Abstract
Rosin is a sustainable resource, which is mainly composed of resin acid. Rosin-modified resin is widely used in adhesives, inks, coatings, and other fields, and its stability is very important for the production, storage, and use of products. Thermal stability and reactivity of three resin acids (levopimaric acid, neoabietic acid, and dehydroabietic acid) and four rosin-modified resins were studied using an accelerating rate calorimeter (ARC). They are stable, and exothermic reactions do not occur even when they were heated to 200 °C under a nitrogen atmosphere, but they are unstable under an oxygen atmosphere. The mechanism of the oxidation reaction process was found: first, resin acids absorb oxygen, and then an exothermic oxidation occurs. The initial exothermic temperature (T0) of levopimaric acid, neoabietic acid, and dehydroabietic acid are 354.01, 353.83, and 398.20 K, the initial oxidation kinetics shows a second-order reaction, and the activation energies (Ea) are 42.90, 58.05, and 46.60 kJ/mol, respectively. Peroxide concentration of three resin acids were determined by iodometry. The T0 values of hydrogenated rosin, disproportionated rosin, hydrogenated rosin glyceride, and hydrogenated rosin pentaerythritol ester, the four rosin-modified resin, are 353.71, 348.32, 412.85, and 412.44 K. Levopimaric acid and neoabietic acid have higher oxidative reactivity and easily undergoes an oxidation reaction at lower temperature. Rosin-modified resins are stable and find it difficult to undergo oxidation reactions.
Introduction
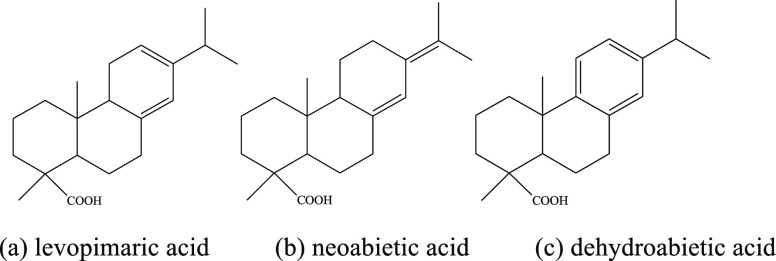
Gum rosin is a viscous liquid secreted from pine trees, and it is a natural resin obtained by distillation. It is widely used in paper sizing, printing inks, soaps, cosmetics, adhesives, emulsifiers, medical supplies, and other fields.1−3 Rosin is mainly composed of resin acids: abietic acid, levopimaric acid, neoabietic acid, dehydroabietic acid, etc.; the structure of levopimaric acid, neoabietic acid, and dehydroabietic acid are shown in Figure 1.
Figure 1.
Structure of the three resin acids.
Levopimaric acid and neoabietic acid are compounds with conjugated double bonds, and they are prone to oxidation,4−6 Diels–Alder addition,7 isomerization,8,9 and other chemical reactions. Dehydroabietic acid is an aromatic compound, and its chemical properties are relatively stable. It is not easily oxidized under oxygen (or air) at room temperature. When dehydroabietic acid is oxidized with chromic acid, the reaction takes place at the α position.10 Resin acids have conjugated double bonds, their chemical properties are active, and they easily oxidize in air or oxygen. Hydroperoxides easily form when rosin acid is oxidized, and they have an allergic reaction to the human body.11,12 Therefore, it is very important to study the stability of resin acids and rosin-modified resins for production, storage, and use of products. Thermogravimetric (TG) analysis13 and colorimetric methods14 have been used for the determination of rosin and the thermal stability of rosin-modified resins. Minn15 determined the stability of six purified rosin acid structures including abietic, neoabietic, palustric, levopimaric, isopimaric, and dehydroabietic acids using high-pressure differential scanning calorimetry (HPDSC).
The stability of resin acid and rosin-modified resin is related to their oxidation reactivity. The products and mechanisms of abietic acid oxidation were investigated under various storage conditions.16 Rate constants were reported for the reaction of abietic acid with singlet oxygen (1O2) in chloroform solution by Monroe.17 There are a series of literature reports on the investigation of the products from the thermal oxidation reaction and photo-oxidation reaction of resin acid.5,18−21 The oxidation kinetics of rosin,22 rosin pentaerythritol ester,23 and rosin glycerol ester24 have been studied under ultraviolet irradiation. The characteristics of the thermal oxidation of abietic acid were determined using IR and GC.25,26 Liu et al.27 reported the thermal stability of gum rosin and abietic acid. Li et al.28 investigated the oxidation characteristics of abietic acid by a mini closed pressure vessel test (MCPVT) method. These research studies mainly focused on the oxidation reaction of rosin and abietic acid and their stability; however, these investigations were less concerned with the initial oxidation process of rosin, such as the oxidation reaction characteristics at room temperature.
It is very important to investigate the oxidation process of rosin from room temperature to 200 °C. This is very significant for the long-term storage of resin acids and rosin-modified resins, control of their production, their application, and research and development of antioxidant technology. In this paper, the accelerating rate calorimeter (ARC) method was used to analyze three resin acids (levopimaric acid, neoabietic acid, and dehydroabietic acid) and four rosin-modified resins (hydrogenated rosin, disproportionated rosin, hydrogenated rosin glyceride, and hydrogenated rosin pentaerythritol ester) and to investigate their temperature and time (T–t), pressure and time (P–t), and pressure and temperature (P–T) behaviors and oxidation characteristics.
Our aim is to understand the oxidation process, oxidation characteristics, and reaction mechanism of resin acids. By investigating the thermal stability of the resin acids and rosin-modified resins, basic data for their storage, transportation, and utilization are provided. This provides a survey method for the thermal stability evaluation of renewable resources.
Results and Discussion
Pressure Effect of Resin Acids Oxidation Process
The melting points of levopimaric acid, neoabietic acid, and dehydroabietic acid are 150, 167, and 171 °C, respectively. The possible reactions of resin acids are as follows:
| R1 |
| R2 |
| R3 |
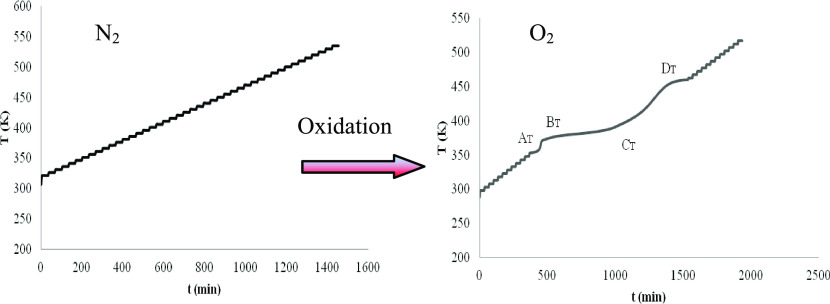
Reaction R1 is an oxidation reaction, Reaction R2 is a polymerization reaction, and reaction R3 is a complex thermal decomposition reaction. At a lower temperature (below the boiling point), if there is no thermal decomposition reaction to generate gas in the initial reaction, reaction R1 is a gas reduction reaction, and reaction R2 would not change the amount of oxygen. Therefore, it can be determined whether there is an oxidation reaction by monitoring the pressure and time (P–t) behavior in the reaction system. As a comparative experiment of the oxidation reaction, an ARC experiment wass carried out in a nitrogen atmosphere. The P–t plots of levopimaric acid, neoabietic acid, and dehydroabietic acid are shown in Figure 2.
Figure 2.
P–t plots of the three resin acids under a nitrogen atmosphere.
Figure 2 shows that none of the three resin acids were detected to absorb (reduce) or thermally decompose (increase) the gas produced. That is to say, thermal decomposition of resin acids was not detected even at temperatures above 200 °C, there is no chemical reaction of resin acid, and they are very stable under a nitrogen atmosphere.
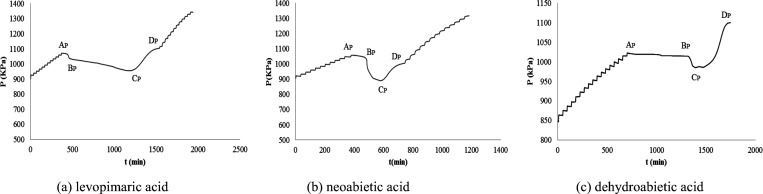
Is the resin acid oxidized under an oxygen atmosphere? The P–t behavior of the three resin acids under an oxygen atmosphere are shown in Figure 3, determined by the ARC method.
Figure 3.
P–t plots of the three resin acids under an oxygen atmosphere.
Figure 3 shows that there are two stages of oxygen reduction: the first is from AP to BP, the second is from BP to CP, and there is a stage of thermal decomposition that causes the pressure to rise, from CP to DP. That is to say, the resin acid has a chemical reaction under an oxygen atmosphere, and the oxidation process is very complex.
Exothermic Effect of Resin Acid Oxidation
When an exothermic chemical reaction occurs, the temperature of the reaction system should increase. Therefore, we can judge whether an exothermic chemical reaction has taken place by measuring the change of temperature with time (T–t behavior). Chemical reactions of resin acids may include oxidation and polymerization. In order to distinguish the oxidation reaction and polymerization reaction of resin acids, first, experiments were carried out under a nitrogen atmosphere. For polymerization reactions, a temperature change is detected under a nitrogen atmosphere. The T–t plots of levopimaric acid, neoabietic acid, and dehydroabietic acid under a nitrogen atmosphere are shown in Figure 4.
Figure 4.
T–t plots of the three resin acids under a nitrogen atmosphere.
Figure 4 shows that exothermic heat from the reaction of the three resin acids was not detected using the ARC; that is to say, there is no chemical reaction of the resin acids under a nitrogen atmosphere. Both Figures 2 and 4 show that the three resin acids are stable without chemical reactions, i.e., polymerization, under a nitrogen atmosphere.
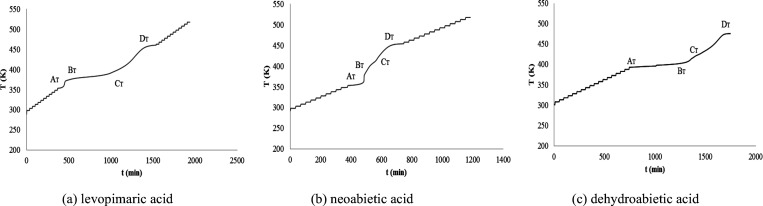
The stability of the resin acids is of great concern under an oxygen or air atmosphere. It is helpful to understand the stability of rosin and its modified resin. Figure 5 shows the T–t plots of the three resin acids under an oxygen atmosphere by the ARC method.
Figure 5.
T–t plots of the three resin acids under an oxygen atmosphere.
Figure 5 shows that the exothermic reaction of the resin acids has taken place, and it is via oxidation because the resin acid has no chemical reaction under a nitrogen atmosphere. The resin acid oxidation reaction has the following characteristics:
(1) The oxidation reaction is divided into three stages: the first stage is the initial oxidation reaction; the second stage is the slow oxidation reaction; and the third stage is the later oxidation reaction.
(2) The first stage is mainly the oxidation reaction of gas oxygen on the surface of the resin acid solid. It is from AT to BT in Figure 5.
(3) The second stage is the oxidation reaction in which oxygen diffuses into the resin acid, and it is controlled by diffusion, so it is slow. It is from BT to CT.
(4) The third stage is the oxidation reaction of the resin acid in the liquid phase because the oxidation reaction temperature is above the melting point. It is from CT to DT.
(5) Dehydroabietic acid is more slowly oxidized than levopimaric acid or neoabietic acid because dehydroabietic acid has an aromatic structure and is more stable.
Oxidation Activity of Resin Acids
The initial oxidation reaction of the resin acids is important for the investigation of thermal stability because the initial oxidation temperature is related to the reaction activity. This is very interesting when we enlarge the initial reaction part of Figure 3. Figure 3 shows that the pressure first decreases and then increases: that is, the amount of gas in the vessel first decreases and then increases. Its reasonable explanation is that the oxidation reaction of resin acid may occur as follows:
| R4 |
| R5 |
| R6 |
where R is the resin acids levopimaric acid, neoabietic acid, and dehydroabietic acid.
The oxidation absorption (4) is a process where oxygen is absorbed. There is no significant exothermic reaction and no change in temperature. However, reaction R4 is a gas reduction process where the pressure is reduced. The initial absorption of oxygen is indicated by Tabs, and the values of Tabs of the three resin acids are shown in Table 1.
Table 1. Initial Oxygen Absorption Temperature of Resin Acids.
| sample | atmosphere | Tabs (K) | T0 (K) | max. SHR (K/min) | tmax (min) |
|---|---|---|---|---|---|
| levopimaric acid | O2 | 353.04 | 354.01 | 1.118 | 57.87 |
| neoabietic acid | O2 | 353.13 | 353.83 | 11.958 | 80.47 |
| dehydroabietic acid | O2 | 387.71 | 398.20 | 0.199 | 572.38 |
| levopimaric acid | N2 | no absorption | not exothermic | 0 | no reaction |
| neoabietic acid | N2 | no absorption | not exothermic | 0 | no reaction |
| dehydroabietic acid | N2 | no absorption | not exothermic | 0 | no reaction |
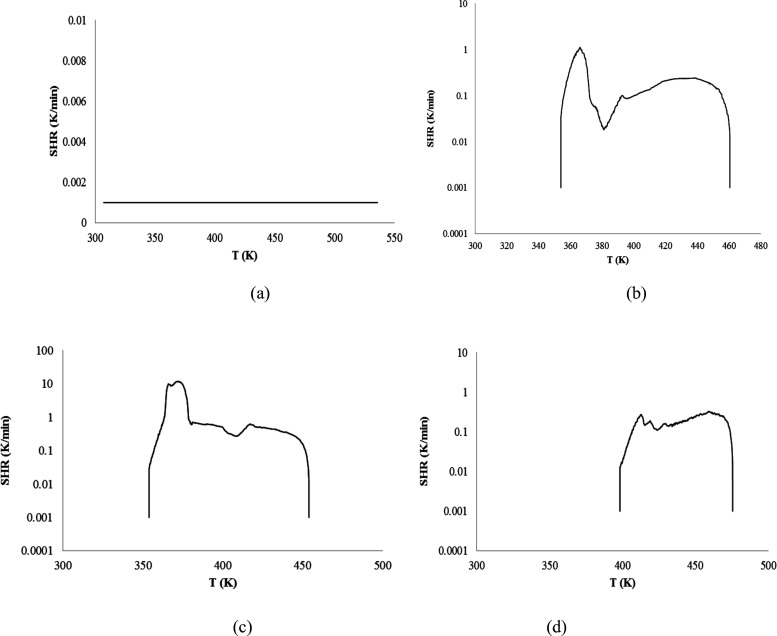
Reaction R5 is an oxidation process, and it is an exothermic process. The characteristics of an exothermic process include the initial exothermic temperature (T0), the maximum self-heat rate (SHR), and the time (tmax) to reach maximum SHR. T0 is related to the reaction activity and maximum SHR, and tmax is related to fast chemical reaction. T0 is one of the important parameters for the chemical reactivity. Therefore, measurements and calculations of the initial oxidation reaction temperature of resin acids are very important. The conventional method for determining the initial oxidation temperature is by determining the initial exothermic temperature using the ARC. Initial exothermic temperature (T0) was obtained from the SHR−T curve when the initial dT/dt > 0.02 K/min. The SHR–T curve of the three resin acids are shown in Figure 6. T0, maximum SHR, and tmax are also shown in Table 1, respectively.
Figure 6.
SHR vs temperature of resin oxidation reaction: (a) levopimaric acid + N2; (b) levopimaric acid + O2; (c) neoabietic acid + O2; (d) dehydroabietic acid + O2.
Table 1 shows that the order of Tabs is levopimaric acid < neoabietic acid < dehydroabietic acid. The results showed that the reactive orders are levopimaric acid < neoabietic acid < dehydroabietic acid. In other words, the initial exothermic temperature (T0) is different from the initial temperature of oxygen absorption (Tabs), and T0 > Tabs.
These experimental results can be explained by the structural characteristics of the three resin acids. Levopimaric acid and neoabietic acid are compounds with conjugated double bonds, which are unstable and are easily oxidized. The structure of levopimaric acid and neoabietic acid are similar, and their reaction activity is similar, so the initial oxidation temperature (T0) is also similar. Dehydroabietic acid is a compound with a benzene ring structure. Its chemical properties are stable, it is not easily oxidized, and its T0 is high. From the view point of chemical structure, the order of oxidation activity of the three kinds of resin acids is levopimaric acid (easy) ≈ neoabietic acid > dehydroabietic acid (difficult).
Their melting points are as follows: levopimaric acid, M.P. of 150–152 °C; neoabietic acid, M.P. of 167–169 °C; dehydroabietic acid therefore, M.P. of 166–168 °C. The oxidation reaction occurs in the gas and solid surface. However, when the resin acids absorbed oxygen, but small exothermic heat was not detected by ARC (when dT/dt < 0.02 K/min.), it is difficult to determine the initial oxidation reaction. How to determine the initial oxidation reaction is an interesting question.
Kinetics of Initial Oxidation
It is assumed that the initial oxidation reaction of resin acid is as follows:
| R7 |
If the oxidation kinetics is a second-order reaction, the kinetic equation is
| 1 |
where t is the time, a is the initial molar number of resin acid, b is the initial molar volume of O2, x is the molar number of the reaction products at t, and k is the rate constant. If we assume that the resin acid vapor pressure is p = 0, its melting point is 448 K (175 °C). Assuming that O2 is an ideal gas in the cell, the molar quantity of O2 can be calculated using the ideal gas equation nt = (b – x) = PV/RT, where P is the pressure, R is the gas constant, and T is the temperature. In the experiment, a and b values of the three resin acids are shown in Table 2 below:
Table 2. Kinetic Equations of the Three Resin Acids.
| resin acids | a | b | kinetic equation | activation energy (kJ/mol) | correlation coefficient R2 |
|---|---|---|---|---|---|
| levopimaric acid | 0.001625 | 0.003800 | ln[(0.001625 – x)/(0.003800 – x)] = –459.8kt – 0.8495 | 42.90 | 0.995 |
| neoabietic acid | 0.001620 | 0.003783 | ln[(0.001620 – x)/(0.003783 – x)] = –462.3kt – 0.8481 | 58.05 | 0.976 |
| dehydroabietic acid | 0.001795 | 0.003433 | ln[(0.001795 – x)/(0.003433 – x)] = –610.5kt – 0.6484 | 46.60 | 0.989 |
Then, eq 2 can be obtained by the integral of eq 1
| 2 |
Oxidation kinetic equations of levopimaric acid, neoabietic acid, and dehydroabietic acid are shown in Table 2.
The rate constant k is
| 3 |
where Ar is the pre-exponential factor and Ea is the activation energy. Equation 4 can be obtained by logarithm of eqs 2 and 3
| 4 |
where Y = ln{[(a – b)/t]ln[(b(a – x)/a(b – x)]}. The linear equations of levopimaric acid, neoabietic acid, and dehydroabietic acid are eqs 5, 6, and 7, respectively.
Levopimaric acid
| 5 |
Neoabietic acid
| 6 |
Dehydroabietic acid
| 7 |
According to eqs 5–7, the activation energies of the initial oxidation of the three resin acids are shown in Table 2. In Table 2, R2 is the correlation coefficient of eqs 5–7.
Initial Oxidation Products
The oxidation products of levopimaric acid and neoabietic acid are complex,4−6 in which peroxide is a very important substance because it decomposes to free radicals and it easily initiates complex chemical reactions. Therefore, the peroxides of three resin acids initial oxidation product were determined by iodometry. The relationship between peroxide concentration and oxidation time is shown in Figure 7 when the reaction temperature is 323.15 K.
Figure 7.
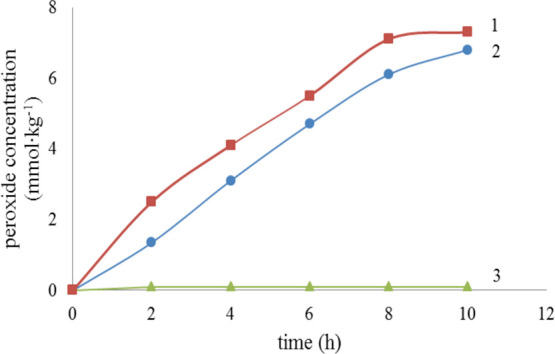
Peroxide concentration vs oxidation time: 1: neoabietic acid; 2: levopimaric acid; 3- dehydroabietic acid.
Figure 7 shows that the oxidation reaction of levopimaric acid and neoabietic acid easily occurs, and the relationship between peroxide concentration and time is linear. However, dehydroabietic acid was stable, no significant oxidation reaction occurred at 323.15 K, and the peroxide concentration was less than 0.2 mmol/kg.
Thermal Stability of Rosin-Modified Resins
Rosin is a very unstable natural resin, and it easily oxidizes, reducing the quality of products. Therefore, stabilization of rosin by modification is a common interest of scientists and enterprises. Product modified with rosin is called rosin-modified resin. Rosin-modified resins include hydrogenated rosin, disproportionated rosin, hydrogenated rosin glyceride, hydrogenated rosin pentaerythritol ester, etc. Hydrogenated rosin is the product obtained by rosin hydrogenation, disproportionated rosin is the product obtained by rosin dehydrogenation with a benzene ring structure, hydrogenated rosin pentaerythritol ester is the product obtained by rosin pentaerythritol ester hydrogenation, and hydrogenated rosin glyceride is the product obtained by rosin pentaerythritol ester hydrogenation.
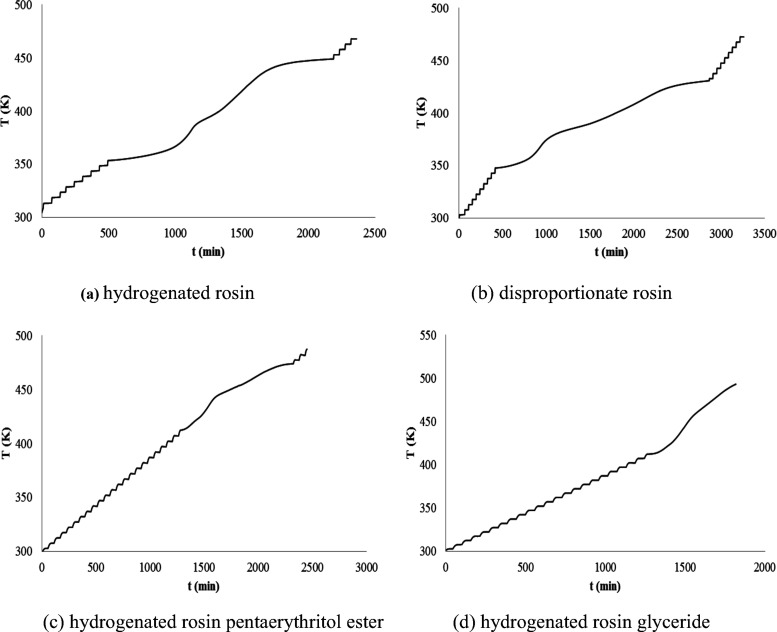
However, the study of their thermal oxidation stability has not been performed. The thermal stability of hydrogenated rosin, disproportionated rosin, hydrogenated rosin glyceride, and hydrogenated rosin pentaerythritol ester was investigated using the ARC. The experimental results are shown in Figure 8.
Figure 8.
Thermal stability of the four rosin- modified resins: (a) hydrogenated rosin, (b) disproportionate rosin, (c) hydrogenated rosin pentaerythritol ester, and (d) hydrogenated rosin glyceride
From the P–T, T–T, and (dT/dt)–t curves in Figure 8, we obtained Tobs, T0, and maximum SHR of the four rosin- modified resins, which are shown in Table 3.
Table 3. Tobs, T0, and Maximum SHR of the Four Rosin-Modified Resins.
| substance | Tobs (K) | T0 (K) | maximum SHR (K/min) |
|---|---|---|---|
| hydrogenated rosin | 353.49 | 353.71 | 0.200 |
| disproportionated rosin | 347.68 | 348.32 | 0.122 |
| hydrogenated rosin pentaerythritol ester | 412.44 | 412.85 | 0.157 |
| hydrogenated rosin glyceride | 412.34 | 412.44 | 0.272 |
| rosin18 | 308.42 | 308.92 | 4.951 |
Table 3 shows that compared with those of rosin, the Tobs and T0 of hydrogenated rosin and disproportioned rosin are significantly improved, those of hydrogenated rosin pentaerythritol ester and hydrogenated rosin glyceride are more significant, and their oxidation temperatures are increased by more than 100 to 412 K. When rosin is stabilized and modified, its thermal stability is significantly improved. This is very meaningful in the adhesive, ink, and coating applications.
Conclusions
In this study, the oxidative stabilities of levopimaric acid, neoabietic acid, dehydroabietic acid, and rosin-modified resins were measured using an ARC. Exothermic reactions do not occur even when they are heated to 523 K; that is, they have high thermal stability under a nitrogen atmosphere. The thermal oxidation process and characteristics of levopimaric acid, neoabietic acid, and dehydroabietic acid were investigated. The results show that, first, the resin acid absorbs oxygen, and then an exothermic reaction takes place. Their initial exothermic temperatures (T0) are, 354.01, 353.83, and 398.20 K. Their initial oxidation kinetics shows a second-order reaction, and their activation energies were calculated using the P–t curve and T–t curve. The peroxides of three resin acids initial oxidation product were determined by iodometry.
The thermal stability of the rosin-modified resins, hydrogenated rosin, disproportionated rosin, hydrogenated rosin glyceride, and hydrogenated rosin pentaerythritol ester, were investigated using an ARC, and the results showed that compared with rosin, the thermal stabilities of the four rosin-modified resins were significantly improved. The thermal stability of hydrogenated rosin glyceride and hydrogenated rosin pentaerythritol ester is high, and this is very valuable for adhesive, ink, and coating applications.
Materials and Methods
Materials
Levopimaric acid was prepared by Qi’s method,29 with a mass purity of >97%. Neoabietic acid was prepared by Loeblich’s method,30 with a purity of >97%. Dehydroabietic acid was prepared by Zhou’s method,31 with a purity of >98.5%. Hydrogenated rosin glyceride and hydrogenated rosin pentaerythritol ester were from, Nanning Pinliu Bio Technology Co., Ltd., China. Hydrogenated rosin and disproportionated rosin were from Wuzhou Sun Shine Forestry & Chemicals Co., Ltd. of Guangxi, China. O2 and N2 gas were obtained from Tomoe Shokai Co., Ltd., Japan, with a mass purity of >99.99%.
Experiment for Thermal Stability Evaluation
The accelerating rate calorimeter (ARC) (Arthur D. Little, Inc. USA, ARC 2000) is a high-precision instrument for monitoring the exothermic and pressure behavior of chemical reaction processes. It was used to evaluate the thermal stability of the resin acids (levopimaric acid, neoabietic acid, and dehydroabietic acid) and the four rosin-modified resins (hydrogenated rosin, disproportionated rosin, hydrogenated rosin glyceride, and hydrogenated rosin pentaerythritol ester). The experimental conditions were as follows:
The sample mass of resin acids (levopimaric acid, neoabietic acid and dehydroabietic acid) or rosin-modified resins in the test bomb (the material is titanium) is 0.4–0.5 g. The atmosphere is oxygen or nitrogen, and the initial pressure at which the experiments were performed were 0.9–0.99 MPa. ARC mode: heat-wait-search’ mode (HWS mode); start temperature: 25 °C; end temperature: 250 °C ; temperature step: 5 °C ; slope sensitivity: 0.02 K·min–1; wait time: 20 min; search time: 10 min.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (11762003), the Major science and technology special project in Guangxi of China (AA17204087-20), the Science and technology fund in Guangxi Education Department of China(2019KY0179), and the Science Foundation of Guangxi University for Nationalities of China (2017MDQN004, XTCX201706).
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
References
- Li Q.; Huang X.; Liu H.; Shang S.; Song Z.; Song J. Properties enhancement of room temperature vulcanized silicone rubber by rosin modified aminopropyltriethoxysilane as a crosslinking agent. ACS Sustainable Chem. Eng. 2017, 5, 10002–10010. 10.1021/acssuschemeng.7b01943. [DOI] [Google Scholar]
- Liu X.; Zhang R.; Li T.; Zhu P.; Zhuang Q. Novel fully biobased benzoxazines from rosin: synthesis and properties. ACS Sustainable Chem. Eng. 2017, 5, 10682–10692. 10.1021/acssuschemeng.7b02650. [DOI] [Google Scholar]
- Lee S.; Lee K.; Kim Y.-W.; Shin J. Preparation and characterization of a renewable pressure-sensitive adhesive system derived from ε-decalactone, L-lactide, epoxidized soybean oil, and rosin ester. ACS Sustainable Chem. Eng. 2015, 3, 2309–2320. 10.1021/acssuschemeng.5b00580. [DOI] [Google Scholar]
- Kanno H.; Schuller W. H.; Lawrence R. V. Some reactions of levopimaric acid dioxide. J. Org. Chem. 1966, 31, 4138–4142. 10.1021/jo01350a061. [DOI] [Google Scholar]
- Schuller W. H.; Lawrence R. V. Air oxidation of resin acids III. The photosensitized oxidation of neoabietic acid and the configurations of the pine gum resin acids. J. Am. Chem. Soc. 1961, 83, 2563–2570. 10.1021/ja01472a032. [DOI] [Google Scholar]
- Herz W.; Ligon R. C.; Kanno H.; Schuller W. H.; Lawrence R. V. Resin acids. XX. The structure of levopimaric acid dioxide. J. Org. Chem. 1970, 35, 3338–3342. 10.1021/jo00835a033. [DOI] [Google Scholar]
- Halbrook N. J.; Lawrence R. V.; Dressler R. L.; Blackstone R. C.; Herz W. Structure and stereochemistry of Diels-Alder adducts of levopimaric acid. J. Org. Chem. 1964, 29, 1017–1021. 10.1021/jo01028a008. [DOI] [Google Scholar]
- Loeblich V. M.; Lawrence R. V. The thermal isomerization of neoabietic acid. J. Am. Chem. Soc. 1957, 79, 1497–1499. 10.1021/ja01563a061. [DOI] [Google Scholar]
- Loeblich V. M.; Baldwin D. E.; O’Connor R. T.; Lawrence R. V. Thermal isomerization of levopimaric acid. J. Am. Chem. Soc. 1955, 77, 6311–6313. 10.1021/ja01628a071. [DOI] [Google Scholar]
- Wenkert E.; Carney R. W. J.; Kaneko C. Dehydroabietic acid derivatives. An unusual α-oxidation of a ketone. J. Am. Chem. Soc. 1961, 83, 4440–4444. 10.1021/ja01482a033. [DOI] [Google Scholar]
- Karlberg A.-T.; Basketter D.; Goosens A.; Lepoittevin J.-P. Regulatory classification of substances oxidised to skin sensitisers on exposure to air. Contact Dermatitis 1999, 40, 183–188. 10.1111/j.1600-0536.1999.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Gafvert E.; Shao L. P.; Karlberg A.-T.; Nilsson U.; Nilsson J. L. G. Contact allergy to resin acid hydroperoxides. Hapten binding via free radicals and epoxides. Chem. Res. Toxicol. 1994, 7, 260–266. 10.1021/tx00038a020. [DOI] [PubMed] [Google Scholar]
- Ma Q.; Liu X.; Zhang R.; Zhu J.; Jiang Y. Synthesis and properties of full bio-based thermosetting resins from rosin acid and soybean oil: the role of rosin acid derivatives. Green Chem. 2013, 15, 1300–1310. 10.1039/C3GC00095H. [DOI] [Google Scholar]
- Swann M. Colorimetric determination of rosin and rosin esters. Anal. Chem. 1951, 23, 885–888. 10.1021/ac60054a014. [DOI] [Google Scholar]
- Minn J. Determination of oxidative stability of rosin products by high-pressure differential scanning calorimetry. Thermochim. Acta 1985, 91, 87–94. 10.1016/0040-6031. [DOI] [Google Scholar]
- Prinz S.; Müllner U.; Heilmann J.; Winkelmann K.; Sticher O.; Haslinger E.; Hüfner A. Oxidation products of abietic acid and its methyl ester. J. Nat. Prod. 2002, 65, 1530–1534. 10.1021/np010656l. [DOI] [PubMed] [Google Scholar]
- Monroe B. Rate constants for the reaction of singlet oxygen with conjugated dienes. J. Am. Chem. Soc. 1981, 103, 7253–7256. 10.1021/ja00414a035. [DOI] [Google Scholar]
- Moore R. N.; Lawrence R. V. Air oxidation of resin acids. I. Photosensitized oxidation of levopimaric acid. J. Am. Chem. Soc. 1958, 80, 1438–1440. 10.1021/ja01539a040. [DOI] [Google Scholar]
- Gigante B.; Marcelo-Curto M. J.; Lobo A. M.; Prabhakar S.; Slawin A.; Rzepa H.; Williams D. Photooxidation of resin acids. J. Nat. Prod. 1989, 52, 85–94. 10.1021/np50061a011. [DOI] [Google Scholar]
- Herz W.; Ligon R. C. Resin acids. XXIII. Oxidation of levopimaric acid withpotassium permanganate and osmium tetroxide. J. Org. Chem. 1972, 37, 1400–1405. 10.1021/jo00974a024. [DOI] [Google Scholar]
- Herz W.; Blackstone R. C. Resin acids. XV. Oxidative transformations of the levopimaric acid-acetylenedicarboxylic ester adduct. J. Org. Chem. 1969, 34, 1257–1266. 10.1021/jo01257a015. [DOI] [Google Scholar]
- Liu J.-L.; Liu X.-M.; Li W.-G.; Ma L.; Shen F. Kinetics of gum rosin oxidation under 365 nm ultraviolet irradiation. Monatsh. Chem. 2014, 145, 209–212. 10.1007/s00706-013-1014-7. [DOI] [Google Scholar]
- Li Y.; Liu X.; Zhang Q.; Wang B.; Yu C.; Rashid H. U.; Xu Y.; Ma L.; Lai F. Characteristics and kinetics of rosin pentaerythritol ester via oxidation process under ultraviolet trradiation. Molecules 2018, 23, 2816. 10.3390/molecules23112816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; Niu M.; Xu X.; Bian H.; Chen J.; Wen J.; Yu C.; Liang M.; Ma L.; Lai F.; Liu X. Characteristics and kinetics of the glycerol ester of rosin via an oxidation process under ultraviolet irradiation. New J. Chem. 2020, 44, 3375–3381. 10.1039/C9NJ04439F. [DOI] [Google Scholar]
- Ren F.; Zheng Y.-f F.; Liu X.-M.; Yang Q.-Q.; Zhang Q.; Shen F. Thermal oxidation reaction process and oxidation kinetics of abietic acid. RSC Adv. 2015, 5, 17123–17130. 10.1039/C4RA16791K. [DOI] [Google Scholar]
- Ren F.; Zheng Y.-F.; Liu X.-M.; Yue X. Y.; Ma L.; Li W.-G.; Lai F.; Liu J.-L.; Guan W.-L. An investigation of the oxidation mechanism of abietic acid using two-dimensional infrared correlation spectroscopy. J. Mol. Struct. 2015, 1084, 236–243. 10.1016/j.molstruc.2014.12.055. [DOI] [Google Scholar]
- Liu P.; Liu X.; Kubota S.; Huang P.; Wada Y. Thermal oxidation process and characteristic of abietic acid and gum rosin by accelerating rate calorimeter (ARC). J. Therm. Anal. Calorim. 2019, 138, 479–488. 10.1007/s10973-019-08195-8. [DOI] [Google Scholar]
- Li Y.; Xu X.; Niu M.; Chen J.; Wen J.; Bian H.; Yu C.; Liang M.; Ma L.; Lai F.; Liu X. Thermal stability of abietic acid and its oxidation products. Energy Fuels 2019, 33, 11200–11209. 10.1021/acs.energyfuels.9b02855. [DOI] [Google Scholar]
- Qi Z.; Wang C.; Jiang J. Isolation and physical-chemical data testing of levopimaric acid. Biomass Chem. Eng. 2019, 53, 45–50. 10.3969/j.issn.1673-5854.2019.06.008. [DOI] [Google Scholar]
- Loeblich V. M.; Lawrence R. V. An improved procedure for the isolation of neoabietic acid from pine oleoresin and rosin. J. Org. Chem. 1956, 21, 610–611. 10.1021/jo01112a003. [DOI] [Google Scholar]
- Zhou D.; Liu X.; Lai F.; Li W.; Lan H. Study on extraction and separation process of dehydroabietic acid from disproportionated rosin. Appl.Chem. Ind. 2015, 44, 1719–1722. 10.16581/j.cnki.issn1671-3206.2015.09.038. [DOI] [Google Scholar]