Abstract
Detection of SARS-CoV-2 viral RNA by RT-PCR assays is the primary diagnostic test for COVID-19. Cycle threshold (CT) values generated by some of these assays provide inversely proportional proxy measurements of viral load. The clinical implications of CT values are incompletely characterized, particularly in solid organ transplant (SOT) recipients. We conducted a retrospective chart review of 25 adult SOT recipients admitted to the Yale New Haven Health System between March 1 and May 15, 2020, analyzing 50 test results to investigate the clinical implications of SARS-CoV-2 CT values in this population. Initial CT values from upper respiratory tract samples were significantly higher in patients on tacrolimus, but were not associated with admission severity nor highest clinical acuity. Viral RNA was detected up to 38 days from symptom onset with a gradual increase in CT values over time. In five patients with serial testing, CT values <35.0 were detected >21 days after symptom onset in 4/5 and ≥27 days in 2/5, demonstrating prolonged RNA detection. These data describe SARS-CoV-2 viral dynamics in SOT patients and suggest that CT values may not be useful to predict COVID-19 severity in SOT patients. SARS-CoV-2 CT values may be more useful in informing infection prevention measures.
KEYWORDS: clinical research/practice, diagnostic techniques and imaging, infection and infectious agents – viral, infectious disease, organ transplantation in general
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CDC, Centers for Disease Control and Prevention; CMV, cytomegalovirus; COVID-19, coronavirus disease 2019; CT, cycle threshold; HFNC, high-flow nasal cannula; hsCRP, high-sensitivity C-reactive protein; ICU, intensive care unit; IQR, interquartile range; IRB, institutional review board; LDT, laboratory developed test; N2, nucleocapsid 2 gene; NRB, non-rebreather; PPE, personal protective equipment; RT-PCR, reverse transcriptase polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SOT, solid organ transplant; WHO OSCI, World Health Organization COVID-19 Ordinal Scale for Clinical Improvement
1. INTRODUCTION
The pandemic of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) presents significant health-care challenges. The primary method to diagnose COVID-19 relies on reverse transcriptase polymerase chain reaction (RT-PCR)-based tests that detect viral RNA in clinical specimens. Some RT-PCR tests yield a cycle threshold (CT) value, the number of cycles required for a fluorescent signal to cross the detection threshold. CT values provide semiquantitative information with values that are inversely proportional to the relative amount of viral RNA present in tested samples. In certain viral respiratory tract infections, such as influenza, CT values may correlate with disease severity.1 In COVID-19, viral replication in upper respiratory tract specimens is highest between 0.6 days prior to symptom onset and 5 days after symptom onset before declining.2 , 3 As COVID-19 progresses, CT values increase in upper respiratory samples, reflecting diminished abundance of viral RNA with time.2, 3, 4, 5, 6, 7, 8 Though CT values may reflect viral burden and time from initial viral acquisition, the utility of individual CT values for clinical decision-making or prognosis has not been established.
Solid organ transplant (SOT) recipients are generally at higher risk of acquiring infectious diseases and developing worse outcomes. While certain series of SOT recipients with COVID-19 report high overall disease severity and poor outcomes,9 , 10 others suggest low mortality among transplant recipients.11 It is unknown if SARS-CoV-2 RT-PCR CT values are associated with disease severity and clinical outcomes as few studies to date have evaluated associations of SARS-CoV-2 CT values in these patients. Additionally, studies detailing viral dynamics using CT values have not routinely included or identified SOT patients. As a proxy for viral load, CT values may have important implications for managing COVID-19 in SOT recipients. Immunosuppression modification and infection prevention measures could be informed by CT values as clinical specimens with lower CT are more likely to yield culturable, and therefore infectious, virus.12, 13, 14, 15 Accordingly, we performed a retrospective study in a single health system to investigate the associations between initial SARS-CoV-2 CT values and clinical parameters in SOT patients as well as viral dynamics over time.
2. METHODS
Adult (≥18 years old) SOT recipients on maintenance immunosuppression diagnosed with symptomatic COVID-19 and admitted to Yale New Haven Health System from March 1 to May 15, 2020 were identified by electronic chart review. Nasopharyngeal or combined nasopharyngeal and oropharyngeal samples were collected by medical professionals using flocked swabs in accordance with guidelines from the Centers for Disease Control and Prevention (CDC). Repeat testing was primarily performed for discharge to extended care facilities due to requirements of two negative SARS-CoV-2 RT-PCR assays before acceptance.
Patients with SARS-CoV-2 RT-PCR testing performed at the Yale New Haven Hospital clinical virology laboratory reported as detected, positive, presumptive positive, or inconclusive were included. SARS-CoV-2 RT-PCR was performed on nasopharyngeal or combined nasopharyngeal and oropharyngeal samples using a laboratory developed test (LDT) derived from the CDC assay and approved via an Emergency Use Authorization from the Food and Drug Administration,16 or the commercially available Cepheid Xpert Xpress SARS-CoV-2 test (hereafter referred to as Xpert).17 The LDT detects two SARS-CoV-2-specific regions of the viral nucleocapsid gene (N1 and N2) and the Xpert detects a pan-Sarbecovirus target in the envelope (E) or the SARS-CoV-2-specific N2 gene. Definitions of positive, inconclusive, and negative tests are provided in the Appendix S1. Evaluation of CT values was performed using N2 CT results after demonstrating assay results were comparable (Appendix S1). We grouped N2 CT into low (0–20.0), moderate (20.1–30.0), and high (30.1–40.0) values based on currently understood clinical correlates. Negative tests demonstrating resolution of viral RNA persistence were not obtained in these patients.
Demographic, transplant-specific, clinical, COVID-19-related management, and outcome data were collected retrospectively through a period of at least 28 days beginning at hospital admission. Initial laboratory values were the first recorded, whereas peak laboratory values were the highest recorded during the hospital admission. Creatinine levels were censored for patients undergoing renal replacement therapy, including hemodialysis or peritoneal dialysis. For laboratory values reported as above or below the detectable range, the upper or lower limit of detection was recorded, respectively. Approximate symptom onset was obtained from admission documentation. The furthest time point of symptom onset from hospital admission was included if a range of dates was documented. Clinical severity in the first 24 h of hospitalization and highest clinical acuity during the hospital course were determined using the World Health Organization COVID-19 Ordinal Scale for Clinical Improvement (WHO OSCI).18 WHO OSCI scores used in this study are provided in the Appendix S1. This score was modified by adding one additional point if a patient was admitted to an intensive care unit (ICU). Patients with a modified WHO OSCI score ≥5 were designated as severe and those with a score of <5 were designated as non-severe. The highest clinical acuity as determined by the modified WHO OSCI score was used to categorize patients into non-severe or severe groups to investigate clinical associations.
2.1. Institutional Review Board (IRB) Approval
This study was approved by the Yale Human Investigation Committee (IRB Protocol Identification 2000028099).
2.2. Statistical analysis
Fisher’s exact, Mann-Whitney, Kruskal-Wallis, and simple linear regression testing was performed with Prism 8 (version 8.4.2, GraphPad) using an alpha value of 0.05. Additional details are provided in the Appendix S1. Descriptive statistics are reported as percentages or medians with interquartile ranges (IQR).
3. RESULTS
We identified 25 hospitalized SOT recipients who met the inclusion criteria. Demographic and clinical data are presented in Table 1. Fifteen patients had severe COVID-19 and 10 patients had non-severe COVID-19 as the highest clinical acuity during hospitalization based on the modified WHO OSCI score. Median patient age was 60 years (range 29–78), and most (13/25, 52.0%) identified as Black or African American. Twelve patients were male (48.0%) and 13 patients were female (52.0%) with significantly more males in the severe group. Twenty-three patients were kidney SOT and two were liver SOT recipients. More patients were on a tacrolimus-based immunosuppressive regimen (18/25, 72.0%) as compared to a belatacept-based regimen (7/25, 28.0%) prior to admission. Regimens were combined with an antimetabolite (17/25, 68.0%; 15 mycophenolate mofetil, one mycophenolic acid, and one azathioprine) and/or prednisone (22/25, 88.0%).
TABLE 1.
Patient demographics and clinical characteristics
| Total (N = 25) | Nonsevere (N = 15) | Severe (N = 10) | p value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, median (IQR), years | 60.0 (54.0–64.5) | 60.0 (48.0–65.0) | 61.5 (54.8–64.2) | .77 |
| Gender, N (%) | ||||
| Male | 12 (48.0) | 4 (26.7) | 8 (80.0) | .01 |
| Female | 13 (52.0) | 11 (73.3) | 2 (20.0) | |
| Race, N (%) | ||||
| Black or African American | 13 (52.0) | 8 (53.3) | 5 (50.0) | .78 |
| White or Caucasian | 4 (16.0) | 3 (20.0) | 1 (10.0) | |
| American Indian or Alaskan | 1 (4.0) | 1 (6.7) | 0 | |
| Other | 7 (28.0) | 3 (20.0) | 4 (40.0) | |
| Ethnicity, N (%) | ||||
| Hispanic or Latino | 7 (28.0) | 3 (20.0) | 4 (40.0) | 0.38 |
| Non-Hispanic | 18 (72.0) | 12 (80.0) | 6 (60.0) | |
| Transplanted organ, N (%) | ||||
| Kidney | 23 (92.0) | 13 (86.7) | 10 (100.0) | .50 |
| Liver | 2 (8.0) | 2 (13.3) | 0 | |
| Transplantation to admission, days, median (IQR) | 1581 (566–2699) | 2298 (789–3359) | 1044 (473–2403) | .12 |
| Admission immunosuppression, N (%) | ||||
| Tacrolimus | 18 (72.0) | 9 (60.0) | 9 (90.0) | .18 |
| Belatacept | 7 (28.0) | 6 (40.0) | 1 (10.0) | .18 |
| Antimetabolite | 17 (68.0) | 9 (60.0) | 8 (80.0) | .40 |
| Prednisone | 22 (88.0) | 12 (80.0) | 10 (100.0) | .25 |
| Microbiology | ||||
| Initial N2 CT, median (IQR) | 22.9 (18.7–25.4) | 22.9 (16.3–29.4) | 23.1 (18.9–24.6) | .97 |
| Laboratory | ||||
| Tacrolimus (ng/ml), median (IQR) [N] | ||||
| Initial | 12.0 (6.5–25.0) [18] | 10.0 (4.0–26.0) [9] | 13.0 (7.0–24.0) [9] | .65 |
| Trough | 4.0 (3.0–6.3) [18] | 6.0 (4.0–8.5) [9] | 3.0 (3.0–4.0) [9] | .02 |
| Creatinine (mg/dl), median (IQR)b | ||||
| Initial | 2.0 (1.3–2.9) | 1.6 (1.0–2.9) | 2.2 (1.5–3.4) | .17 |
| Peak | 2.4 (1.8–5.0) | 1.9 (1.2–3.4) | 3.9 (2.2–5.7) | .03 |
| AST (U/L), median (IQR) | ||||
| Initial | 35.0 (29.5–51.0) | 34.0 (27.0–43.0) | 45.0 (32.2–60.0) | .09 |
| Peak | 57.0 (44.0–120.5) | 50.0 (37.0–58.0) | 109.0 (58.2–144.5) | <.01 |
| ALT (U/L), median (IQR) | ||||
| Initial | 25.0 (18.5–34.5) | 22.0 (12.0–29.0) | 34.0 (21.8–42.0) | .06 |
| Peak | 37.0 (25.5–119.0) | 32.0 (23.0–44.0) | 101.5 (36.2–158.8) | .02 |
| hsCRP (mg/L), median (IQR) | ||||
| Initial | 88.6 (50.8–191.6) | 76.1 (11.5–146.4) | 156.7 (88.2–225.8) | .02 |
| Peak | 154.4 (78.8–213.2) | 139.2 (52.9–202.1) | 184.4 (98.9–237.0) | .22 |
| Ferritin (ng/ml), median (IQR) | ||||
| Initial | 1,366.0 (571.0–2186.0) | 1,127.0 (227.0–1958.0) | 1,488.0 (921.3–4714.0) | .26 |
| Peak | 2,206.0 (1,184.0–3656.0) | 2,206.0 (719.0–3390.0) | 2,243.0 (1,226.0–8589.0) | .40 |
| D-dimer (mg/L), median (IQR) | ||||
| Initial | 1.0 (0.6–1.7) | 1.2 (0.6–2.2) | 0.8 (0.6–1.4) | .34 |
| Peak | 2.3 (1.5–13.3) | 1.6 (1.3–4.2) | 11.6 (2.3–21.6) | .02 |
| Peak IL-6 (pg/ml), median (IQR) | 44.5 (5.0–189.8) | 10.0 (5.0–107.5) | 159.5 (19.2–218.0) | .09 |
| COVID-19 therapies | ||||
| Atazanavir, N (%) | 6 (24.0) | 4 (26.7) | 2 (20.0) | >.99 |
| Convalescent plasma, N (%) | 3 (12.0) | 0 | 3 (30.0) | .05 |
| Hydroxychloroquine, N (%) | 24 (96.0) | 14 (93.3) | 10 (100.0) | >.99 |
| Methylprednisolone, N (%) | 7 (28.0) | 1 (6.7) | 6 (60.0) | .01 |
| Remdesivir, N (%) | 1 (4.0) | 0 | 1 (10.0) | .4 |
| Tocilizumab, N (%) | 17 (68.0) | 8 (53.3) | 9 (90.0) | .08 |
| Clinical | ||||
| Hospitalization days, median (IQR)a | 10.0 (5.8–21.0) | 7.0 (5.0–15.0) | 11.0 (10.0–27.0) | .09 |
| Modified WHO OSCI, first 24 h, N (%) | ||||
| Score <5 (initially nonsevere) | 20 (80.0) | 15 (100.0) | 5 (50.0) | .01 |
| Score ≥5 (initially severe) | 5 (20.0) | 0 | 5 (50.0) | |
| Critical care in ICU during hospitalization, N (%) | ||||
| Did not require critical care | 15 (60.0) | 15 (100.0) | 0 | <.01 |
| Required critical care | 10 (40.0) | 0 | 10 (100.0) | |
| Highest oxygen required, N (%) | ||||
| Room air – 6L nasal cannula | 15 (60.0) | 15 (100.0) | 0 | <.01 |
| NRB, HFNC, or intubation | 10 (40.0) | 0 | 10 (100.0) | |
| 28 days mortality, N (%) | 4 (16) | 0 | 4 (40.0) | .02 |
Note: Percentages are calculated per grouping (all, nonsevere, and severe). Non-severe and severe categorization represent the highest acuity COVID-19 severity during the entire hospital course as determined by the modified World Health Organization COVID-19 Ordinal Scale for Clinical Improvement (WHO OSCI). P values represent comparisons between nonsevere and severe groups. Individual p values were calculated for immunosuppressive agents and therapies as patients received multiple medications simultaneously.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CT, cycle threshold; HFNC, high-flow nasal cannula; hsCRP, high-sensitivity C-reactive protein; IQR, interquartile range; N2, nucleocapsid 2 gene; NRB, nonrebreather.
Creatinine values for two patients in the non-severe group were censored due to dialysis.
Days of admission were censored for three patients who remained inpatient at the time of analysis.
Patients with severe disease had significantly higher peak creatinine, aspartate aminotransferase (AST), and alanine aminotransferase (ALT). Inflammatory markers also correlated with severity, with significantly higher initial high-sensitivity C-reactive protein (hsCRP) and D-dimer in the severe group. The median hospital length-of-stay was 10.0 days for patients discharged during the study period, with no difference between nonsevere and severe groups. Three patients remained hospitalized at the time of analysis, with hospital lengths-of-stay exceeding the 28-day analysis period. Four patients included in this study died. The median hospital length-of-stay for the patients who died was 18.5 days (IQR 10.0–27.0 days). All patients who died were in the severe group and required critical care during their course. No deaths occurred in the nonsevere group during admission, and no additional deaths occurred in either group following discharge. One patient had low-level cytomegalovirus (CMV) viremia without end-organ disease.
Antimetabolite immunosuppressive medications (mycophenolate mofetil, mycophenolic acid, or azathioprine) were discontinued after diagnosis with COVID-19 per institutional protocol. Immunosuppression was otherwise managed at the discretion of the inpatient transplant providers. No acute graft rejection developed upon decrease in immunosuppressive regimens. Type of immunosuppression on admission was not associated with highest clinical acuity, although more patients in the non-severe group were on a belatacept-based regimen (severe 1/10 vs. non-severe 6/15; p = .18). Of the patients on a tacrolimus-based regimen at admission (N = 18), there was no difference between initial tacrolimus levels in non-severe or severe groups. However, patients in the severe group had significantly lower tacrolimus trough levels during their hospital course. Significantly, more patients in the severe group received convalescent plasma (severe 3/10 vs. non-severe 0/15; p = .05) and methylprednisolone (severe 6/10 vs. non-severe 1/15; p = .01). More patients in the severe group received tocilizumab, which trended toward but did not reach statistical significance (severe 9/10 vs. non-severe 8/15; p = .08). Of the 25 patients, all but one received hydroxychloroquine.
Initial RT-PCR testing was performed prior to or within the first 5 days of hospitalization in all 25 patients, totaling 25 initial tests. A majority of these 25 tests were performed on the first hospital day (19/25 tests on hospital day 1, 5/25 tests on hospital days 2–5; for one patient, the initial test was performed 4 days prior to hospitalization for COVID-19 by screening that followed a nosocomial exposure during a separate hospitalization). One sample (1/25) was reported as inconclusive (LDT N1 CT >40, N2 CT 37.9), whereas all other samples (24/25) were positive.
Initial N2 CT values did not differ when categorizing patients by admission disease severity via applying the modified WHO OSCI score to the first 24 h of hospitalization (initial non-severe N2 CT median 22.9 IQR 18.9–25.1 [N =20] vs. initial severe median N2 CT 21.6 IQR 17.8–36.2 [N =5]; p = .77). Initial N2 CT values also did not differ between severity groups categorized by highest clinical acuity during hospitalization (non-severe median N2 CT 22.9 IQR 16.3–29.4 [N =15] vs. severe median N2 CT 23.1 IQR 18.9–24.6 [N =10]; p = .97). Although significantly more males were in the severe group for highest clinical acuity during hospitalization, no difference was found between gender and initial N2 CT values (male median 23.5 IQR 20.0–25.6 vs. female median 20.5 IQR 16.0–27.9, p = .28).
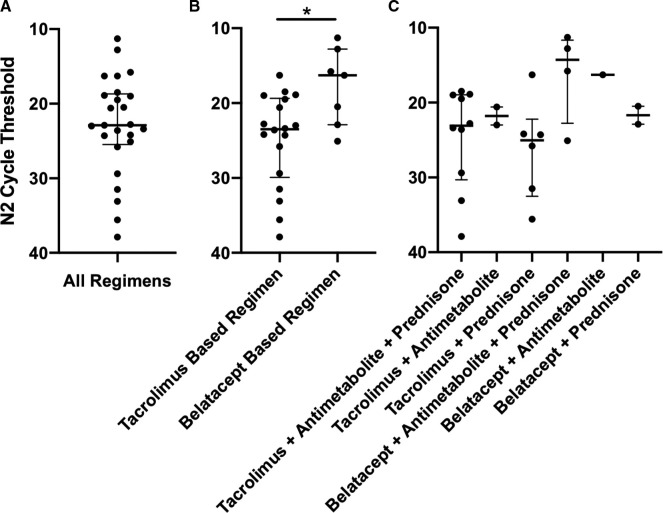
Separating initial N2 CT values by tacrolimus- or belatacept-based immunosuppression revealed significantly higher CT values with tacrolimus-based regimens (tacrolimus-based regimen median 23.5 IQR 19.4–29.9 [N =18] vs. belatacept-based regimen median 16.3 IQR 12.8–22.9 [N =7], p = .02). However, no significant difference was found when separating by individual immunosuppressive regimen (tacrolimus or belatacept with or without an antimetabolite and/or prednisone) ( Figure 1).
FIGURE 1.
Initial N2 CT per immunosuppressive regimen at admission. Error bars represent median and interquartile ranges (IQR). Panel A: Combined N2 CT values. Initial N2 CT values presented in panels B and C are aggregated in panel A. Panel B: N2 CT values as a function of tacrolimus or belatacept. Values were compared using Mann-Whitney test, p = .02 (*). N2 CT values presented in panel C are aggregated in panel B. Panel C: N2 CT values as a function of individual immunosuppressive regimens. Values were compared using Kruskal-Wallis test, p = .19
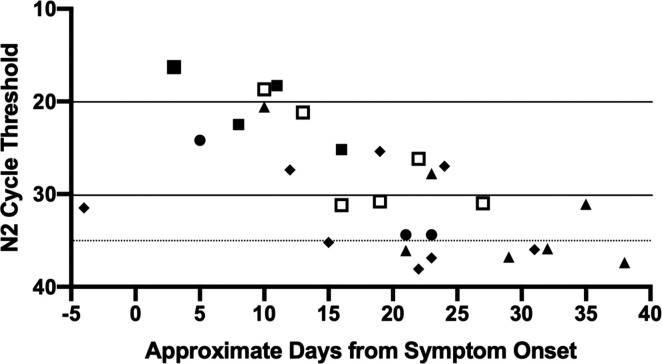
Five patients (four non-severe and one severe) had three or more positive SARS-CoV-2 RT-PCR test results during their admission. Plotting N2 CT values as a function of days from symptom onset demonstrated an increase in CT values over time ( Figure 2). N2 CT values were grouped into low CT (0–20.0) over days 1–11 from approximate symptom onset (three values), moderate CT (20.1–30.0) over days 5–24 (10 values), and high CT (30.1–40.0) over days 15–38 (14 values; one value obtained due to contact tracing following a nosocomial exposure during a non-COVID-19-associated hospital admission was also grouped with high CT values). Four of five patients had N2 CT values <35.0 beyond 21 days after symptom onset and two of five patients had CT values <35.0 beyond 27 days (and up to 35 days). Viral RNA was detected up to 38 days from symptom onset.
FIGURE 2.
N2 CT values from symptom onset. Individual shapes represent five different patients. Solid lines separate values into low N2 CT (0–20.0), moderate N2 CT (20.1–30.0), and high N2 CT (30.1–40.0). One value obtained following a nosocomial exposure but 4 days prior to symptom onset is grouped with high N2 CT viral load values. The dotted line separates values at N2 CT 35.0
4. DISCUSSION
The dynamics of SARS-CoV-2 infection in the setting of solid organ transplantation are not well characterized. At this time, it is unclear whether transplant patients are at higher risk for more severe disease due to uncontrolled viral replication in the setting of compromised immune systems or at lower risk due to altered cytokine production from dampened immune responses. The majority of data suggest that SOT patients experience more severe manifestations of COVID-19, including increased overall mortality.9 , 10 , 19 Identifying markers of severity is particularly important in risk stratifying this vulnerable population. Though clinical and epidemiologic factors have been associated with more severe disease, there are little data on whether CT values correlate with disease severity. Use of CT values as a biomarker to predict disease severity in the general population has been explored, with some studies reporting higher viral loads significantly associated with degree of clinical severity6 , 8 and others finding no association.3 , 4 , 7 A study of renal transplant patients did not find an association with nasopharyngeal CT values and severe disease.20 Similarly, our analysis did not find an association of initial CT values with clinical severity in the first 24 hours of hospitalization or highest clinical acuity during hospitalization among SOT recipients.
We found a significantly higher CT in SOT patients on tacrolimus-based immunosuppressive regimens as compared to those on belatacept-based regimens, though interpretation of this result is limited by the small sample size and confounding factors. Although tacrolimus trough levels were statistically lower in severe patients, the clinical implications of this finding are unclear. Of note, there is biologic plausibility to a lower viral load in SOT patients administered tacrolimus, as tacrolimus has been demonstrated to diminish non-SARS-CoV-2 coronavirus replication in vitro.21 Others have proposed that tacrolimus could diminish SARS-CoV-2 replication in SOT patients.22 Potential positive or negative consequences of immunosuppressive regimens for SOT patients with COVID-19, including effects on clinical outcomes and viral dynamics, require further investigation.
Studies in non-SOT patients consistently describe decreasing viral load over time regardless of sample collection site or assay method.2, 3, 4, 5, 6, 7, 8 We present data on viral dynamics in SOT patients and similarly extrapolate a decrease in viral load, based on higher CT values from upper respiratory tract samples related to time from symptom onset, in an immunocompromised population. All five patients assessed over time continued to have detectible virus greater than 14 days after symptom onset. Virus was detected as long as 38 days after symptom onset, which is consistent with known prolonged PCR positivity in immunocompetent patients as well as recent reports in SOT patients. In a small study of 10 kidney transplant recipients with COVID-19 matched with 10 infected family members, the mean duration of viral shedding was more than twice as long in the SOT group (28 vs. 12 days).23 Similarly, SARS-CoV-2 was detected in a renal transplant patient as distant as 63 days after symptom onset.24 Whereas viral dynamics in immunocompetent patients generally demonstrate a rapid decrease in viral load, dynamics in the five patients in this study suggest a delayed rate of decay.
Some studies have demonstrated that SARS-CoV-2 could not be successfully cultured from clinical specimens with high CT values, particularly in those with CT values ≥33–35.12, 13, 14, 15 Publicly available data from the CDC utilizing the assay on which the Yale New Haven Hospital LDT is based similarly demonstrate the inability to reliably culture SARS-CoV-2 from samples with CT values above this range.25 Since several patients in this study had CT values falling within a potentially “culturable range” (<35.0) beyond 21–27 days after symptom onset, our findings raise the possibility that viable virus may continue to be shed beyond 28 days after symptom onset in SOT patients. This has important implications for transmissibility and infection prevention measures. Our data suggest that current CDC time-based strategies to relieve infection prevention measures (20 days after symptom onset for immunocompromised patients25) may prematurely end precautions for SOT patients since CT values <35, and thus potentially culturable virus, could be present up to 27 days after symptom onset in SOT recipients. Accordingly, prolonged periods of isolation and continued personal protective equipment (PPE) use, potentially 28 days or longer from symptom onset, may be a safer approach for SOT recipients with COVID-19. Alternatively, some have proposed a CT-based approach suggesting CT values of 33–34, interpreted in clinical context, as a cutoff for relaxing infection control measures.26 , 27 This approach could reduce isolation time and PPE use for SOT recipients rather than adopting a 28 days minimum from symptom onset. However, there is currently no universal standardized RT-PCR assay detecting nor quantifying SARS-CoV-2 viral RNA, RT-PCR assays detect differing targets and can yield varying CT values, CT values beyond which SARS-CoV-2 cannot be cultured may be assay dependent, and some assays do not report CT values at all.28 , 29 Furthermore, respiratory samples are not uniform, and CT values differ based on sample acquisition from the upper or lower respiratory tract.6 , 7 Sample collection is operator dependent and quality can vary, likely impacting CT values as well. Together these factors significantly limit the ability to recommend specific CT cutoff values for clinical decision-making. Regardless, optimized infection control practices for SOT recipients could further prevent the spread of SARS-CoV-2, particularly iatrogenic spread among this population.
This study has several limitations, including small sample size, single health system retrospective design, and lack of comparison with matched non-immunosuppressed patients. Reliance on admission documentation to determine approximate symptom onset for comparing baseline CT values is limited by the variability in time from symptom onset to presentation. Though a modified scoring system was used for clinical severity, the modification accounted for patients who would have been classified as non-severe despite requiring ICU-level care and did not affect classification of patients who were already considered severe. Censoring the three patients who remained inpatient at the study conclusion from hospital length-of-stay analysis impacts this result. Administration of tocilizumab to non-severe patients potentially confounds disease severity classification by highest clinical acuity, as tocilizumab may subvert the cytokine release syndrome currently understood as the primary pathophysiologic process of severe COVID-19.30 However, the lack of difference between initial N2 CT values in patients presenting with severe or non-severe COVID-19 during the first 24 h of admission argues against this possibility. Combining N2 CT values obtained from differing assays (LDT vs. Xpert) may be viewed as a limitation, but N2 CT values generated by the Yale New Haven Hospital Clinical Virology laboratory are comparable. Combining N2 values represents a real-world approach as many clinical microbiology laboratories currently utilize multiple assays to test for SARS-CoV-2. Finally, there is no standard classification for SARS-CoV-2 CT values, and our classification of high, moderate, or low CT values is based on currently understood CT value ranges and clinical correlates.
This study describes SARS-CoV-2 CT dynamics specifically in SOT recipients. We found no association of initial N2 CT values with clinical severity in the first 24 h of admission or with highest clinical acuity, suggesting that this is not a reliable biomarker predicting clinical severity in SOT recipients. N2 CT values remained positive for up to 38 days after symptom onset with CT <35.0 up to 35 days after symptom onset. Infection prevention practices based on CT or time-based approaches may need to be prolonged for SOT patients, potentially 28 days or longer after symptom onset. Further studies are needed to elucidate these important questions.
ACKNOWLEDGMENTS
We are grateful for statistical consultation provided by the Yale University StatLab.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Funding information No sources of funding were used to support this work.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Kumar D, Ferreira VH, Blumberg E, et al. A 5-year prospective multicenter evaluation of influenza infection in transplant recipients. Clin Infect Dis. 2018;67(9):1322–1329. doi: 10.1093/cid/ciy294. [DOI] [PubMed] [Google Scholar]
- 2.Zou L, Ruan F, Huang M, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26(5):672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 4.To KK, Tsang OT, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu F, Yan L, Wang N, et al. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis. 2020;71(15):793–798. doi: 10.1093/cid/ciaa345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng S, Fan J, Yu F, et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang JT, Ran RX, Lv ZH, et al. Chronological changes of viral shedding in adult inpatients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020:ciaa631. doi: 10.1093/cid/ciaa631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Yan LM, Wan L, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20(6):656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crespo M, Jose Perez-Saez M, Redondo-Pachon D, et al. COVID-19 in elderly kidney transplant recipients. Am J Transplant. 2020;20(10):2883–2889. doi: 10.1111/ajt.16096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20(7):1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhoori S, Rossi RE, Citterio D, Mazzaferro V. COVID-19 in long-term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol. 2020;5(6):532–533. doi: 10.1016/S2468-1253(20)30116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 13.La Scola B, Le Bideau M, Andreani J, et al. Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards. Eur J Clin Microbiol Infect Dis. 2020;39(6):1059–1061. doi: 10.1007/s10096-020-03913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bullard J, Dust K, Funk D, et al. Predicting infectious SARS-CoV-2 from diagnostic samples. Clin Infect Dis. 2020:ciaa638. doi: 10.1093/cid/ciaa638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singanayagam A, Patel M, Charlett A, et al. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro Surveill. 2020;25(32):2001483. doi: 10.2807/1560-7917.ES.2020.25.32.2001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yale New Haven Hospital. Accelerated Emergency Use Authorization (EUA) Summary SARS-CoV-2 RT-PCR Assay. 2020. https://www.fda.gov/media/136602/download. Accessed August 29, 2020
- 17.Cepheid. Xpert Xpress SARS-CoV-2 Instructions for Use, For Use Under an Emergency Use Authorization (EUA) Only. 2020. https://www.Cepheid.com/Package%20Insert%20Files/Xpress-SARS-CoV-2/Xpert%20Xpress%20SARS-CoV-2%20EUA%20PI%20GX%20System%20rev%20D.pdf. Accessed August 29, 2020.
- 18.World Health Organization (WHO). WHO R&D Blueprint novel Coronavirus COVID-19 Therapeutic Trial Synopsis Draft February 18, 2020. 2020. https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf. Accessed August 29, 2020.
- 19.Tschopp J, L’Huillier AG, Mombelli M, et al. First experience of SARS-CoV-2 infections in solid organ transplant recipients in the Swiss Transplant Cohort Study. Am J Transplant. 2020;20(10):2876–2882. doi: 10.1111/ajt.16062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benotmane I, Gautier Vargas G, Wendling MJ, et al. In-depth virological assessment of kidney transplant recipients with COVID-19. Am J Transplant. 2020. 10.1111/ajt.16251 [DOI] [PMC free article] [PubMed]
- 21.Carbajo-Lozoya J, Muller MA, Kallies S, Thiel V, Drosten C, von Brunn A. Replication of human coronaviruses SARS-CoV, HCoV-NL63 and HCoV-229E is inhibited by the drug FK506. Virus Res. 2012;165(1):112–117. doi: 10.1016/j.virusres.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willicombe M, Thomas D, McAdoo S. COVID-19 and calcineurin inhibitors: should they get left out in the storm? J Am Soc Nephrol. 2020;31(6):1145–1146. doi: 10.1681/ASN.2020030348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu L, Gong N, Liu B, et al. Coronavirus disease 2019 Pneumonia in immunosuppressed renal transplant recipients: a summary of 10 confirmed cases in Wuhan, China. Eur Urol. 2020;77(6):748–754. doi: 10.1016/j.eururo.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang M, Zhang J, Shi H, Liu B, Zeng F. Viral shedding prolongation in a kidney transplant patient with COVID-19 pneumonia. Am J Transplant. 2020;20(9):2626–2627. doi: 10.1111/ajt.15996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention (CDC), USA. Duration of Isolation Precautions for Adults with COVID-19. Coronavirus Disease 2019 (COVID-19). 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html. Accessed August 29, 2020
- 26.Tom MR, Mina MJ. To interpret the SARS-CoV-2 test, consider the cycle threshold value. Clin Infect Dis. 2020:ciaa619. doi: 10.1093/cid/ciaa619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mermel LA. Disposition of patients with COVID-19 infection whose respiratory specimens remain SARS-CoV-2 PCR-positive. Infect Control Hosp Epidemiol. 2020:1–8. doi: 10.1017/ice.2020.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nalla AK, Casto AM, Huang MW, et al. Comparative performance of SARS-CoV-2 detection assays using seven different primer-probe sets and one assay kit. J Clin Microbiol. 2020;58(6) doi: 10.1128/JCM.00557-20. e00557-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rhoads D, Peaper DR, She RC, et al. College of American Pathologists (CAP) Microbiology Committee Perspective: caution must be used in interpreting the Cycle Threshold (Ct) value. Clin Infect Dis. 2020:ciaa1199. doi: 10.1093/cid/ciaa1199. [DOI] [PubMed] [Google Scholar]
- 30.Price CC, Altice FL, Shyr Y, et al. Tocilizumab treatment for cytokine release syndrome in hospitalized COVID-19 patients: survival and clinical outcomes. Chest. 2020;158(4):1397–1408. doi: 10.1016/j.chest.2020.06.006. S0012-3692(20)31670-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.