Summary
The reported incidence rate of venous and arterial thrombotic events in critically ill patients with COVID‐19 infections is high, ranging from 20% to 60%. We adopted a patient‐tailored thromboprophylaxis protocol based on clinical and laboratory presentations for these patients in our institution. We hypothesised that patients who received high‐intensity thromboprophylaxis treatment would experience fewer thrombotic events. The aims of our study were to explore the incidence of thrombotic events in this population; to assess independent factors associated with thrombotic events and to evaluate the incidence of haemorrhagic events. A retrospective review of all adult patients with confirmed SARS‐CoV‐2 infection admitted to the intensive care unit (ICU) between 1 March and 29 May 2020 was performed. The primary outcome was a composite of venous and arterial thrombotic events diagnosed during the ICU stay. Multivariable logistic regression was used to identify the independent factors associated with thrombotic events. A total of 188 patients met the inclusion criteria. All received some type of thromboprophylaxis treatment except for six patients who did not receive any prophylaxis. Of the 182 patients who received thromboprophylaxis, 75 (40%) received high‐intensity thromboprophylaxis and 24 (12.8%) were treated with therapeutic anticoagulation. Twenty‐one patients (11.2%) experienced 23 thrombotic events (incidence rate of 12.2% (95%CI 7.9–17.8)), including 12 deep venous thromboses, 9 pulmonary emboli and 2 peripheral arterial thromboses. The multivariable logistic regression analysis showed that only D‐dimer (OR 2.80, p = 0.002) and high‐intensity thromboprophylaxis regimen (OR 0.20, p = 0.01) were independently associated with thrombotic events. Thirty‐one patients (16.5%) experienced haemorrhagic events; among them, 13 were classified as major bleeding according to the International Society on Thrombosis and Haemostasis criteria. Therapeutic anticoagulation, but not the high‐intensity thromboprophylaxis regimen, was associated with major bleeding. A proactive approach to the management of thromboembolism in critically ill COVID‐19 patients utilising a high‐intensity thromboprophylaxis regimen in appropriately selected patients may result in lower thrombotic events without increasing the risk of bleeding.
Keywords: COVID‐19, deep venous thrombosis, D‐dimer, pulmonary embolism, thromboembolic events
Introduction
The SARS‐CoV‐2 pandemic has resulted in an influx of patients to hospitals and intensive care units (ICU) globally. While the respiratory manifestations and cytokine storm associated with this virus have been the main concern, the underlying involvement of other organs and systems has also been the subject of much interest and the reason why some patients succumb to the illness [1, 2]. In particular, the unique haematologic presentations observed in some of the sickest patients and the high incidence of thrombotic events have led to a global effort to identify optimal proactive management strategies [3, 4, 5]. The haematologic changes reported in COVID‐19 (including prolongation of prothrombin time and activated partial thromboplastin time, elevations of D‐dimers and fibrinogen and a mild to moderate thrombocytopenia) are not identical to that of acute disseminated intravascular coagulopathy which is commonly seen in ICU [5, 6]. This is supported by the fact that while some of these haematologic abnormalities may mimic disseminated intravascular coagulopathy, the most common manifestation of COVID‐19 coagulopathy is thrombosis rather than bleeding with a high incidence of thrombotic events in critically ill patients [2, 5, 7, 8, 9].
A recent multinational consensus statement from the International Society on Thrombosis and Haemostasis highlighted the high incidence of venous thromboembolism, the severity of the occurrence, and called for a systematic approach to venous thromboembolism prevention, diagnosis and treatment for patients with COVID‐19 [10]. At our institution, we adopted an anticoagulation protocol that provides a patient‐tailored algorithm, utilising stratification based on clinical and laboratory presentations, balancing bleeding and thrombotic risk. While the cut‐off values for these diagnostic tests and the response to them continues to be modified as more data emerge, we derived risk stratification values based on the available evidence [11, 12].
We hypothesised that patients who received high‐intensity thromboprophylaxis treatment would experience fewer venous and arterial thrombotic events. The aims of our study were to explore the incidence of thrombotic events in our critically ill COVID‐19 patients; to assess factors that are independently associated with thrombotic events and to evaluate the incidence of the occurrence of haemorrhagic events.
Methods
We performed a retrospective review of all adult patients admitted to the ICU at Cleveland Clinic, Abu Dhabi, with confirmed SARS‐CoV‐2 infection, as detected by a real‐time reverse transcription‐polymerase chain reaction assay, between 1 March and 29 May 2020. Patients were not included if they had a brief (< 24 h) ICU stay or if the COVID‐19 infection was deemed to be incidental and did not impact their ICU admission. In addition, we did not include patients who had a confirmed thrombotic event diagnosis before ICU admission. The institutional Ethics Committee of Cleveland Clinic Abu Dhabi approved the study and waived the need for informed consent due to the retrospective nature of the study.
The primary outcome was a composite of venous and arterial thrombotic events diagnosed during ICU stay. Venous thromboembolism included pulmonary embolism and deep venous thrombosis. Central venous catheter‐related thrombosis and haemodialysis venous thrombosis, as well as ischaemic stroke and myocardial infarction that occurred before ICU admission, were not considered as part of thrombotic events and were not analysed. The secondary outcome was the overall occurrence of haemorrhagic events and the rate of major bleeding, which was defined according to the International Society on Thrombosis and Haemostasis criteria [13].
Data were collected on baseline characteristics including the reason for ICU admission and the presence of medical comorbidities. Laboratory values including full blood count, coagulation parameters and inflammatory markers (C‐reactive protein, interleukin 6 and ferritin) were collected on admission to ICU and day 7 of ICU stay (or at time of the thrombotic event or at ICU discharge, whichever was sooner). We also recorded the maximal D‐dimer value (max D‐dimer) at any point during ICU stay. Clinical outcomes captured included ICU mortality; the need for and duration of mechanical ventilation; ICU length of stay; the need for vasopressors; renal replacement therapy; prone position; the use of neuromuscular blocking drugs; and extracorporeal membrane oxygenation. The strategy used for medical thromboprophylaxis was also documented.
The hospital’s established protocol (Fig. 1) provides an algorithm that uses D‐dimer limits to determine appropriate candidates for standard (enoxaparin 40 mg daily) or high‐intensity thromboprophylaxis (enoxaparin 40 mg twice daily) and those who need to undergo venous thromboembolism imaging studies. This was performed using deep venous compression ultrasonography for upper and lower limb extremities and computed tomography pulmonary angiography. Patients with suspected peripheral arterial ischaemia also had a computed tomography angiogram.
Figure 1.
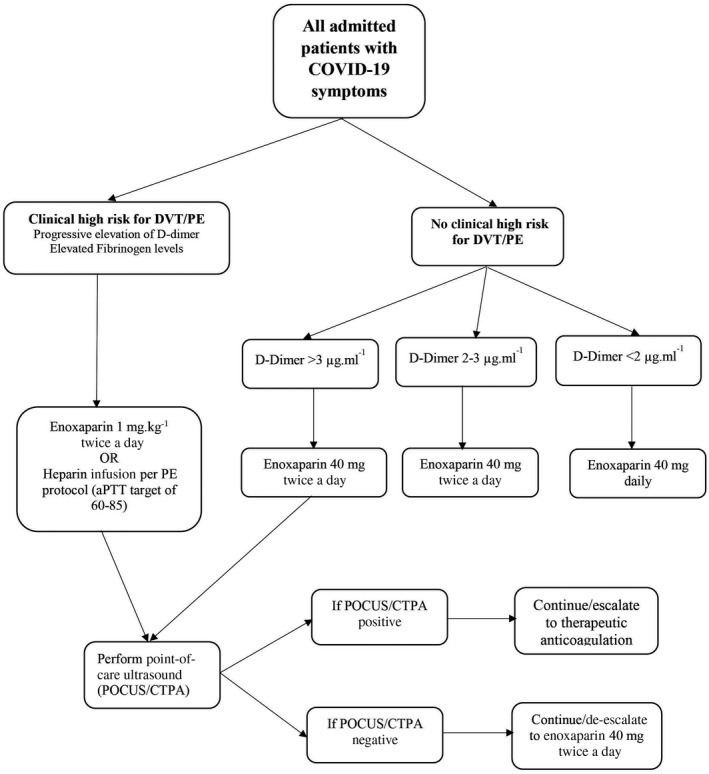
Hospital thromboprophylaxis protocol for COVID‐19 patients. DVT, deep venous thrombosis; PE, pulmonary embolism; BID: twice a day; CTPA, computed tomography pulmonary angiogram; POCUS, point of care ultrasound; apTT, activated partial thromboplastin time.
Patients who were deemed at high risk for bleeding (defined as venous thromboembolism bleed score > 2 [14]) (see online Supporting Information Appendix S1), platelet count < 50 × 109.l−1 or INR > 2, were not included. Similar to routine ICU practice, those patients presenting with a high clinical suspicion of venous thromboembolism, or pulmonary embolism were placed on therapeutic anticoagulation while awaiting confirmatory studies to guide continuation or de‐escalation.
Dosing adjustments in case of renal impairment or extreme body weight were also provided (see online Supporting Information Appendix S1). The hospital protocol for anticoagulation of patients receiving extracorporeal oxygenation membrane is intravenous heparin infusion aiming for activated partial thromboplastin time between 40 and 60 s.
Statistical analyses were performed using SPSS software version 20.0 (IBM corporation, Armonk, NY, USA). Normality of data distribution was assessed using the Shapiro–Wilk test and by visually checking the distribution (histogram) of each variable. Comparisons of values between independent groups were performed by the two‐tailed Student t test or the Mann–Whitney U test, as appropriate. Analysis of the discrete data was performed by χ2 test or Fisher's exact test when the numbers were small. Pairwise comparisons between different study times were assessed using the paired Student’ t test or Wilcoxon’s test, as appropriate. The Bonferroni method was used to adjust for multiple comparisons. No imputation was made for missing data. Variables with > 25% of patients missing data were not reported or included in the analysis.
A receiver operating characteristics (ROC) curve was constructed to evaluate D‐dimer's ability to predict thrombotic events. The best cut‐off for a ROC curve was chosen with the highest Youden index [15]. Sensitivity, specificity, positive predictive value, negative predictive value and their 95%CIs were calculated for the best cut‐off value. A multivariable logistic regression analysis was used to identify significant independent factors that were associated with thrombotic events. Variables that were associated with thrombotic events (p < 0.1) in univariate analysis, and that are known to influence the thrombotic event occurrence, were entered in the model. The potential problem of co‐linearity was evaluated using Spearman or Pearson correlation coefficient before running the analysis. Goodness of fit of the model was assessed using the Hosmer–Lemeshow’s test. A value of p < 0.05 was considered statistically significant and all reported p values are two‐sided.
Results
From 1 March to 29 May 2020, 188 adult patients with COVID‐19 were admitted to ICU and included in this study (Fig. 2). The main characteristics of the cohort are summarised in Table 1.
Figure 2.
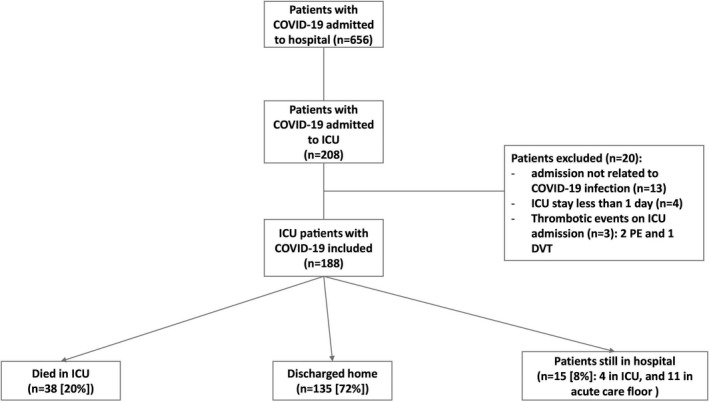
Flow chart of COVID‐19 patients admitted to the intensive care unit (ICU) and their outcomes. PE, pulmonary embolism; DVT, deep venous thrombosis.
Table 1.
Baseline characteristics in thromboembolic events and non‐thrombotic event groups. Values are median (IQR [range]) or number (proportion).
| Variables |
All patients n = 188 |
Thrombotic events n = 21 |
Non‐thrombotic events n = 167 |
p value |
|---|---|---|---|---|
| Age; years | 49 (40–61 [22–102]) | 50 (36–54 [31–84]) | 49 (40–62 [22–102]) | 0.42 |
| Male | 154 (82%) | 18 (86%) | 136 (81%) | 0.63 |
| BMI; kg.m−2 | 26.4 (24.0–30.5 [14.6–54.1]) | 26.0 (23.3–30.1 [18.2–34.3]) | 26.5 (24.1–30.5 [14.6–54.1]) | 0.30 |
| Any comorbidities | 118 (63%) | 12 (57%) | 106 (64%) | 0.52 |
| Diabetes mellitus | 73 (39%) | 7 (33%) | 66 (40%) | 0.64 |
| Hypertension | 76 (41%) | 8 (38%) | 68 (41%) | 0.82 |
| Chronic artery disease | 22 (12%) | 1 (5%) | 21 (13%) | 0.48 |
| Chronic kidney disease | 23 (12%) | 1 (5%) | 22 (13%) | 0.48 |
| Malignancy | 8 (4%) | 2 (10%) | 6 (4%) | 0.22 |
| Reasons for ICU admission; n (%) | ||||
| Acute respiratory distress syndrome | 143 (76%) | 17 (81%) | 126 (75%) | 0.85 |
| Other | 45 (24%) | 4 (19%) | 41 (25%) | |
| Laboratory data on ICU admission | ||||
| C‐reactive protein; mg.l‐1 | 110 (43–204 [0–509]) | 107 (21–221 [5–509]) | 111 (43–204 [0–479]) | 0.97 |
| Leucocytes count; × 109.l‐1 | 8.8 (6.3–12.1 [2.1–30.4]) | 9.5 (6.7–14.8 [3.2–30.4]) | 8.8 (6.2–12.2 [2.1–25.0]) | 0.50 |
| Lymphocytes count; ×109.l‐1 | 0.9 (0.6–1.3 [0.1–6.0) | 0.9 (0.5–1.4 [0.1–2.7]) | 0.9 (0.6–1.3 [0.1–6.0]) | 0.90 |
| Lymphocytes ≤ 1 × 109.l‐1; | 111 (59%) | 13 (65%) | 98 (59%) | 0.56 |
| Platelet count; ×109.l‐1 | 226 (174–285 [60–548]) | 246 (181–310 [128–482]) | 226 (173–285 [60–548]) | 0.55 |
| INR | 1.2 (1.1–1.3 [0.9–10]) | 1.2 (1.1–1.3 [0.9–1.3]) | 1.2 (1.1–1.3 [0.9–10]) | 0.70 |
| Activated partial thromboplastin time; s | 34 (30–38) [23–179]) | 31 (28–38 [24–59]) | 34 (30–38 [23–179]) | 0.40 |
| D‐dimer; µg.ml‐1 [normal reference:<0.05] | 1.8 (0.8–3.9 [0.3–4.0]) | 4.0 (1.8–4.0 [0.5–4.0]) | 1.5 (0.8–3.5 [0.3–4.0]) | 0.004 |
| D‐dimer (max); µg.ml‐1 | 3.5 (1.3–4.0 [0.3–4.0]) | 4.0 (4.0–4.0 [0.5–4.0]) | 3.0 (1.2–4.0 [0.3–4.0]) | <0.001 |
| D‐dimer (max) >3 µg.ml‐1 | 97/179 (52%) | 18/21 (86%) | 79/158 (50%) | 0.002 |
| Fibrinogen; g.l‐1 | 6.0 (4.5–7.1 [0.8–8.0]) | 5.4 (2.3–6.7 [1.5–8.0]) | 6.0 (4.7–7.2 [0.8–8.0]) | 0.22 |
| Ferritin; µg.l‐1 [reference range: 36–480] | 995 (494–1904 [30–8000]) | 843 (529–2333 [180–3625]) | 1009 (492–1809 [30–8000]) | 0.70 |
| Interleukin‐6; ng.l‐1 | 155 (54–620 [2–10748]) | 668 (45–2264 [15–10748]) | 153 (55–437 [2–7946]) | 0.25 |
| ICU treatments | ||||
| Invasive mechanical ventilation | 98 (52%) | 15 (71%) | 83 (50%) | 0.06 |
| Extracorporeal membrane oxygenation | 9 (5%) | 3 (14%) | 6 (4%) | 0.065 |
| Thromboprophylaxis strategy | ||||
| None | 6 (3%) | 0 | 6 (4%) | 0.46 |
| Standard prophylactic dose | 83 (44%) | 11 (52%) | 72 (43%) | |
| High‐intensity prophylactic dose | 75 (40%) | 6 (29%) | 69 (41%) | |
| Therapeutic anticoagulation | 24 (13%) | 4 (19%) | 20 (12%) | |
| ICU outcomes | ||||
| ICU mortality | 38 (20%) | 5 (24%) | 33 (20%) | 0.77 |
| Duration of mechanical ventilation, day | 14 (7–29 [1–81]) | 16 (10–32 [1–74]) | 13 (6–28 [1–81]) | 0.24 |
| ICU length of stay, days | 9 (5–21 [2–75]) | 20 (8–30 [3–74]) | 9 (5–19 [2–75]) | 0.01 |
All patients received thromboprophylaxis except for six; four of these did not receive thromboprophylaxis due to a high risk of gastrointestinal bleeding, one patient had severe thrombocytopenia and one had an epidural haematoma. A standard prophylactic dose of enoxaparin 40 mg daily (Fig. 1, online Supporting Information Appendix S1) was used in 83 (44 %) patients; 75 (40%) received high‐intensity thromboprophylaxis and 24 (12.8%) were treated with therapeutic doses of heparin, including four who were on oral anticoagulation at home for atrial fibrillation. Among 179 patients (nine missing values), 111 (62%) had a D‐dimer max ≥ 2µg.ml−1.
Twenty‐one of 188 patients (11.2%) experienced a total of 23 thrombotic events, giving an incidence of 12.2% (95%CI 7.9–17.8), including 12 (52.2%) deep venous thromboses, 9 (39%) pulmonary emboli and 2 (8.7%) peripheral arterial thromboses. The median (IQR [range]) time from ICU admission to thrombotic events was 6 (1–13 [0–24]) days. Eighty‐two patients (43.6%) underwent 92 venous thromboembolism imaging studies. No significant differences were found in baseline characteristics between patients who did and did not experience thrombotic events except for the D‐dimer level, which was significantly higher in the thrombotic events group (Table 1). Invasive mechanical ventilation, prone position and vasopressor support were not significantly associated with thrombotic events although the use of neuromuscular blocking drugs was significantly higher in those who had thrombotic events (52.4% vs. 30.5%, p = 0.045).
The use of high‐intensity thromboprophylaxis was higher in the non‐thrombotic events group (40.7%) than in the thrombotic events group (28.6%); however, thromboprophylaxis regimen was not significantly associated with thrombotic events. There was no difference in ICU mortality and duration of mechanical ventilation between the two groups although the median ICU length of stay was significantly longer in the thrombotic events group (Table 1). As of 10 July, 150 patients were alive (mortality rate 20%); among them, 135 (90%) were discharged from hospital. Median (IQR [range]) ICU length of stay was 9 (5–21[2–75]) days.
Multivariable logistic regression analysis was performed to determine the factors that were independently associated with the occurrence of thrombotic events. In the univariate analysis (Table 1), D‐dimer, invasive mechanical ventilation, neuromuscular blocking drugs and extracorporeal membrane oxygenation were associated with thrombotic events (p < 0.1). Thus, these variables were entered into the multivariable model. Thromboprophylaxis regimen was also included in the model, even if it was not statistically associated with thrombotic events (p > 0.1), as it is a well‐known factor that can influence the thrombotic events occurrence. Table 2 shows the results of the multivariable analysis. Only D‐dimer max and thromboprophylaxis regimen were independently associated with thrombotic events. The Hosmer and Lemeshow’s test was not statistically significant (p = 0.76), testifying to the goodness of fit of the model. Elevated D‐dimers increased the risk of thrombotic events (OR = 2.80), whereas high‐intensity thromboprophylaxis regimen, but not therapeutic dose, was associated with a lower‐risk of thrombotic events compared with the regular prophylactic regimen (OR = 0.20; Table 2). Replacing D‐dimer max by D‐dimer level on ICU admission in the multivariable model yielded the same findings (see online Supporting Information Table S1).
Table 2.
Multivariable logistic regression analysis.
| OR | 95%CI | p value | |
|---|---|---|---|
| D‐dimer (maximum); µg.ml‐1 | 2.80 | 1.45–5.38 | 0.002 |
| Mechanical ventilation (refer: no) | 0.80 | 0.18–3.52 | 0.77 |
| Neuromuscular blocking drugs; (refer: no) | 1.15 | 0.28–4.76 | 0.84 |
| Extracorporeal membrane oxygenation; (refer: no) | 1.91 | 0.28–12.90 | 0.51 |
| Thromboprophylaxis strategy | |||
| Standard prophylactic dose (refer) | – | ||
| High‐intensity prophylaxis dose | 0.20 | 0.06–0.69 | 0.01 |
| Therapeutic anticoagulation | 0.40 | 0.08–1.86 | 0.24 |
The ability of D‐dimer max to predict thrombotic events occurrence was acceptable with area under the ROC curve of 0.730 (95%CI 0.659–0.793) (Fig. 3). The best cut‐off value (according to Youden index) was > 3.93 µg.ml−1 (95%CI: >3.84 µg.ml−1 to > 3.93 µg.ml−1) with a sensitivity of 86% (95%CI 64–97%), specificity of 61% (95%CI 53–68%), positive predictive value of 21% (95%CI 13–32%) and negative predictive value of 97% (95%CI 92–99%). A cut‐off of D‐dimer > 1.57 µg.ml−1 had a sensitivity of 95% (95%CI 76–100%), specificity of 34% (95%CI 27–42%), positive predictive value of 15% (95%CI 10–23%) and negative predictive value of 98% (95%CI 90–100%).
Figure 3.
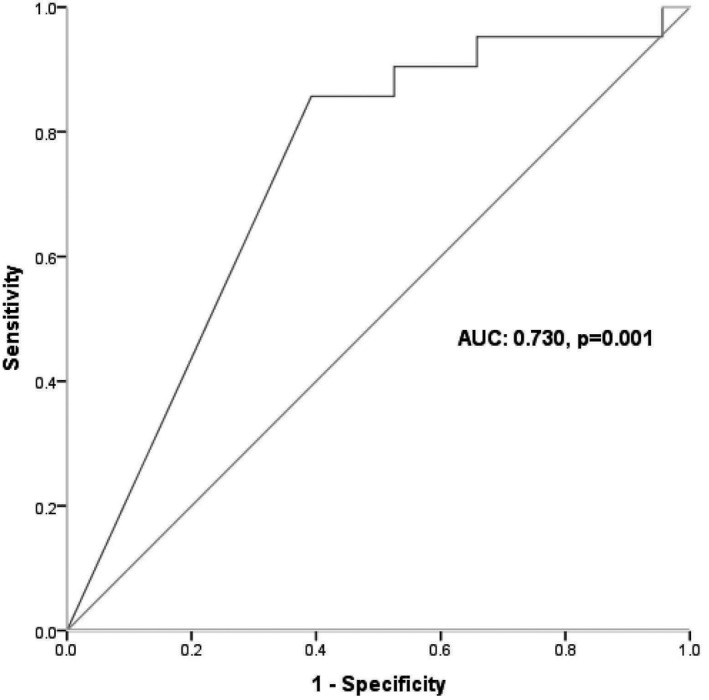
Receiver operating characteristic curve showing the ability of D‐dimer (maximum) to predict the occurrence of thromboembolic events.
Figure 4 shows the changes in blood results between ICU admission and time of thrombotic events occurrence or day 7 in both thrombotic events and non‐thrombotic events groups. D‐dimer increased significantly in the thrombotic events group (p = 0.017), although it was higher than the non‐thrombotic group (p < 0.001) which did not change during admission. Fibrinogen decreased significantly in the non‐thrombotic events group (p < 0.001), and did not change in the thrombotic events group.
Figure 4.
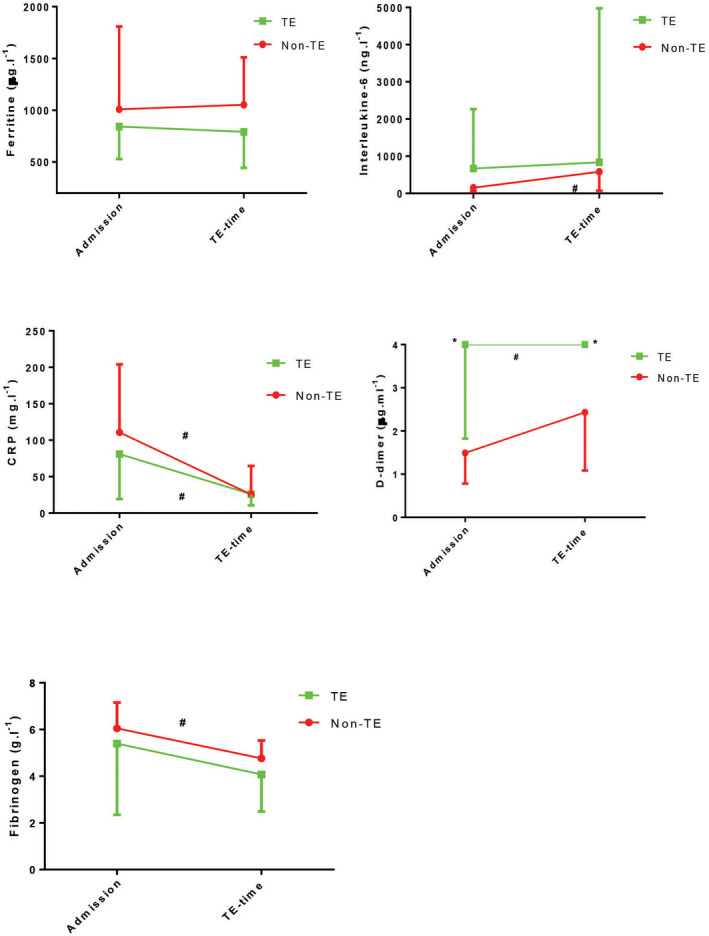
Results of inflammatory and coagulation markers between ICU admission and time of thromboembolic thromboembolic (TE) events in thromboembolic and non‐thromboembolic groups.*p < 0.025 between thromboembolic and non‐thromboembolic comparisons (Bonferroni method). #p < 0.05 within‐group comparisons. Data are median (IQR).
Thirty‐one patients (16.5%) experienced haemorrhagic events during their ICU stay; among them, 13 were classified as major bleeding according to the International Society on Thrombosis and Haemostasis criteria. The median (IQR [range]) time from ICU admission to haemorrhagic events was 12 (3–21 [0–52]) days. Out of the 24 patients who received therapeutic anticoagulation, five (21%) had major haemorrhagic events compared with 8 out of 164 patients (5%) who did not receive therapeutic anticoagulation (p = 0.014). Among the 75 patients who received high‐intensity prophylactic regimen, only 2 (2.7%) experienced major bleeding. Five patients (38.5%) out of the 13 who experienced major bleeding received extracorporeal membrane oxygenation compared with 4 (2.3%) out of 175 patients who did not experience major bleeding (p < 0.001). Renal replacement therapy was higher in patients with major bleeding (6/13) than in patients without major bleeding (23/175) (46% vs. 13%, p = 0.006). Intensive care mortality was significantly higher in patients who experienced major bleeding (9/13) compared with those with no major bleeding (29/174) (69% vs. 17%, p < 0.001).
Discussion
In our COVID‐19 ICU patients, we found that the overall incidence of thrombotic events was 12.2% and that D‐dimers were independent predictors of thrombotic events. The use of high‐intensity prophylactic treatment was associated with a lower incidence of thrombotic events without increasing major bleeding. However, therapeutic anticoagulation was associated with major bleeding.
The impact of COVID‐19 on coagulation and haemostasis is multifactorial and includes systemic inflammation and activation of the complement system (cytokine storm). Heparin is known to have a role as an anti‐inflammatory agent through mechanisms that include binding to inflammatory cytokines [16, 17, 18, 19] as well as its inherent anticoagulant properties. It also potentially prevents viral attachment through binding to host or viral glycoproteins [20].
The overall thrombotic rate observed in our study of 12.2% is lower than that previously reported with severe COVID‐19 in ICU, which has ranged from 20% to 69% [5, 26]. The lower rate of thrombotic events may be explained by the impact of our hospital thromboprophylaxis protocol. Indeed, our results showed that high‐intensity thromboprophylaxis regimen was independently associated with a lower risk of thrombotic events. Maatman et al., in their observational study of 109 COVID‐19 patients admitted to ICU, demonstrated the inadequacy of routine thromboembolic prophylaxis regimens reporting a venous thromboembolism rate of 28% [26]. Another retrospective study also reported a reduction in venous thromboembolism after the intensification of thromboprophylaxis [27]. Nevertheless, Trigonis et al. observed no association between thromboprophylaxis anticoagulation regimen and the rate of deep venous thrombosis in 45 critically ill COVID‐19 patients [25].
The ability of D‐dimer to predict the occurrence of thrombotic events was acceptable (area under the ROC curve = 0.730) and similar to previous reports (area under the ROC curve = 0.729 and 0.760) [26, 28]. We found that a D‐dimer value of 1.57 µg.ml−1 had an excellent negative predictive value (98%) to rule out thromboembolism. This value of D‐dimer is close to that in our hospital protocol (2 µg.ml−1) on which we base the decision to use a high‐intensity thromboprophylaxis regimen (Fig. 1). We also found that a high value of D‐dimer (> 3.93 µg.ml−1) had a poor positive predictive value (22%) to ascertain the presence of thrombotic events. Our findings suggest that therapeutic anticoagulation based only on elevated D‐dimer levels cannot be recommended. It is difficult to compare these results with those of previous reports since most of them have used a different definition of thrombotic events and different thromboprophylaxis regimens [25, 28, 29].
The occurrence of major haemorrhagic events was significantly higher in those who received therapeutic anticoagulation but not in those who received the high‐intensity prophylactic regimen. Our clinical algorithm does not lead to full‐dose anticoagulation, except in patients who have venous thromboembolism confirmed through imaging or when there is a high clinical suspicion based on the presentation, where it is given until confirmatory imaging studies are performed. The indications for therapeutic anticoagulation included imaging‐confirmed thrombotic events, extracorporeal membrane oxygenation and clotted renal replacement therapy circuits. Helms et al. observed a low rate of haemorrhagic events (2.7%) among 150 critically ill COVID‐19 patients [8]; however, the authors did not report the number of patients who received therapeutic anticoagulation. In a different recent study, haemorrhagic complications were reported in 21% of COVID‐19 ICU patients [9] where therapeutic anticoagulation was used. The rate of major bleeding among these haemorrhagic events was high (77%) [9]. Therefore, we cannot recommend the liberal use of therapeutic anticoagulation in critical COVID‐19 patients.
Our study is the first to demonstrate the utility and safety of a patient‐tailored thromboprophylaxis regimen with a high‐intensity prophylactic dosage based on a D‐dimer level to reduce the rate of thrombotic events in critically ill COVID‐19 patients. In addition, therapeutic anticoagulation was associated with a higher rate of major bleeding without decreasing thrombotic events rate. However, further studies are needed to confirm our findings.
Our study has some important limitations. It is a single‐centre retrospective study conducted at a quaternary care facility in the Middle East, and thus management and outcomes do not necessarily reflect those at other centres. Less than half of the patients underwent imaging for thrombotic events, which may have resulted in missing events in some patients. Also, we did not include central venous and haemodialysis catheter‐related thrombosis as part of thrombotic events. This might, in part, explain the lower observed rate of thrombotic events in our study than previously reported. However, even though many ICUs observed an increase in such thrombosis in COVID‐19 patients, its real incidence is still unknown. While we implemented an institutional protocol that prompts providers to order imaging based on D‐dimer cut‐off values (D‐dimer > 3 µg.l−1, Fig. 1), the ultimate decision to prescribe anticoagulation was still at the discretion of the provider.
In conclusion, we have found that a proactive approach to thromboembolism in COVID‐19 critically ill patients through utilising a high‐intensity pharmacological thromboprophylaxis in appropriately selected patients may result in reduced thrombotic events without increasing the risk of bleeding. Screening patients through imaging studies based on elevated D‐dimer (> 6 times the upper limit of normal) may also aid in the early detection of thrombotic events in the highest risk patients. However, more trials are needed to establish the best thromboprophylaxis approach in COVID‐19 ICU patients.
Supporting information
Appendix S1. Protocol: Information related to the Institutional thromboprophylaxis protocol.
Table S1.Results of the multivariate logistic regression results when D‐dimer value on admission was included in the model instead of max D‐dimer.
Acknowledgements
No external funding or competing interests declared
Contributor Information
B. Atallah, @batallah08.
W. Almahmeed, @wmahmeed.
J. Mallat, Email: mallatjihad@gmail.com, @mallat_jihad.
References
- 1. Connors JM, Levy JH. COVID‐19 and its implications for thrombosis and anticoagulation. Blood 2020; 135: 2033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lax SF, Skok K, Zechner P, et al. Pulmonary arterial thrombosis in COVID‐19 with fatal outcome: results from a prospective, single‐center, clinicopathologic case series. Annals of Internal Medicine 2020; 14: M20–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Atallah B, Mallah SI, AlMahmeed W. Anticoagulation in COVID‐19. European Heart Journal Cardiovascular Pharmacotherapy 2020; 6: 260–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Paranjpe I, Fuster V, Lala A, et al. Association of treatment dose anticoagulation with in‐hospital survival among hospitalized patients with COVID‐19. Journal of the American College of Cardiology 2020; 76: 122–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klok FA, Kruip MJ, van der Meer NJ, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thrombosis Research 2020; 191: 145–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang C, Wang Y, Li C, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Poissy J, Goutay J, Caplan M, et al. Pulmonary embolism in COVID‐19 patients: awareness of an increased prevalence. Circulation 2020; 142: 184–6. [DOI] [PubMed] [Google Scholar]
- 8. Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS‐CoV‐2 infection: a multicenter prospective cohort study. Intensive Care Medicine 2020; 46: 1089–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fraissé M, Logre E, Pajot O, Mentec H, Plantefève G, Contou D. Thrombotic and hemorrhagic events in critically ill COVID‐19 patients: a French monocenter retrospective study. Critical Care 2020; 24: 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. International Society on Thrombosis and Haemostasis (ISTH) . A systematic approach for managing venous thromboembolism in patients with COVID‐19. July 15, 2020. https://www.isth.org/news/517212/A‐Systematic‐Approach‐for‐Managing‐Venous‐Thromboembolism‐in‐Patients‐with‐COVID‐19.htm (accessed 17/07/2020).
- 11. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. Journal of Thrombosis and Haemostasis 2020; 18: 844–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Velavan TP, Meyer CG. Mild versus severe COVID‐19: laboratory markers. International Journal of Infectious Diseases 2020; 95: 304–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. Journal of Thrombosis and Haemostasis 2005; 3: 692–4. [DOI] [PubMed] [Google Scholar]
- 14. Klok FA, Barco S, Turpie AGG, et al. Predictive value of venous thromboembolism (VTE)‐BLEED to predict major bleeding and other adverse events in a practice‐based cohort of patients with VTE: results of the XALIA study. British Journal of Haematology 2018; 183: 457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ray P, Le Manach Y, Riou B, Houle TT. Statistical evaluation of a biomarker. Anesthesiology 2010; 112: 1023–40. [DOI] [PubMed] [Google Scholar]
- 16. Mummery RS, Rider CC. Characterization of the heparin‐binding properties of IL‐6. Journal of Immunology 2000; 165: 5671–569. [DOI] [PubMed] [Google Scholar]
- 17. Young E. The anti‐inflammatory effects of heparin and related compounds. Thrombosis Research 2008; 122: 743–52. [DOI] [PubMed] [Google Scholar]
- 18. Poterucha TJ, Libby P, Goldhaber SZ. More than an anticoagulant: do heparins have direct anti‐inflammatory effects? Thrombosis and Haemostasis 2017; 117: 437–44. [DOI] [PubMed] [Google Scholar]
- 19. Mousavi S, Moradi M, Khorshidahmad T, Motamedi M. Anti‐inflammatory effects of heparin and its derivatives: a systematic review. Advances in Pharmacological Sciences 2015; 2015: 507151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shukla D, Spear PG. Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. Journal of Clinical Investigation 2001; 108: 503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID‐19 patients admitted to an academic hospital in Milan, Italy. Thrombosis Research 2020; 191: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. Journal of Thrombosis and Haemostasis 2020; 18: 1421–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Llitjos J‐F, Leclerc M, Chochois C, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID‐19 patients. Journal of Thrombosis and Haemostasis 2020; 18: 1743–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Klok FA, Kruip MJHA, van der Meer NJM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID‐19: An updated analysis. Thrombosis Research 2020; 191: 148–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Trigonis RA, Holt DB, Yuan R, et al. Incidence of venous thromboembolism in critically ill coronavirus disease 2019 patients receiving prophylactic anticoagulation. Critical Care Medicine 2020; 48: e805–e808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maatman TK, Jalali F, Feizpour C, et al. Routine venous thromboembolism prophylaxis may be inadequate in the hypercoagulable state of severe coronavirus disease 2019. Critical Care Medicine 2020; 48: e783–e790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Filippidis P, Kampouri E, Viala B, et al. Reduction of venous thromboembolic events in hospitalized patients with coronavirus disease 2019 after intensification of thromboprophylaxis. Research and Practice in Thrombosis and Haemostasis 2020; 4(Suppl 1). [Google Scholar]
- 28. Demelo‐Rodríguez P, Cervilla‐Muñoz E, Ordieres‐Ortega L, et al. Incidence of asymptomatic deep vein thrombosis in patients with COVID‐19 pneumonia and elevated D‐dimer levels. Thrombosis Research 2020; 192: 23–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Artifoni M, Danic G, Gautier G, et al. Systematic assessment of venous thromboembolism in COVID‐19 patients receiving thromboprophylaxis: incidence and role of D‐dimer as predictive factors. Journal of Thrombosis and Thrombolysis 2020; 50: 211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Protocol: Information related to the Institutional thromboprophylaxis protocol.
Table S1.Results of the multivariate logistic regression results when D‐dimer value on admission was included in the model instead of max D‐dimer.


