab. Proc Natl Acad Sci U S A 2020;117:10970‐10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Roche’s phase III EMPACTA study showed Actemra/RoActemra reduced the likelihood of needing mechanical ventilation in hospitalised patients with COVID‐19 associated pneumonia [global web site media]. Basel, Switzerland: Roche; September 18, 2020. Available at: https://www.roche.com/media/releases/med‐cor‐2020‐09‐18.htm. Accessed September 20, 2020. [Google Scholar]
- 32. Lan T, Chang L, Wu L, Yuan YF. IL‐6 plays a crucial role in HBV infection. J Clin Transl Hepatol 2015;3:271‐276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang ML, Wang CT, Yang SJ, Leu CH, Chen SH, Wu CL, et al. IL‐6 ameliorates acute lung injury in influenza virus infection. Sci Rep 2017;7:43829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 2020;130:2620‐2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18:844‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Paranjpe I, Fuster V, Lala A, Russak A, Glicksberg BS, Levin MA, et al. Association of treatment dose anticoagulation with in‐hospital survival among hospitalized patients with COVID‐19. J Am Coll Cardiol 2020;76:122‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bloom PP, Meyerowitz EA, Reinus Z, Daidone M, Gustafson J, Kim AY, et al. Liver biochemistries in hospitalized patients with COVID‐19. Hepatology 2020; 10.1002/hep.31326. [DOI] [PubMed] [Google Scholar]
- 38. Moon AM, Webb GJ, Aloman C, Armstrong MJ, Cargill T, Dhanasekaran R, et al. High mortality rates for SARS‐CoV‐2 infection in patients with pre‐existing chronic liver disease and cirrhosis: preliminary results from an international registry. J Hepatol 2020;73:705‐708 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


