Dear Editor,
The link between SARS‐CoV‐2 and the reported cutaneous manifestations has not been established. We assessed a possible correlation between the paediatric dermatological manifestations and the biological investigations, using for the first time three different SARS‐CoV‐2 tests.
From April to June, 2020, minors presenting with skin manifestations and symptoms of COVID‐19 themselves or any of first‐degree relatives (i.e. fever, influenza‐like, respiratory, Ear–Nose–Throat and/or digestive symptoms), were enrolled. Epidemiological and clinical information, description of households and biological results including three types of SARS‐CoV‐2 tests [nasal PCR (systemic symptoms within the past 48 h), serology (IgG, techniques: Abbott ARCHITECT) and interferon‐γ(IFN‐γ)‐ELISPOT‐assay] were collected. IFN‐γ‐ELISPOT‐assay, an early (since day 5) qualitative and quantitative analysis, evaluates specific memory T cells by quantifying the IFN‐γ production after a short‐term stimulation with SARS‐COV‐2 peptide. At least one test among serology and IFN‐γ‐ELISPOT‐assay was performed on patients with chilblains.
Thirty patients (20 boys, average = 9.5 years) representing 28 households were included. Thirty‐seven symptomatic first‐degree relatives were analysed. In 23/30 patients (77%) and 14/17 (82%) of chilblains patients, COVID‐19 was suspected in at least one first‐degree relative and confirmed in four including two with chilblains.
Chilblains were reported in 17 patients with a large spectrum of severity (Fig. 1). Lesions occurred before (n = 2, average: 19 days), simultaneously (n = 2, 20%) or after systemic manifestations (60%, average: 22 days). Spontaneous resolution was complete in an average of 27 days (10–50). Two patients relapsed in 15 and 45 days, respectively. Other cutaneous manifestations occurred before (20%, average: 18 days), during (30%) or after systemic manifestations (50%, average: 25 days). Two patients, including one child, presented with a linear pattern of urticarial lesions, both also presented with chilblains (Fig. 1).
Figure 1.
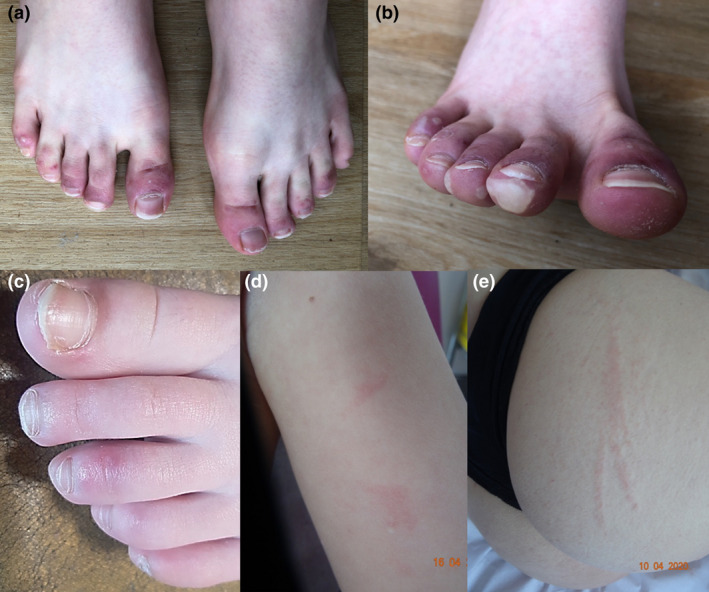
Clinical pictures of our paediatric series during the COVID‐19 pandemic: chilblain‐like lesions associated with livedo (a–c), spontaneous urticarial lesions with linear disposition (d, e).
Elevated CRP [average 14 mg/L (0–200)] and/or increased inflammatory cytokines were noted in 11 children (37%) including 8/17 with chilblains (47%). Cytokine levels were increased in 58%, 50%, 40% and 33% of chilblains patients tested for: TNF‐α (range 20–60 pg/mL), IL‐1 (range 7–280 pg/mL), type 1 IFN (range 2–6 UI/mL) and IL‐6 (range 10–127 pg/mL), respectively (Table 1). In chilblains, tests were performed in an average of 18 days (5–98) and 21 days (6–51) after skin lesions and systemic manifestations onset respectively.
Table 1.
Clinical and biological characteristics of the patients
| Total patients, | Chilblain‐like patients, | |
|---|---|---|
| n = 30 (%) | n = 17 (%) | |
| Mean age (range), sex ratio F: H | 9.5 years (1.8–17.3), 0.5 | 11.2 years (1.8–17.3), 0.4 |
| Household contact with a case of COVID‐19: | ||
| ‐ Probable cases | 23 (77) | 14 (82) |
| ‐ Confirmed cases: PCR/serology/both | 2 (7)/1 (3)/1 (3) | 2 (12)/0/0 |
| Households description: | ||
| ‐ Number of households | 28 | 15 |
| ‐ Total of first‐degree relatives | 79 | 13 |
| Past medical history of patients: | ||
| ‐ Raynaud phenomenon, photosensitivity | 0 | 0 |
| ‐ Auto‐immune disease | 0 | 0 |
| ‐ Inflammatory bowel disease | 1 (3) | 0 |
| ‐ Asthma/atopic dermatitis | 3 (10)/2 (7) | 3 (19)/2 (12) |
| ‐ Urticaria | 1 (3) | 1 (6) |
| ‐ Obesity | 0 | 0 |
| Dermatological manifestations: | ||
| ‐ Chilblains: total/feet/hands/both | 17 (57)/14 (47)/2 (7)/1(3) | 17 (100)/14 (82)/2 (12)/1 (6) |
| ‐ Eccrine hidradenitis | 2 (7) | – |
| ‐ Maculo‐papular rash | 8 (27) | – |
| ‐ Urticaria | 1 (3) | – |
| ‐ Livedo | 2 (7) | – |
| ‐ Targetoid lesions | 2 (7) | – |
| ‐ Vascular/ecchymotic purpura | 1 (3)/1 (3) | – |
| ‐ Erythema nodosum | 1 (3) | – |
| ‐ Mucosal manifestations | 0 | – |
| Average time of cutaneous complete remission | 22 days (1–50) | 27 days (10–50) |
| Symptoms: | ||
| ‐ Mean pruritus scale from 1 to 10 | 7 (1–10), n = 11 (33) | 6 (1–10), n = 6 (62) |
| ‐ Mean VAS pain scale from 1 to 10 | 6 (2–10), n = 9 (27) | 5 (3–8), n = 4 (50) |
| Systemic manifestations: | n = 20 (67) | n = 10 (59) |
| ‐ Fever | 10 (33) | 3 (18) |
| ‐ Influenza‐like symptoms | 13 (43) | 7 (41) |
| ‐ Respiratory symptoms | 10 (33) | 6 (35) |
| ‐ ENT symptoms/anosmia | 10 (33)/1 (3) | 7 (41)/0 |
| ‐ Digestive symptoms | 7 (23) | 3 (18) |
| ‐ Articular symptoms | 1 (3) | 0 |
| Mean time lapse from systemic symptoms to lesions: | n = 20 (67) | n = 10 (59) |
| ‐ Systemic manifestations before | 25 days (3–77), n = 10 (50) | 22 days (5–46), n = 6 (60) |
| ‐ Cutaneous manifestations before | 18 days (2–30), n = 4 (20) | 19 days, n = 2 (20) |
| ‐ Simultaneous manifestations | 0 day, n = 6 (30) | 0 day, n = 2 (20) |
| Laboratory tests: | n = 25 (80) | n = 16 (94) |
| ‐ Anaemia (Hb < 11 g/dL) | 1 (4) | 0 |
| ‐ Hyperlymphocytosis (>5.2 G/L) | 2 (8) | 0 |
| ‐ Neutrophilic hyperleukocytosis (>8 G/L) | 1 (4) | 1 (6) |
| ‐ Elevated liver enzymes (ALT, AST) | 0 | 0 |
| ‐ Elevated creatinine | 1 (4) | 0 |
| ‐ Elevated CRP (>5 mg/L), mean (extremes) | 3 (12), 14 (0–200) | 1 (6), 5 (0–49) |
| ‐ Low PT (<70%) | 2 (8) | 1 (6) |
| ‐ Elevated aPTT (ratio >1.2) | 6 (24) | 3 (19) |
| ‐ Elevated fibrinogène (>3.5 g/L) (n = 15) | 0 | 0 |
| ‐ Elevated d‐dimer level (>500 ng/mL) (n = 15) | 1 (7) | 0 |
| ‐ Positive antinuclear antibodies, mean title (extremes), specificity (n = 17) | 14/17 (82), 264 (100–800), 0 | 11/14 (79), 263 (100–800), 0 |
| ‐ APLA syndrome (β2GP1, cardiolipin, lupus anticoagulant) (n = 15) | 1/15 (7) | 1/12 (8) |
| ‐ Positive C‐ANCA, specificity (n = 17) | 4/17 (23), 0 | 2/14 (14), 0 |
| ‐ Complement anomalies: C3, C4, CH50 (n = 9) | 1 low CH50 at 66 (4) | 1 low CH50 at 66 (7) |
| ‐ Elevated cytokines serum concentrations: | ||
| ○ IL1 (>15 pg/mL), mean (range) | 6/14 (43), 105 (17–280) | 6/12 (50), 104 (7–280) |
| ○ IL6 (>10 pg/mL), mean (range) | 5/15 (33), 59 (10–127) | 4/12 (33), 62 (10–127) |
| ○ TNF‐α (>20 pg/mL), mean (range) | 7/15 (47), 33 (20–60) | 7/12 (58), 31 (20–60) |
| ○ Type 1 IFN (α) (>2 UI/mL), mean (range) | 4/12 (33), 4 (2–6) | 4/10 (40), 4 (2–6) |
| Tests of SARS‐CoV‐2: | ||
| ‐ PCR positive | 0/8 | 0/3 |
| ‐ IgG positive (Abbott ARCHITECT) | 1/26 (4) | 1/16 (6) |
| ‐ IFN‐γ‐ELISPOT‐assay positive | 0/11 (100) | 0/10 (100) |
| Mean duration of follow‐up | 34 day (8–72) | 42 day (11–72) |
The 3/3 nasal PCR were negative. Serology was positive in only 1/16 chilblains patient among the 26 patients tested. IFN‐γ‐ELISPOT‐assay was negative in all the 10 chilblains patients tested. In children with chilblains, these tests were performed in an average of 45 days from lesions onset (5–82) and 56 days from systemic manifestations (35–89).
High levels of cytokines, mostly TNF‐α, IL‐1, type 1 IFN and IL‐6 were noted in 47% of chilblains patients. Biological inflammation was not correlated with: (i) time lapses from cutaneous or systemic symptoms to the blood test, (ii) severity of chilblains. A cytokine storm was described in adults with COVID‐19 1 and in the paediatric inflammatory multisystem syndrome temporally associated with SARS‐COV‐2 infection (PIMS‐TS): elevated CRP and IL‐6 levels. 2
The peak of incidence of COVID‐19 and the reported chilblains occurred simultaneously. 3 , 4 , 5 , 6 , 7 , 8 , 9 In 28/68 reported patients presenting chilblains, 7 , 9 , 10 serology was negative. Only 1/16 chilblains children serology was positive. Sensitivity of our technique varies from 100 to 85% in severe or mild symptomatic patients respectively. Moreover, it is known to be positive after 19 and 30 days of evolution in 85% and 94% of the patients, respectively. In all our patients, the COVID‐19 was confirmed only once, using three different methods. Our result might reflect the estimated prevalence of seropositivity for SARS‐CoV‐2 in the general French population.
While epidemiological data, clinical manifestations and elevated cytokines level suggest an association with SARS‐CoV‐2, no evident link could have been made.
Conflicts of interest
None.
Funding sources
None.
Acknowledgement
The patients in this manuscript have given written informed consent to the publication of their case details.
References
- 1. Huang C, Wang Y, Li X et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Lond Engl 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. ECDC . Paediatric inflammatory multisystem syndrome and SARSCoV‐ 2 infection in children, 2020. URL https://www.ecdc.europa.eu/sites/default/files/documents/covid‐19‐risk‐assessment‐paediatricinflammatory‐multisystem‐syndrome‐15‐May‐2020.pdf (last accessed: May 2020).
- 3. Dong Y, Mo X, Hu Y et al. Epidemiology of COVID‐19 among children in China. Pediatrics 2020; 145. [DOI] [PubMed] [Google Scholar]
- 4. Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J Eur Acad Dermatol Venereol 2020; 34: 212–213. [DOI] [PubMed] [Google Scholar]
- 5. De Masson A, Bouaziz J‐D, Sulimovic L et al. Chilblains are a common cutaneous finding during the COVID‐19 pandemic: a retrospective nationwide study from France. J Am Acad Dermatol 2020; 83: 667–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Galván Casas C, Català A, Carretero Hernández G et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol 2020; 183: 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Andina D, Noguera‐Morel L, Bascuas‐Arribas M et al. Chilblains in children in the setting of COVID‐19 pandemic. Pediatr Dermatol 2020; 37: 406–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Colonna C, Monzani NA, Rocchi A, Gianotti R, Boggio F, Gelmetti C. Chilblain‐like lesions in children following suspected COVID‐19 infection. Pediatr Dermatol 2020; 37: 437–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garcia‐Lara G, Linares‐González L, Ródenas‐Herranz T, Ruiz‐Villaverde R. Chilblain‐like lesions in pediatrics dermatological outpatients during the COVID‐19 outbreak. Dermatol Ther 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. El Hachem M, Diociaiuti A, Concato C et al. A clinical, histopathological and laboratory study of 19 consecutive Italian paediatric patients with chilblain‐like lesions: lights and shadows on the relationship with COVID‐19 infection. J Eur Acad Derlatik Venereol 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]


