Abstract

The present manuscript pertains to the design and synthesis of a series of 3-hydroxyindole-substituted β-carbolines/tetrahydro-β-carbolines with an aim to explore their antiproliferative structure–activity relationship against breast cancer. The conjugate with an optimum combination of a flexible tetrahydro-β-carboline core, a tertiary alcoholic group along with a chloro substituent on the indole ring, proved to be the most active compound. It displayed IC50 values of 13.61 and 22.76 μM against MCF-7 (ER+) and MDA-MB-231 (ER−) cells, respectively. The docking studies were found to be consistent with experimental results owing to the stronger binding affinity of the synthesized conjugates via hydrophobic and H-bonding interactions.
Introduction
Among various life-threatening diseases in women, the increasing incidence of breast cancer is considered as a global health concern. The current statistics of breast cancer are alarming with an estimated 271 270 new cases diagnosed with approximately 42 260 deaths in the United States in 2019. According to a survey conducted by the Health Ministry of India, the situation is even worse in India as breast cancer ranks number one among Indian females with an incidence rate of 25.8 per 100 000 women and the figure is expected to get double by 2020. India also faces a challenge of a low survival rate of only 66.1% as compared to the United States and Australia that have a survival rate of 90%.1 Two-thirds of patients suffering from breast cancer exhibit immune-histochemical expression of estrogen receptors (ER+), progesterone receptors (PR+), and human epidermal growth factor receptor 2 (HEGF2), thus enabling an effective targeted therapy,2 while 10–20% are diagnosed with an aggressive form of triple-negative breast cancer (TNBC), which lacks the expression of targeted proteins.3 TNBC is characterized by a high rate of recurrence and poor prognosis, making this disease an enticing area of research among the scientific fraternity.4 Doxorubicin (DOX) and triarylethylenes such as tamoxifen and raloxifene are US FDA-approved selective estrogen receptor modulators (SERMs) for the treatment of BC. However, the frequent occurrence of myelosuppression along with insomnia, dizziness, hot flashes, and chances of endometrial cancer has limited the clinical application of these drugs.5 To date, there are very few drug candidates in the literature that can effectively target both TNBC and non-TNBC.6−9 This puts forward the need for the augmentation of new alternative treatments for the development of novel antibreast cancer drugs to tackle this lethal disease.
β-Carboline, containing a common tricyclic 9H-pyrido[3,4-β]indole ring, was originally isolated from Peganum harmala and had been traditionally used for the treatment of alimentary tract cancer. The antineoplastic potential of β-carbolines is linked via diverse mechanisms such as the inhibition of DNA topoisomerases I and II,10 intercalation to DNA,11,12 or targeting specific cancer signaling pathways.13−16 The DNA intercalation potential of β-carboline is linked with its high risk of cytotoxicity to normal cells, while its saturated analogue tetrahydro-β-carboline (THβC) exhibit much lower cytotoxicity as evident by peganumine A with an IC50 of 38.5 μM against MCF-7.17
Substituted 3-hydroxy-oxindol-2-ones are considered important pharmacophores in various bioactive heterocycles such as donaxaridine, dioxibrassinine, arundaphine, and maremycin B, displaying promising antitumor potential.18,19 Recently, we have disclosed the synthesis and antiproliferative evaluation of 1H-1,2,3-triazole tethered tetrahydro-β-carboline–isatin conjugates with varying stereochemistry at C-1 of the THβC ring. The conjugates with the unsubstituted THβC ring displayed better activities than conjugates with a C-1-substituted THβC core.20 In logical extension to this and in continuation with our interest,21 the present work includes the structural modifications on unsubstituted THβC via amalgamating it with an oxindole core for improving their antiproliferative potential. The corresponding unsaturated counterparts have also been synthesized to compare the effect of a rigid planar backbone of the β-carboline core with a flexible nonplanar backbone of THβC, as depicted in Figure 1, on the activities against MCF-7 (ER+) and MDA-MB-231 (ER−) cell lines.
Figure 1.

Designed prototype of the synthetic work.
Results and Discussion
Chemistry
The synthetic protocol for the synthesis of desired conjugates 8a–f and 9a–f/10a–f involved an initial synthesis of precursors spiroepoxyoxindole 3, 2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylic acid ethyl ester 6, and 9H-β-carboline-3-carboxylic acid ethyl ester 7. The synthesis of functionalized spiro-epoxyindoles 3 involved an initial base-promoted N-alkylation of C-5-substituted isatin 1 to afford 2 with subsequent Corey–Chaykovsky epoxidation using trimethyl sulfoxonium iodide, as depicted in Scheme 1.22 The second set of precursors 6 and 7 was prepared via initial base-promoted Pictet–Spengler cyclization of l-tryptophan with 30% aqueous solution of formalin at room temperature to yield 2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylic acid 5. The esterification of the corresponding acid with thionyl chloride in absolute ethanol afforded 2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylic acid ethyl ester 6. Oxidation of 6 with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) in dry chloroform yielded 9H-β-carboline-3-carboxylic acid ethyl ester 7 (Scheme 1).23
Scheme 1. Synthetic Route to Precursors Spiroindoline-oxirane-2-ones 3, 2,3,4,9-Tetrahydro-1H-β-carboline-3-carboxylic Acid Ethyl Ester 6, and 9H-β-Carboline-3-carboxylic Acid Ethyl Ester 7.

The synthesis of the first series of target scaffolds, viz., β-carboline-3-hydroxy-oxindole (8a–f), was attempted under different reaction conditions. Interestingly, the use of p-toluene sulfonic acid in different solvents like dimethylformamide (DMF), tetrahydrofuran (THF), or acetonitrile did not result in any significant transformation owing to the poor nucleophilicity of N-1 of the β-carboline nucleus. The use of NaH as a base in dry DMF afforded the corresponding products 8a–fvia regioselective ring opening from a less hindered site. The crude products thus obtained were purified via column chromatography using ethyl acetate/hexane (70:30) as an eluent to afford 8a–f in good yields (Scheme 2).
Scheme 2. Synthesis of Substituted β-Carboline–Oxindole Conjugates 8a–f.
The structural assignment to synthesized compounds was done on the basis of spectral data and analytical evidence. For example, compound 8b displayed a molecular ion peak at m/z 492.1823 [M + 1]+ in its high-resolution mass spectrum (HRMS). Its 1H nuclear magnetic resonance (NMR) showed the presence of a triplet at δ 1.47 corresponding to methyl, a quartet at δ 4.50 corresponding to methylene (−OCH2), doublets at δ 4.67 and 4.91 corresponding to methylene protons (−NCH2) attached to the β-carboline ring, and a pair of doublets at δ 4.81 and 4.99 corresponding to benzylic CH2. Its 13C NMR showed the appearance of characteristic signals at δ 165.7 and 176.5 corresponding to the carbonyl carbon of oxindole and β-carboline core, respectively.
To optimize the reaction conditions for the second set of target compounds, a model reaction between 6a and 3a in a different set of solvent conditions was carried out. Solvents were found to have a significant effect on the progress of the reaction. No product was obtained using nonpolar solvents like toluene or xylene. The use of bases, viz., K2CO3 or NaH, in polar aprotic solvents like THF, DMF, or dichloromethane (DCM) did not result in any significant reaction, even under reflux. The use of polar protic solvents like methanol however gave a mixture of 9a and 10a, in the ratio of 45:55 as evident from their crude 1H NMR spectra, the careful chromatographic separation of which afforded the corresponding THβC–oxindole conjugates 9a and 10a. After optimizing the reaction condition, a library of THβC–oxindole conjugates 9b–f and 10b–f was synthesized, as shown in Scheme 3. The structural assignment was carried out using spectral data and analytical evidence. 1H NMR of 9b showed characteristic resonances at δ 3.07 and 3.17 corresponding to methylenes protons of the THβC ring (H-4), δ 3.40 corresponding to N–CH2 linked to the THβC ring (H-6), and doublets at δ 4.68 and 5.06 corresponding to N–CH2 linked to the oxindole ring. Its 13C NMR displayed the characteristic resonances at 32.0 (N–CH2), 43.6 (−CH2), 48.2 (benzylic CH2), 60.8 (−OCH2), and 75.3 (quarternary carbon), further validated by 13C DEPT-135 along with the requisite number of aromatic carbons. The characteristic signals of 10b appeared at δ 2.50 and 3.00 corresponding to methylene of the THβC ring (H-4), 3.56 corresponding to −OCH2 (H-6), and 4.48 and 5.20 corresponding to N–CH2 linked to the oxindole ring (H-7). Its 13C and DEPT-135 NMR spectra revealed the presence of characteristic resonances at δ 43.9 (−CH2), 47.8 (benzylic CH2), 60.6 (−OCH2), 62.4 (O–CH2), and 76.3 (quarternary carbon) along with the required number of aromatic carbons.
Scheme 3. Tetrahydro-β-carboline–Oxindole-2-one Conjugates 9a–f and 10a–f.
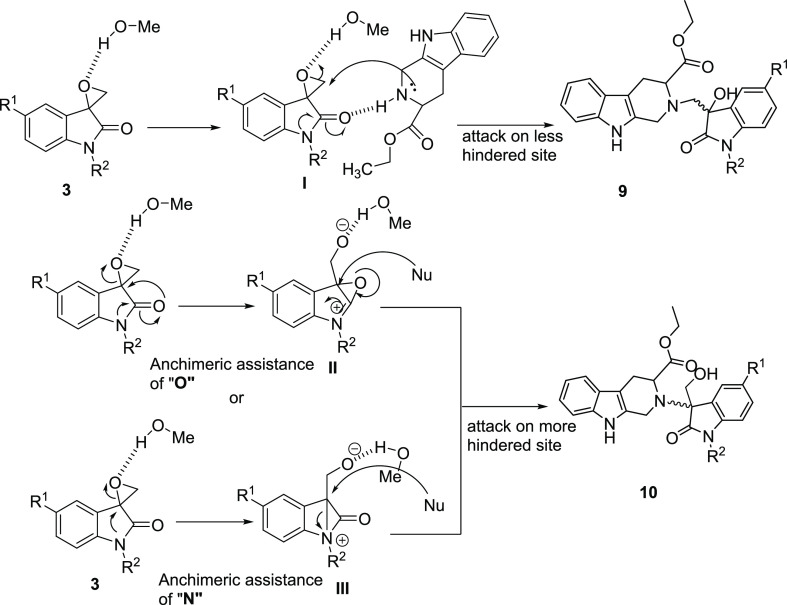
Mechanistically, the use of a polar protic solvent is known to activate the oxirane ring via H-bonding, zeroing in the proximity of both reactants and stabilizing the transition state.24 Thus, the formation of 9 can be assumed to result from an initial solvent-promoted activation of the epoxide ring, resulting in the transition state I with subsequent nucleophilic ring opening by THβC from the less hindered site. The plausible mechanism for the formation of 10 was proposed to proceed via anchimeric assistance provided by either oxygen (intermediate II) or nitrogen (intermediate III) with subsequent ring opening by the THβC nucleus (Scheme 4).25 To improve the regioselectivity of this reaction, it was considered worthy to carry out the transformation in the presence of the catalytic amount of p-toluene sulfonic acid. The reaction however led to the formation of a mixture of products, viz., 10 and 11. Compound 11 was formed by the participation of a solvent as a nucleophile (Scheme 5).
Scheme 4. Mechanistic Pathway to Tetrahydro-β-carboline–Oxindole-2-one Conjugates 9 and 10.
Scheme 5. p-TSA-Promoted Reaction of Tetrahydro-β-carboline 6 and Spiroindoline-oxirane-2-ones 3.
Mechanistically, the reaction is thought to proceed via an initial activation of the epoxide ring with p-toluene sulfonic acid to result in IV with subsequent ring opening to yield another intermediate V. The nucleophilic addition of THβC-carboline on intermediate V gave the corresponding THβC–isatin conjugate 10, while the role of methanol as nucleophile afforded 11 (Scheme 6).26
Scheme 6. Mechanistic Pathway to the Reaction between 3 and 6 in the Presence of an Acid Catalyst.
In Vitro Antiproliferative Evaluation
The synthesized series of β-carboline-spiro-indolineoxirane-2-ones 8a–f and tetrahydro-β-carboline-3-hydroxyindoline-2-ones 9a–f and 10a–f were assessed for their anti-antiproliferative activities against MCF-7 and MDA-MB-231 cell lines using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay.27 The growth inhibition graphs of target compounds against MCF-7 and MDA-MB-231 cells are summarized in Figures S28–S33 (please see the Supporting Information) using plumbagin as the positive control. The antiproliferative results were compared to standard drugs, viz., plumbagin, tamoxifen, and peganumine A. The IC50 (μM) (concentration required to achieve 50% tumor growth inhibition) values of the synthesized compounds and standard drugs plumbagin and tamoxifen and natural product peganumine A are listed in Table 1.
Table 1. IC50 Values of the Test Compounds in MCF-7 and MDA-MB-231 Cell Lines.


A careful analysis of the antiproliferative activities revealed an interesting structure–activity relationship (SAR) with activities mainly dependent upon the nature of the core (β-carboline/THβC), the nature of the substituent at N-1 and C-5 positions, as well as the nature of alcohol (1° or 3°) on the 3-hydroxyindole ring. Among β-carboline-3-hydroxy indoles 8a–f, the compounds having a methyl substituent at the N-1 position of the indole ring proved to be inactive against the MCF-7 cell line. The replacement of methyl with a benzyl substituent at the N-1 position improved the activity profile against MCF-7 with compounds 8d and 8f exhibiting IC50 values of 30.26 and 25.01 μM, respectively. A similar trend was also observed against MDA-MB-231 cells with a preference of the N-benzyl core over the N-methyl core as evident from compounds 8b and 8f exhibiting IC50 values of 45.33 and 43.36 μM, respectively. Compound 8f proved to be the most promising among β-carboline-3-hydroxyindole series, being potent on both ER+ (MCF-7) and ER– (MDA-MB-231) cells with IC50 values of 25.01 and 43.36 μM, respectively.
Comparing the observed activities of β-carboline-3-hydroxyindolin-2-ones 8a–f with their saturated ring counterpart THβC-3-hydroxyindolin-2-ones 9a–f revealed a general improvement in their antiproliferative activities. The activities of 9a–f were found to depend upon the nature of the substituent at the C-5 position of the 3-hydroxyindole ring, while the substituent at N-1 seems to have a lesser effect. The introduction of a halogen substituent (F, Cl) at the C-5 position improved the activities irrespective of the nature of the substituent at the N-1 position as evident by 9d, 9e, and 9f exhibiting better activities than tamoxifen on both MCF-7 and MDA-MB-231 cell lines. Changing the nature of alcohol from 3° (9a–f) to 1° (10a–f) at the C-3 position of hydroxyindole resulted in, generally, a decrease in antiproliferative efficacy, while the nature of the substituent at the C-5 position of hydroxyindole seemed to have a favorable influence with the order of preference Cl > F > H. Compound 10d having a chloro substituent at the C-5 position of hydroxyindole displayed an IC50 value of 15.14 μM against MCF-7 and 23.30 μM against MDA-MB-231 cells. Compound 9d with an optimum combination of the chloro substituent at C-5 and benzyl at the N-1 position of 3-hydroxyindole, a saturated THβC ring along with tertiary alcohol, proved to be the most active among the series exhibiting IC50 values of 13.61 and 22.76 μM against MCF-7 and MDA-MB-231 cells, respectively.
The generalized SAR of the synthesized series against MCF-7 and MDA-MB-231 is depicted in Figure 2.
Figure 2.
Generalized SAR of the synthesized 3-hydroxyindole-substituted β-carboline and tetrahydro-β-carboline.
Molecular Docking and ADMET Properties
To rationalize the observed anticancer activities and to identify the novel binding cores, we performed in silico studies to explore the binding pattern of the active compounds (8f, 9e, 9d, 9f, and 10d) including the native ligand into the active site of human kinase Akt1 receptor. Akt, also known as protein kinase B (PKB), is a class of serine/threonine kinases, consists of three isoforms, Akt1 (PKBa), Akt2 (PKBb), and Akt3 (PKBc).28 AKT regulates cellular processes that are features of breast cancer such as cell cycle progression, apoptosis, and growth factor-mediated cell survival. Activation of the Akt pathway is associated with erbB2 overexpression in breast cancer.29 In addition, activation of Akt alters important cellular processes such as cell proliferation, migration, metabolism, and signal transduction, leading to cancer growth in the human breast; thus, its blockade can improve breast cancer treatment and thus have been exploited as a potential target for drug discovery.30 More, also, indole analogues have been reported to block the activation of AKT.30,31 It is worth saying that all of the docked compounds have a similar binding landscape with the native ligand (Figure 3). The predicted binding energies for the active compounds (8f, 9d, 9e, 9f, 10d = −8.5, −8.9, −8.6, −8.8, −8.7 kcal/mol, respectively) demonstrated a high degree of correlation with the experimental IC50 values as well as high thermodynamic and conformational stability of complexes. For example, 9d, the most potent compound against MCF-7 and MDA-MB-231 cancer cell lines, exhibited the strongest binding with Akt1 based on the lowest binding energy of its complex (BE = −8.9 kcal/mol). This compound also displayed an interesting ligand–receptor interaction profile with its carboline ring fitting well into the hydrophobic pocket of amino acids Val164, Ala177, Met281, and Leu156 by establishing π–alkyl and π–σ interactions. This was accompanied by one hydrogen bond between the hydrogen of the ethoxyl group and the carboxylate side chain of Glu234 (2.01 Å). The Cl-substituent orientated toward the C-helix region of Akt1 via hydrophobic interactions with the Phe236 residue. Surprisingly, the change in the position of methylene in compound 10d decreased its binding affinity toward Akt1 receptor. The phenyl ring attached to the nitrogen of the indole moiety formed hydrophobic interactions with amino acids Val164, Met281, and Ala177. Additionally, hydrogen bond network was observed between the sp2-hybridized oxygen of Asp292 with carboline moiety, sp3-hybridized methylene group attached to nitrogen on the indole ring as well as the proton acceptor amino acid Asn279 and the sp2-hybridized nitrogen of the carboline ring (bond distance = 2.44, 3.43, and 3.64 Å). Noticeable electrostatic interaction exists between amino acid Lys272 and the carboline ring, whereas the benzyl ring interacts with Val164, which was sandwiched between π–alkyl and π–σ interactions as well as Ala177 and Met281 via π-alkyl and π–σ interactions. The C–Cl bond stretched out to form hydrophobic interaction with amino acid Phe161, as reported previously.28 As observed for compounds 9d and 10d, these formed a favorable interaction with the electron-rich sulfur of Met281 via hydrophobic interaction (Figure 3). The replacement of the benzyl group with the methyl substituent on the nitrogen group of the indole moiety decreased the binding affinity of compound 9e. This compound formed hydrogen bonding with Asp279, Asp292, and Lys276 through its carboline and indole moieties. Besides, the carboline moiety formed π–alkyl interaction with Ala177 as well as π–σ interaction with Met281 and Val164.
Figure 3.
Predicted binding of active compounds: 8f, 9e, 9d, 9f, 10d, and tamoxifen (gray) within the AKT1 receptor (PDB: 3COB).
The orientation of compound 9f within the binding domain of Akt showed that the carboline moiety formed hydrophobic interaction with amino acids Met281, Ala177, and Val164, whereas the methylene linker formed ionic interaction with Glu234. Surprisingly, the fluorine substituent only occupied space without any interaction with the receptor. The potency of an analogue of β-carboline-spiro-indolineoxirane-2-ones completely altered after the introduction of a fluoro substituent. The benzyl group on the indole core of compound 8f occupied a similar hydrophobic pocket to 10d, consisting of amino acids Val164, Ala177, Leu156, and Met281. In addition, the fluorine substituent and carboline moiety on 8f interacted via hydrogen bond with Gly159 and ionic interaction with Glu234 (Figure 3). In this way, studies performed with docking were in agreement with the experimental results and therefore reinforced the proposal that compounds 9d, 9e, and 10d can be promising antibreast cancer agents upon further derivatization.
Furthermore, the evaluation of pharmacokinetic properties and drug likeness of the promising conjugates via ADMET profiling (Table S1, Supporting Information) revealed that they possess druglike properties. For instance, 9d and 10d presented good intestinal absorption and were safe in terms of hepatotoxicity and skin sensitization. In addition, ease of permeation of 9d and 10d through biological membranes is clearly demonstrated by their good log P values. The profiling of 8f, 9d, 9f, and 10d toward CYP (cytochrome P450) isoforms revealed that they are inhibitors of CYP2C19 and CYP3A4, whereas 8f additionally inhibited CYP2C9.
Conclusions
In conclusion, the present manuscript describes the synthesis and antiproliferative evaluation of a series of β-carboline/tetrahydro-β-carboline-3-hydroxyindole-2-ones via epoxide ring-opening reactions under varied reaction conditions. Most of the synthesized conjugates showed promising activities against the tested cell lines with a few exhibiting comparable activities to or even better activities than standard drug tamoxifen. Compounds 8c (β-carboline–oxindole) and 9c (THβC–oxindole) with IC50 values of 35.40 and 24.1 μM against estrogen unresponsive cells could effectively serve as molecular templates for the development of new selective inhibitors of TNBC. The comparison of the present set with the previously reported 1H-1,2,3-triazole-tethered tetrahydro-β-carboline–isatin conjugates showed improvement in cytotoxic activities against both ER+ and TNBC cells.20 In addition, the comparison of rigidity/flexibility among the present set of synthesized conjugates (β-/THβ-carbolines) as well as the nature of free hydroxyl groups (1°/3°) has shown to play an important role in determining their antiproliferative activities. These interesting SAR findings can provide a rationale for the optimization and development of efficient anti-BC agents.
Experimental Section
General Note
All of the reactions were carried out using standard techniques. Column chromatography was performed using silica gel (60–120 mesh) with ethyl acetate/hexane as an eluent mixture. The melting points were recorded in capillaries and were uncorrected. The 1H NMR and 13C NMR spectra were recorded on JEOL 400 and Bruker 500 and 125 MHz spectrometers using CDCl3 solutions with tetramethylsilane (TMS) as an internal standard. Chemical shifts were reported in parts per million (ppm) and coupling constants J were indicated in hertz. Mass spectroscopy data was collected on a Bruker micrOTOF-QII equipment using electrospray ionization (ESI) as the source.20
Cell Culturing
MCF-7 cells were cultured in complete Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin, and MDA-MB-231 cells were cultured in 3:1 DMEM and Ham’s F12 with 10% FBS and 1% penicillin–streptomycin; both cell lines were incubated at 37 °C and 5% carbon dioxide.
MTT Assay
Cells were seeded in 96-well plates at a density of 5000 cells per well in triplicate in media. After 24 h, the test compounds (dissolved in dimethyl sulfoxide (DMSO)), diluted in DMEM, were added to each well. Cells were treated with a range of different concentrations of each compound (5, 10, 20, 50, 100 μM). Following a 24 h incubation with the test compounds, sterile 5 mg/mL MTT (Sigma-Aldrich) dissolved in phosphate-buffered saline (PBS) was added to each well and incubated with cells for 2 h. A solubilization solution (10% sodium dodecyl sulfate (SDS), 10 mM HCl) of equal volume to the wells was then added to each well, which was incubated with cells for 16 h at 37 °C. The optical density of each well at 570 nm was determined using a microtiter plate reader (Tecan Sunrise Microplate Reader, Magellan software).27
Statistical Analysis
The statistical analysis was performed using Excel, and IC50 values were estimated using GraphPad Prism 5 software (Hearne Scientific Software). The experiments were performed in duplicate (with three replicates each), and the statistical significance was calculated using a one-way ANOVA test, Bartlett’s equal variance test, and a posthoc analysis (Dunnett’s multiple comparison test) on all runs. A p-value of less than 0.05 was used to estimate the significance of the observations. The Z-factor calculated for each 96-well plate and assays having a Z-factor >0.6 were included in the statistical analysis.32
Molecular Docking
The X-ray coordinates of Akt were retrieved from the protein data bank (www.rcsb.org) with its cocrystallized ligand (PDB: 3COB) at a resolution of 2.7 Å. All docking simulations were performed using AutodockVina, and the results are summarized in Table S2.33 The docking systems were validated by docking the native ligand of the protein crystal structure into the respective binding site. SwissADME34 and pkCSM35 web-based applications were used to predict the ADMET properties of active compounds.
Acknowledgments
The financial assistance from the Council of Scientific and Industrial Research, New Delhi, India, under CSIR-SRF fellowship CSIR Ref No. 09/254(0257)/2016-EMR-I is gratefully acknowledged.
Glossary
Abbreviations
- BC
breast cancer
- TNBC
triple-negative breast cancer
- THβC
tetrahydro-β-carboline
- SERMs
selective estrogen receptor modulators
- ER
estrogen receptor
- IC50
50% inhibitory concentration
- SAR
structure–activity relationship
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c01226.
Physicochemical and ADMET properties of compounds 8f, 9e, 9f, 9d, 10d (Table S1); binding energy, hydrogen bonding interactions, and important residues involved in the active compounds docked on AKT receptor (Table S2); 1H and 13C NMR data of all of the synthesized conjugates along with scanned (1H, 13C, DEPT) NMR spectra for representative compounds, viz., 8a, 8b, 8c, 8f, 9b, 9c, 9f, 10b, 10f, 11 (Figures S1–S27); and percentage inhibition graphs (Figures S28–S33) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a American Cancer Society. Cancer Facts & Figures; 2019.; b Malvia S.; Bagadi S. A.; Dubey U. S.; Saxena S. Epidemiology of breast cancer in Indian women. Asia-Pac. J. Clin. Oncol. 2017, 13, 289–295. 10.1111/ajco.12661. [DOI] [PubMed] [Google Scholar]
- Dai X.; Li T.; Bai Z.; Yang Y.; Liu X.; Zhan J.; Shi B. Breast cancer intrinsic subtype classification, clinical use and future trends. Am. J. Cancer Res. 2015, 5, 2929–2943. [PMC free article] [PubMed] [Google Scholar]
- Carey L. A.; Dees E. C.; Sawyer L.; Gatti L.; Moore D. T.; Collichio F.; Ollila D. W.; Sartor C. I.; Graham M. L.; Perou C. M. The triple negative paradox: primary tumor chemo-sensitivity of breast cancer subtypes. Clin. Cancer Res. 2007, 13, 2329. 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- Cleator S.; Heller W.; Coombes C. Triple Negative Breast Cancer: therapeutic options. Lancet Oncol. 2007, 8, 235–244. 10.1016/S1470-2045(07)70074-8. [DOI] [PubMed] [Google Scholar]
- DeMichele A.; Troxel A. B.; Berlin J. A.; Weber A. L.; Bunin G. R.; Turzo E.; Schinnar R.; Burgh D.; Berlin M.; Rubin S. C.; Rebbeck T. R.; Strom B. L. Impact of raloxifene or tamoxifen use on endometrial cancer risk: a population-based case-control study. J. Clin. Oncol. 2008, 26, 4151. 10.1200/JCO.2007.14.0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldehna W. M.; Almahli H.; Al-Ansary G. H.; Ghabbour H. A.; Aly M. H.; Ismael O. E.; Al-Dhfyan A.; Abdel-Aziz H. A. Synthesis and in vitro anti-proliferative activity of some novel isatins conjugated with quinazoline/phthalazinehydrazines against triple-negative breast cancer MDA-MB-231 cells as apoptosis-inducing agents. J. Enzyme Inhib. Med. Chem. 2017, 32, 600–613. 10.1080/14756366.2017.1279155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles-Escajeda E.; Das U.; Ortega N. M.; Parra K.; Francia G.; Dimmock J. R.; Ramirez A. V.; Aguilera R. J. A novel curcumin-like dienone induces apoptosis in triple-negative breast cancer cells. Cell. Oncol. 2016, 39, 265–277. 10.1007/s13402-016-0272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cázares-Marinero J. J.; Top S.; Vessières A.; Jaouen G. Synthesis and antiproliferative activity of hydroxyferrocifen hybrids against triple-negative breast cancer cells. Dalton Trans. 2014, 43, 817–830. 10.1039/C3DT52070F. [DOI] [PubMed] [Google Scholar]
- Gormen M.; Pigeon P.; Wang Y.; Vessieres A.; Top S.; Martial F.; Gros C.; McGlinchey M. J.; Jaouen G. Side-Chain Effects on the 1-(Bis-aryl-methylidene)- [3]ferrocenophane Skeleton: Antiproliferative Activity against TNBC Cancer Cells and Comparison with the Acyclic Ferrocifen Series. Eur. J. Inorg. Chem. 2017, 2, 454–465. 10.1002/ejic.201601088. [DOI] [Google Scholar]
- Kamal A.; Sathish M.; Nayak V. L.; Srinivasulu V.; Kavitha B.; Tangella Y.; Thummuri D.; Bagul C.; Shankaraiah N.; Nagesh N. Design and synthesis of dithiocarbamate linked β-carboline derivatives: DNA topoisomerase II inhibition with DNA binding and apoptosis inducing ability. Bioorg. Med. Chem. 2015, 23, 5511–5526. 10.1016/j.bmc.2015.07.037. [DOI] [PubMed] [Google Scholar]
- Shankaraiah N.; Siraj K. P.; Nekkanti S.; Srinivasulu V.; Sharma P.; Senwar K. R.; Sathish M.; Vishnuvardhan M. V. P. S.; Ramakrishna S.; Jadala C.; Nagesh N.; Kamal A. DNA-binding affinity and anticancer activity of β-carboline-chalcone conjugates as potential DNA intercalators: Molecular modeling and synthesis. Bioorg. Chem. 2015, 59, 130–139. 10.1016/j.bioorg.2015.02.007. [DOI] [PubMed] [Google Scholar]
- Cao R.; Peng W.; Chen H.; Ma Y.; Liu X.; Hou X.; Guan H.; Xu A. DNA binding properties of 9-substituted harmine derivatives. Biochem. Biophys. Res. Commun. 2005, 338, 1557–1563. 10.1016/j.bbrc.2005.10.121. [DOI] [PubMed] [Google Scholar]
- Song Y.; Kesuma D.; Wang J.; Deng Y.; Duan J.; Wang J. H.; Qi R. Z. Specific inhibition of cyclin-dependent kinases and cell proliferation by harmine. Biochem. Biophys. Res. Commun. 2004, 317, 128–132. 10.1016/j.bbrc.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Li Y.; Liang F.; Jiang W.; Yu F.; Cao R.; Ma Q.; Dai X.; Jiang J.; Wang Y.; Si S. DH334, a beta-carboline anti-cancer drug, inhibits the CDK activity of budding yeast. Cancer Biol. Ther. 2007, 6, 1193–1199. [PubMed] [Google Scholar]
- Zhang J.; Li Y.; Guo L.; Cao R.; Zhao P.; Jiang W.; Ma Q.; Yi H.; Li Z.; Jiang J.; Wu J.; Wang Y.; Si S. DH166, a β-carboline derivative, inhibits the kinase activity of PLK1. Cancer Biol. Ther. 2009, 8, 2374–2383. 10.4161/cbt.8.24.10182. [DOI] [PubMed] [Google Scholar]
- Barsanti P. A.; Wang W.; Ni Z.; Duhl D.; Brammeier N.; Martin E.; et al. The discovery of tetrahydro-beta-carbolines as inhibitors of the kinesin Eg5. Bioorg. Med. Chem. Lett. 2010, 20, 157–160. 10.1016/j.bmcl.2009.11.012. [DOI] [PubMed] [Google Scholar]
- a Mahale S.; Bharate S. B.; Manda S.; Joshi P.; Jenkins P. R.; Vishwakarma R. A.; Chaudhuri B. Antitumour potential of BPT: a dual inhibitor of cdk4 and tubulin polymerization. Cell Death Dis. 2015, 6, 1743. 10.1038/cddis.2015.96. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Kumar S.; Singh A.; Kumar K.; Kumar V. Recent insights into synthetic β-carbolines with anti-cancer activities. Eur. J. Med. Chem. 2017, 142, 48. 10.1016/j.ejmech.2017.05.059. [DOI] [PubMed] [Google Scholar]; c Wang K. B.; Di Y. T.; Bao Y.; Yuan C. M.; Chen G.; Li D. H.; Bai J.; He H. P.; Hao X. J.; Pei Y. H.; Jing Y. K.; Li Z. L.; Hua H. M. Peganumine A, a β-carboline dimer with a new octacyclic scaffold from Peganum harmala. Org. Lett. 2014, 16, 4028–4031. 10.1021/ol501856v. [DOI] [PubMed] [Google Scholar]
- a Peddibhotla S. 3-Substituted-3-hydroxy-2-oxindole, an Emerging New Scaffold for Drug Discovery with Potential Anti-Cancer and other Biological Activities. Curr. Bioact. Compd. 2009, 5, 20–38. 10.2174/157340709787580900. [DOI] [Google Scholar]; b Singh G. S.; Desta Z. Y. Isatins As Privileged Molecules in Design and Synthesis of Spiro-Fused Cyclic Frameworks. Chem. Rev. 2012, 112, 6104–6155. 10.1021/cr300135y. [DOI] [PubMed] [Google Scholar]
- Bronte G.; Passiglia F.; Galvano A.; Barraco N.; Listì A.; Castiglia M.; Rizzo S.; Fiorentino E.; Bazan V.; Russo A. Nintedanib in NSCLC: evidence to date and place in therapy. Ther. Adv. Med. Oncol. 2016, 8, 188–197. 10.1177/1758834016630976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma B.; Singh A.; Gu L.; Saha S. T.; Pillay A. S.; Cele N.; Singh P.; Kaur M.; Kumar V. Diastereoselective approach to rationally design tetrahydro-β-carboline–isatin conjugates as potential SERMs against breast cancer. RSC Adv. 2019, 9, 9809–9819. 10.1039/C9RA00744J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Sharma B.; Gu L.; Pillay R. P.; Cele N.; Awolade P.; Singh P.; Kaur M.; Kumar V. Design, synthesis, and anti-proliferative evaluation of 1H-1,2,3-triazole grafted tetrahydro-β-carboline-chalcone/ferrocenylchalcone conjugates in estrogen responsive and triple negative breast cancer cells. New J. Chem. 2020, 44, 11137–11147. 10.1039/D0NJ00879F. [DOI] [Google Scholar]; b Shalini; Pankaj; Saha S. T.; Kaur M.; Oluwakemi E.; Awolade P.; Singh P.; Kumar V. Synthesis and in vitro anti-proliferative evaluation of naphthalimide–chalcone/pyrazoline conjugates as potential SERMs with computational validation. RSC Adv. 2020, 10, 15836–15845. 10.1039/D0RA01822H. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Kumar S.; Gu L.; Palma G.; Kaur M.; Pillay A. S.; Singh P.; Kumar V. Design, synthesis, anti-proliferative evaluation and docking studies of 1H-1,2,3-triazole tethered ospemifene–isatin conjugates as selective estrogen receptor modulator. New J. Chem. 2018, 42, 3703–3713. 10.1039/C7NJ04964A. [DOI] [Google Scholar]; d Kumar S.; Saha S. T.; Gu L.; Palma G.; Perumal S.; Pillay A. S.; Singh P.; Anand A.; Kaur M.; Kumar V. 1H-1,2,3-Triazole Tethered Nitroimidazole-Isatin Conjugates: Synthesis, Docking, and Anti-Proliferative Evaluation against Breast Cancer. ACS Omega 2018, 3, 12106–12113. 10.1021/acsomega.8b01513. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Singh A.; Saha S. T.; Perumal S.; Kaur M.; Kumar V. Azide-Alkyne Cycloaddition En Route to 1H-1,2,3-Triazole-Tethered Isatin-Ferrocene, Ferrocenylmethoxy-Isatin, and Isatin-Ferrocenylchalcone Conjugates: Synthesis and Antiproliferative Evaluation. ACS Omega 2018, 3, 1263–1268. 10.1021/acsomega.7b01755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouhan M.; Senwar K. R.; Sharma R.; Grover V.; Nair V. A. Regiospecific epoxide opening: a facile approach for the synthesis of 3-hydroxy-3-aminomethylindolin-2-one derivatives. Green Chem. 2011, 13, 2553–2560. 10.1039/c1gc15416h. [DOI] [Google Scholar]
- a Song H.; Liu Y.; Liu Y.; Wang L.; Wang Q. Synthesis and Antiviral and Fungicidal Activity Evaluation of β-Carboline, Dihydro-β-carboline, Tetrahydro-β-carboline Alkaloids, and Their Derivatives. J. Agric. Food Chem. 2014, 62, 1010–1018. 10.1021/jf404840x. [DOI] [PubMed] [Google Scholar]; b Van Linn M. L.; Cook J. M. Mechanistic Studies on the Cis to Trans Epimerization of Trisubstituted 1,2,3,4-Tetrahydro-β-carbolines. J. Org. Chem. 2010, 75, 3587–3599. 10.1021/jo1003778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P.; Senwar K. R.; Jeengar M. K.; Reddy T. S.; Naidu V. G. M.; Kamal A.; Shankaraiah N. H2O-mediated isatinspiro-epoxide ring opening with NaCN: Synthesis of novel 3-tetrazolylmethyl-3-hydroxy-oxindole hybrids and their anticancer evaluation. Eur. J. Med. Chem. 2015, 104, 11–24. 10.1016/j.ejmech.2015.09.025. [DOI] [PubMed] [Google Scholar]
- a Hajra S.; Roy S. S.; Biswas A.; Saleh S. K. A. Catalyst-Free Ring Opening of SpiroaziridineOxindoles by Heteronucleophiles: An Approach to the Synthesis of Enantiopure 3-Substituted Oxindoles. J. Org. Chem. 2018, 83, 3633–3644. 10.1021/acs.joc.7b03288. [DOI] [PubMed] [Google Scholar]; b Hajra S.; Maity S.; Maity R. Efficient Synthesis of 3,3′-Mixed Bisindoles via Lewis Acid Catalyzed Reaction of Spiro-epoxyoxindoles and Indoles. Org. Lett. 2015, 17, 3430–3433. 10.1021/acs.orglett.5b01432. [DOI] [PubMed] [Google Scholar]; c Azizi N.; Saidi M. R. Highly Chemoselective Addition of Amines to Epoxides in Water. Org. Lett. 2005, 7, 3649–3651. 10.1021/ol051220q. [DOI] [PubMed] [Google Scholar]
- Tak R. K.; Gupta N.; Kumar M.; Kureshy R. I.; Khan N. H.; Suresh E. Regioselective Alcoholysis and Hydrochlorination Reactions of Spiro-Epoxy Oxindoles at the Spiro-Centre: Synthesis of 3,3DisubstitutedOxindoles and Application for Anticancer Agent. Eur. J. Org. Chem. 2018, 5678–5687. 10.1002/ejoc.201801002. [DOI] [Google Scholar]
- Sagar S.; Esau L.; Moosa B.; Khashab N. M.; Bajic V. B.; Kaur M. Cytotoxicity and Apoptosis Induced by a Plumbagin Derivative in Estrogen Positive MCF-7 Breast Cancer Cells. Anticancer Agents Med. Chem. 2014, 14, 170–180. 10.2174/18715206113136660369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S.; Zhang Z.; Zhuang X.; Luo J.; Cao X.; Li H.; Tu Z.; Lu X.; Ren X.; Ding K. New thiazolecarboxamides as potent inhibitors of Akt kinases. Bioorg. Med. Chem. Lett. 2012, 22, 1208–1212. 10.1016/j.bmcl.2011.11.080. [DOI] [PubMed] [Google Scholar]
- a Pérez-Tenorio G.; Stål O.; Activation of AKT/PKB in breast cancer predicts a worse outcome among endocrine treated patients. Br. J. Cancer 2002, 86, 540–545. 10.1038/sj.bjc.6600126. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Stål O.; Pérez-Tenorio G.; Åkerberg L.; Olsson B.; Nordenskjöld B.; Skoog L.; Rutqvist L. E. Akt kinases in breast cancer and the results of adjuvant therapy. Breast Cancer Res. 2003, 5, R37 10.1186/bcr569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K. N.; Mehta K. R.; Peterson D.; Evangelista J. C.; Livesey J.; Faridi S. AKT-Induced Tamoxifen Resistance Is Overturned by RRM2 Inhibition. Mol. Cancer Res. 2014, 12, 394–407. 10.1158/1541-7786.MCR-13-0219. [DOI] [PubMed] [Google Scholar]
- Chao W. R.; Yean D.; Amin K.; Green C.; Jong L. Computer-aided rational drug design: a novel agent (SR13668) designed to mimic the unique anticancer mechanisms of dietary indole-3-carbinol to block Akt signaling. J. Med. Chem. 2007, 50, 3412–3415. 10.1021/jm070040e. [DOI] [PubMed] [Google Scholar]
- Zhang J. H.; Chung T. D. Y.; Oldenburg K. R. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J. Biomol. Screening 1999, 4, 67–73. 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- Trott O.; Olson A. J. AutoDockVina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- http://www.swissadme.ch/.
- http://biosig.unimelb.edu.au/pkcsm/prediction.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.