Abstract
Introduction
The impact of atrial arrhythmias on coronavirus disease 2019 (COVID‐19)‐associated outcomes are unclear. We sought to identify prevalence, risk factors and outcomes associated with atrial arrhythmias among patients hospitalized with COVID‐19.
Methods
An observational cohort study of 1053 patients with severe acute respiratory syndrome coronavirus 2 infection admitted to a quaternary care hospital and a community hospital was conducted. Data from electrocardiographic and telemetry were collected to identify atrial fibrillation (AF) or atrial flutter/tachycardia (AFL). The association between atrial arrhythmias and 30‐day mortality was assessed with multivariable analysis.
Results
Mean age of patients was 62 ± 17 years and 62% were men. Atrial arrhythmias were identified in 166 (15.8%) patients, with AF in 154 (14.6%) patients and AFL in 40 (3.8%) patients. Newly detected atrial arrhythmias occurred in 101 (9.6%) patients. Age, male sex, prior AF, renal disease, and hypoxia on presentation were independently associated with AF/AFL occurrence. Compared with patients without AF/AFL, patients with AF/AFL had significantly higher levels of troponin, B‐type natriuretic peptide, C‐reactive protein, ferritin and d ‐dimer. Mortality was significantly higher among patients with AF/AFL (39.2%) compared to patients without (13.4%; p < .001). After adjustment for age and co‐morbidities, AF/AFL (adjusted odds ratio [OR]: 1.93; p = .007) and newly detected AF/AFL (adjusted OR: 2.87; p < .001) were independently associated with 30‐day mortality.
Conclusion
Atrial arrhythmias are common among patients hospitalized with COVID‐19. The presence of AF/AFL tracked with markers of inflammation and cardiac injury. Atrial arrhythmias were independently associated with increased mortality.
Keywords: atrial fibrillation, atrial flutter, COVID‐19, mortality, outcomes
Abbreviations
- AF
atrial fibrillation
- AFL
atrial flutter/tachycardia
- BNP
B‐type natriuretic peptide
- CoV
coronavirus
- COVID‐19
coronavirus disease 2019
- CRP
C‐reactive protein
- ESR
erythrocyte sedimentation rate
- MERS
Middle East respiratory syndrome
- SARS
severe acute respiratory syndrome
1. INTRODUCTION
Since the initial description of cases of severe acute respiratory syndrome‐novel coronavirus 2 (SARS‐CoV‐2) infection in late 2019, a total of almost 24 million confirmed cases with over 800,000 deaths has been documented globally as of August 25, 2020. 1 During the course of the coronavirus disease 2019 (COVID‐19) pandemic, cardiovascular manifestations have been recognized as one of the leading complications among patients hospitalized with the disease. Elevated cardiac troponin levels or electrocardiographic changes has been reported in 7.2%–27.8% of hospitalized patients with COVID‐19. 2 , 3 , 4 Arrhythmias have previously been described with SARS‐CoV and Middle East respiratory syndrome coronavirus (MERS‐CoV) infection 5 , 6 and have been reported to occur in 7%–16.7% of hospitalized patients with SARS‐CoV‐2 infection. 2 , 3 However, data on atrial arrhythmias are limited. 7 , 8 We sought to clarify the incidence of and risk factors for atrial arrhythmias among patients hospitalized with COVID‐19, and to evaluate the association of atrial arrhythmias with hospital outcomes including mortality.
2. METHODS
2.1. Data source
This retrospective observational cohort study consisted of all consecutive patients with confirmed COVID‐19 who were admitted to New York‐Presbyterian (NYP)/Weill Cornell Medicine (WMC), a quaternary referral center and 862‐bed teaching hospital, and NYP/Lower Manhattan Hospital (LMH), a 180‐bed community hospital between March 3 and April 6, 2020. This study was approved by the WMC Institutional Review Board, which waived informed consent. All cases of COVID‐19 were confirmed through real‐time reverse‐transcriptase polymerase chain reaction assays on nasopharyngeal swabs. Using REDCap, 9 patient data were manually abstracted from NYP electronic health records to develop a COVID‐19 registry as previously described. 10 The data that support the findings of this study are available from the corresponding author upon reasonable request.
2.2. Data collection
Demographics (age, sex and race) and pre‐existing comorbid conditions (coronary artery disease, heart failure, hypertension, diabetes, prior history of atrial fibrillation (AF), pulmonary disease, renal disease (defined as creatinine ≥2.0 mg/dl or need for hemodialysis) and active cancer were abstracted from the electronic health record. Hypoxia on presentation was defined as use of supplemental oxygen in the emergency department within 3 h of presentation as abstracted from respiratory flowsheets. Chest radiographic findings were abstracted from the initial and any follow‐up radiology reports and categorized based on the most abnormal findings.
All standard 12‐lead electrocardiograms (ECGs) recorded during each hospitalization were reviewed. ECGs were recorded at 25 mm/s and 1 mV/cm according to standard protocol. ECG measurements were performed using MUSE software (GE Healthcare) including QT interval and heart rate. Hospitalization events that occurred through May 10, 2020 were determined based on review of clinical progress notes and discharge summaries. The hospital course of patients discharged but readmitted during the clinical study period was also included in the analysis. Use of antiviral medications, hydroxychloroquine, steroids, and vasopressors during hospitalization was abstracted. Laboratory values including serum troponin I (TnI), C‐reactive protein (CRP), B‐type natriuretic peptide (BNP), d‐dimer, erythrocyte sedimentation rate (ESR), and ferritin were abstracted. Echocardiographic data on left ventricular ejection fraction and right ventricular function were recorded. Complications including intensive care unit (ICU) admission, respiratory failure requiring mechanical ventilation, bacteremia, venous thromboembolism, arterial thromboembolism, stroke or transient ischemic attack (TIA), and acute kidney injury requiring renal replacement therapy were abstracted.
2.3. Primary outcome
The primary outcome of the study was 30‐day all‐cause mortality. Since data collection was complete through May 10, 2020, 30‐day in‐hospital mortality data were available for all patients. The main explanatory variable was atrial arrhythmia, which was defined as AF or atrial flutter/tachycardia (AFL). Newly detected atrial arrhythmia was a secondary explanatory variable. Arrhythmias were identified by review of all ECGs performed during hospitalization and of all telemetry strips with documentation of arrhythmias in the electronic medical records. Arrhythmias were defined to be ≥30 seconds in duration and was diagnosed on the basis of ECG or telemetry strips. ECG and telemetry findings were adjudicated by study investigators (BP, KM, XY, JK, and JWC). Disagreements on adjudication were resolved by consensus. Patients with AF or AFL who did not have a prior history of atrial arrhythmias were considered to have newly detected AF/AFL. Secondary endpoints included arterial thromboembolic, stroke or TIA events.
2.4. Statistical analysis
Categorical variables are shown as frequencies, and continuous variables are presented as mean ± SD or median (interquartile range [IQR]) depending on normality of distribution. For comparisons of categorical variables, the χ 2 or Fisher exact tests were used. For comparisons of continuous variables, the Student t test or Wilcoxon rank‐sum test were used. First, we examined predictors of AF/AFL. We created multivariable logistic regression models by including baseline demographic characteristics (age, sex, and race), co‐morbid conditions (coronary artery disease, heart failure, prior history of AF/AFL, hypertension, diabetes, pulmonary disease, renal disease, immunosuppression, smoking status, and cancer), and marker of disease severity at presentation (hypoxia in emergency department) that had univariate significance (p < .10) for the explanatory variables of interest. Finally, we examined whether AF/AFL was independently associated with the primary endpoint of 30‐day mortality. This was performed by creating multivariable logistic regression models by including presence of arrhythmias, baseline demographic characteristics, co‐morbid conditions and disease severity at presentation that had univariate significance for the primary endpoint. Survival curves were generated using the Kaplan–Meier method and compared by using the log‐rank statistic. All analyses were performed using SAS version 9.4 (SAS Institute) and SPSS version 24 (IBM). All tests were two‐sided with p < .05 indicating statistical significance.
3. RESULTS
3.1. Atrial arrhythmias in the study population
Between March 3 and April 6, 2020, 1053 consecutive patients with COVID‐19 were admitted to NYP‐WMC and NYP‐LMH and included in the study analysis. As of May 10, 2020, 723 (68.6%) patients were discharged, 146 (13.9%) were still hospitalized and 184 (17.5%) had died. The median length of follow‐up was 7 (IQR: 3–18; range: 0–62) days. A total of 154 (14.6%) patients had AF (Figure 1) and 40 (3.8%) had AFL, with an overall frequency of atrial arrhythmias of 15.8%. Among the AF/AFL patients, 101 (61%) had no known history of AF or AFL before hospitalization. Of these patients, 5 (5%) were in AF/AFL at the time of admission while the remaining 96 patients developed AF/AFL later during the course of the hospitalization.
Figure 1.
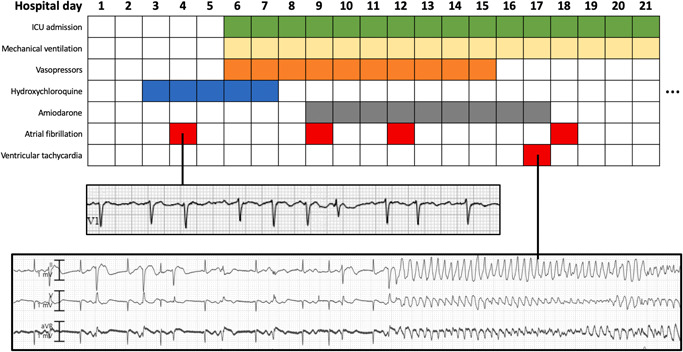
Newly detected atrial fibrillation in a patient with coronavirus disease 2019 (COVID‐19). A 76‐year‐old female admitted with hypoxia and COVID‐19 developed atrial fibrillation with rapid ventricular response (top strip: V1 electrocardiogram) on hospital day 2 and was treated with amiodarone and digoxin. She then developed respiratory failure requiring mechanical ventilation. On hospital day 17, in the setting of potassium level of 2.2 mEq/ml and digoxin level of 0.62 ng/ml, she had ventricular bigeminy and torsade de pointes with prolonged QT interval (bottom strip: telemetry). Time course of treatments are shown.
The clinical characteristics of the study patients stratified by the presence or absence of atrial arrhythmias are summarized in Table 1. Compared to patients without AF/AFL, patients with AF/AFL were older with a higher proportion of males and whites. Patients with AF/AFL also had significantly more co‐morbidities including coronary artery disease, heart failure, prior history of AF/AFL, prior history of stroke, hypertension, pulmonary disease, and renal disease. Finally, a higher proportion of patients with AF/AFL were on medications, such as angiotensin converting enzyme inhibitors or angiotensin receptor blockers and statins. In multivariable regression analysis, age, male sex, prior history of AF, renal disease, and hypoxia on presentation were independently associated with the occurrence of AF/AFL (Table 2).
Table 1.
Comparison of baseline characteristics stratified by presence of atrial arrhythmia
| AF/AFL (n = 166) | No AF/AFL (n = 887) | p Value | |
|---|---|---|---|
| Age, year, mean | 74.5 ± 13.0 | 60.1 ± 17.0 | <.001 |
| Male | 120 (72.3) | 536 (60.4) | .004 |
| Body mass index, kg/m2 | 27.9 ± 6.2 | 28.6 ± 7.0 | .176 |
| Race | <.001 | ||
| White | 73 (44.0) | 301 (33.9) | |
| Black | 11 (6.6) | 107 (12.1) | |
| Asian | 39 (23.5) | 118 (13.3) | |
| Other | 19 (11.4) | 209 (23.6) | |
| Not specified | 24 (14.5) | 152 (17.1) | |
| Coronary artery disease | 45 (27.1) | 112 (12.6) | <.001 |
| Congestive heart failure | 23 (13.9) | 56 (6.3) | <.001 |
| Prior history of AF | 65 (39.2) | 29 (3.3) | <.001 |
| Prior stroke | 21 (12.6) | 52 (5.9) | .002 |
| Diabetes mellitus | 50 (30.1) | 263 (29.7) | .903 |
| Hypertension | 114 (68.7) | 454 (51.2) | <.001 |
| Pulmonary disease | 44 (26.5) | 174 (19.6) | .044 |
| Renal disease | 29 (17.5) | 68 (7.7) | <.001 |
| Cirrhosis | 2 (1.2) | 10 (1.1) | 1.000 |
| Active cancer | 9 (5.4) | 55 (6.2) | .700 |
| Prior organ transplant | 7 (4.2) | 19 (2.1) | .114 |
| Rheumatologic disease | 9 (5.4) | 38 (4.3) | .515 |
| Immunosuppressed status | 5 (3.0) | 24 (2.7) | .796 |
| Active smoking | 3 (1.8) | 37 (4.2) | .144 |
| ACE/ARB use | 56 (33.7) | 244 (27.5) | .103 |
| Statin use | 84 (50.6) | 277 (31.2) | <.001 |
| Hypoxia on presentation | 110 (66.3) | 461 (52.0) | <.001 |
Abbreviations: ACE, angiotensin converting enzyme inhibitor; AF, atrial fibrillation; AFL, atrial flutter; ARB, angiotensin receptor blocker.
Table 2.
Clinical factors independently associated with atrial fibrillation or flutter among atients hospitalized with COVID‐19
| Univariate | Multivariable | |||
|---|---|---|---|---|
| Unadjusted OR (95% CI) | p Value | Adjusted OR (95% CI) | p Value | |
| Age | 1.06 (1.05–1.07) | <.001 | 1.05 (1.03–1.06) | <.001 |
| Male sex | 1.71 (1.19–2.46) | .005 | 1.86 (1.20–2.87) | .005 |
| Prior history of atrial fibrillation | 19.0 (11.7–30.9) | <.001 | 12.4 (7.1–21.5) | <.001 |
| Renal disease | 2.55 (1.59–4.08) | <.001 | 1.84 (1.01–3.38) | .048 |
| Hypoxia on presentation | 1.82 (1.28–2.57) | <.001 | 1.79 (1.18–2.71) | .006 |
| White | 1.53 (1.09–2.14) | .014 | ||
| Coronary artery disease | 2.57 (1.73–3.82) | <.001 | ||
| Congestive heart failure | 2.39 (1.42–4.00) | .001 | ||
| Prior stroke | 2.33 (1.36–3.98) | .002 | ||
| Hypertension | 2.09 (1.47–2.98) | <.001 | ||
| Pulmonary disease | 1.48 (1.01–2.17) | .045 | ||
| Baseline statin use | 2.26 (1.61–3.16) | <.001 | ||
Abbreviations: CI, confidence interval; COVID‐19, coronavirus disease 2019; OR, odds ratio.
3.2. Radiographic, echocardiographic, and laboratory findings
Compared to patients without AF/AFL, patients with AF/AFL more frequently had abnormal chest radiographs (90.3% vs. 83.6%; p = .028) (Table 3). Among 146 (13.9%) patients who underwent echocardiographic imaging, there were no significant differences in left ventricular ejection fraction or proportion of patients with left or right ventricular dysfunction between patients with and without AF/AFL. Myocardial injury with peak troponin I levels ≥0.5 ng/ml were seen in 26.3% of patients with AF/AFL compared to 11.1% of patients without AF/AFL (p < .001). Overall, the AF/AFL group had significantly higher peak levels of TnI, CRP, BNP, d ‐dimer, ESR and ferritin when compared to patients without arrhythmias (p < .001 for all) (Figure 2). Patients with AF/AFL were more likely to undergo treatment with hydroxychloroquine, remdesivir, steroids, IL‐6 inhibitors, and intravenous gamma globulin as outlined in Table 4.
Table 3.
Radiographic, echocardiographic, and laboratory findings
| AF/AFL (n = 166) | No AF/AFL (n = 887) | p Value | |
|---|---|---|---|
| Chest radiography results | |||
| Abnormal chest radiograph no./total no. (%) | 149/165 (90.3) | 728/871 (83.6) | .028 |
| Bilateral infiltrate | 127/165 (77.0) | 619/871 (71.1) | .122 |
| Pleural effusion | 13/165 (7.9) | 40/871 (4.6) | .079 |
| Echocardiography results | |||
| Decreased LVEF < 50%, no./total no. (%) | 16/49 (32.6) | 25/97 (25.8) | .382 |
| Lowest LVEF during hospitalization, %, median (IQR) | 62.5 (47.5–65) | 58.5 (49.5–66.5) | .615 |
| Decreased RV function, no./total no. (%) | 7/49 (14.3) | 16/97 (16.5) | .729 |
| Laboratory values | |||
| Troponin I ≥0.5 ng/ml, no./total no. (%) | 41/156 (26.3) | 81/730 (11.1) | <.001 |
| Troponin I, ng/ml, median (IQR) | 0.13 (0.04–0.52) | 0 (0–0.09) | <.001 |
| C‐reactive protein, mg/dl, median (IQR) | 31.8 (18.7–139) | 17.5 (7.6–38.6) | <.001 |
| B‐type natriuretic peptide, pg/ml, median (IQR) | 225 (101–490) | 48 (17.0–164) | <.001 |
| d ‐Dimer, ng/ml, median (IQR) | 3261 (1195–8591) | 1139 (430–4545) | <.001 |
| ESR, s, median (IQR) | 103 (68–130) | 86 (54–118) | <.001 |
| Ferritin, ng/ml median (IQR) | 1449 (592–2207) | 1011 (454–1758) | <.001 |
Abbreviations: AF, atrial fibrillation; AFL, atrial flutter; ESR, erythrocyte sedimentation rate; IQR, interquartile range; LVEF, left ventricular ejection fraction; RV, right ventricle.
Figure 2.
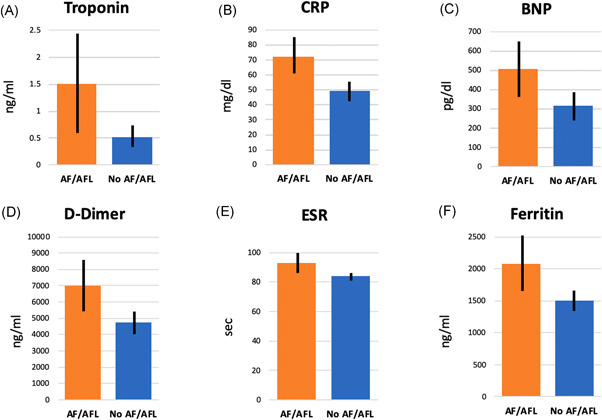
Biomarker levels among patients with and without atrial fibrillation/atrial flutter (AFL) during hospitalization for COVID‐19. Bar graphs comparing mean peak levels of (A) cardiac troponin I, (B) C‐reactive protein, (C) B‐type natriuretic peptide, (D) d ‐dimer, (E) erythrocyte sedimentation rate, (F) ferritin are shown. Error bars indicate 95% confidence intervals for mean. p < .001 for all comparisons. COVID‐19, coronavirus disease 2019
Table 4.
Comparison of hospital course of patients with and without atrial fibrillation (AF)/AFL
| AF/AFL (n = 166) | No AF/AFL (n = 887) | p Value | |
|---|---|---|---|
| Treatment | |||
| Hydroxychloroqine | 129 (77.7) | 616 (69.4) | .032 |
| Remdesivir | 12 (7.2) | 40 (4.5) | .138 |
| Steroids | 65 (39.2) | 176 (19.8) | <.001 |
| IL‐6 inhibitor | 16 (9.6) | 49 (5.5) | .043 |
| Intravenous gamma globulin | 4 (2.4) | 6 (0.7) | .058 |
| Complications | |||
| ICU admission | 100 (60.2) | 249 (28.1) | <.001 |
| Hypotension requiring vasopressor therapy | 99 (60.0) | 224 (25.3) | <.001 |
| Respiratory failure requiring mechanical ventilation | 100 (60.2) | 227 (25.6) | <.001 |
| Bacteremia | 28 (16.9) | 72 (8.1) | <.001 |
| Venous thromboembolism | 13 (7.8) | 41 (4.6) | .085 |
| Stroke/TIA | 10 (6.0) | 8 (0.9) | <.001 |
| Acute kidney injury requiring new RRT | 10 (6.0) | 24 (2.7) | .026 |
| Death | 65 (39.2) | 119 (13.4) | <.001 |
Abbreviations: AFL, atrial flutter/tachycardia; ICU, intensive care unit; RRT, renal replacement therapy; TIA, transient ischemic attack.
3.3. In‐hospital complications and outcomes of patients with arrhythmias
Compared with patients without AF/AFL, patients with AF/AFL had significantly more complications during their hospital course including respiratory failure requiring mechanical ventilatory support, hypotension requiring vasopressors, bacteremia, stroke or TIA, and venous thromboembolism (Table 4). Consistent with these findings, they were also more likely to be admitted to ICUs. Among patients with AF/AFL, 10 (6.0%) had documented stroke or TIA. Of these patients, two (20%) were on therapeutic anticoagulation at the time of diagnosis of the event (one patient was on direct oral anticoagulant therapy and the other patient was on subcutaneous enoxaparin). Of the remaining eight patients, four were not on any anticoagulation at the time of stroke or TIA due to concerns for active bleeding while the remaining four were on prophylaxis dosing of subcutaneous heparin or enoxaparin (Table 5).
Table 5.
Association of atrial arrhythmias with 30‐day all‐cause mortality
| Arrhythmia | Event rates based on arrhythmia (%) | Unadjusted OR (95% CI) | p Value | Adjusted OR (95% CI) a | p Value | |
|---|---|---|---|---|---|---|
| Yes | No | |||||
| Atrial fibrillation (AF) | 37.7 | 12.8 | 4.12 (2.82–6.02) | <.001 | 2.16 (1.33–3.52) | .002 |
| AFL | 22.5 | 16.2 | 1.50 (0.70–3.22) | .293 | 0.65 (0.27–1.55) | .335 |
| Any AF/AFL | 35.5 | 12.9 | 3.74 (2.57–5.43) | <.001 | 1.93 (1.20–3.11) | .007 |
| Newly detected AF/AFL | 36.6 | 14.3 | 3.47 (2.23–5.41) | <.001 | 2.87 (1.74–4.74) | <.001 |
Adjusted for age, body mass index, race, coronary artery disease, congestive heart failure, prior stroke, prior AF, hypertension, lung disease, renal disease, active cancer, immunosuppression, angiotensin converting enzyme inhibitor or angiotensin receptor blocker use, nonsteroidal inflammatory use, proton pump inhibitor use, statin use or hypoxia on presentation based on presence of univariate significance (p < .10).
Abbreviations: AFL, atrial flutter/tachycardia; CI, confidence interval; OR, odds ratio.
Overall in‐hospital mortality was significantly greater among patients with AF/AFL compared with those without AF/AFL (39.2% vs. 13.4%; p < .001). Mortality was significantly higher for patients with AF/AFL (log rank p < .001) (Figure 3). After adjustment for age, race, and co‐morbidities, AF/AFL (adjusted odds ratio [OR]: 1.93; 95% CI: 1.20–3.11; p = .007), and newly detected AF/AFL (adjusted OR: 2.87; 95% CI: 1.74–4.74; p < .001) were independently associated with 30‐day all‐cause mortality.
Figure 3.
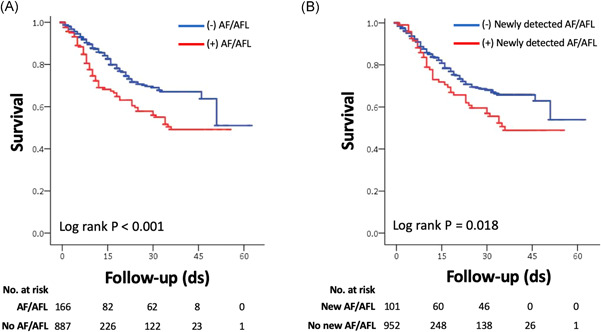
Survival among hospitalized COVID‐19 patients stratified by presence of atrial fibrillation and AFL. (A) Kaplan–Meier survival curves of patients with and without any atrial fibrillation or AFL. (B) Kaplan–Meier survival curves of patients with and without newly detected AFL. AFL, atrial flutter; COVID‐19, coronavirus disease 2019
4. DISCUSSION
In this study of atrial arrhythmia identified in a multi‐center cohort of over 1000 consecutive patients hospitalized with COVID‐19, we identified several important findings. First, AF/AFL was seen in over 15% of patients, with greater than 60% of these occurring in patients without any prior history of AF. Second, age, male sex, prior history of AF, renal disease, and hypoxia on presentation were all independently associated with occurrences of atrial arrhythmias. Third, the presence of AF/AFL tracked with markers of disease severity, such as abnormal chest radiographs, need for ICU admission, vasopressors, mechanical ventilation, and new requirement for renal replacement therapy. Fourth, 6% of patients with AF/AFL experienced stroke or TIA during their hospitalization. Fifth, patients with AF/AFL demonstrated significantly higher indices of myocardial injury and systemic inflammation, without findings of left or right ventricular function impairment when compared to patients without AF/AFL. Finally, after adjustment age and co‐morbidities, the presence of any AF/AFL and newly detected AF/AFL were each independently associated with mortality.
4.1. Comparisons with arrhythmias associated with other coronavirus syndromes
Varying prevalence of arrhythmias associated with previous coronavirus epidemics involving SARS‐CoV and MERS‐CoV infections have been reported in limited series. In a study of 121 patients with SARS, only one patient had AF. 5 In a series of 70 patients with MERS‐CoV infection, 15.7% patients had arrhythmias, although the frequency of atrial arrhythmias was not reported. 6 With the global pandemic from COVID‐19, the cardiovascular complications from SARS‐CoV‐2 infection have been better characterized with larger studies but data on atrial arrhythmias remain limited. In a series of 138 patients hospitalized with COVID‐19, Wang et al. reported that 16.7% of patients had arrhythmias but the frequency of atrial arrhythmias was not reported. 2 Colon et al. 7 published their findings of 115 patients admitted for COVID‐19 and found a 16.5% frequency of atrial arrhythmias, all of whom were admitted to an ICU. Similar to our findings, this study found an association between atrial arrhythmia occurrence and severity of COVID‐19. Their study did not identify a significant difference in troponin or CRP levels among patients with and without atrial arrhythmias, which may likely reflect the limited size of their study cohort. Recently, Bhatla et al. studied 700 patients and identified only 25 incident AF events corresponding to an incidence of only 3.5%. On multivariable analysis, they did not find incident AF to be independently associated with mortality which may have been due to smaller sample size.
4.2. Factors associated with atrial arrhythmias in hospitalized COVID‐19 patients
There are multiple factors leading to COVID‐19‐associated atrial arrhythmias. Age and co‐morbidities, such as pulmonary and renal disease increase the risk of developing atrial arrhythmias regardless of the presence of COVID‐19. In our study, nearly two thirds of the patients with arrhythmias were treated in ICUs. Cardiac arrhythmias have been reported to occur in 14.3%–19.2% of ICU patients, with the majority being AF, 11 which would be consistent with the incidence of AF seen in our study. Independent of the underlying COVID‐19 disease process, alterations in intravascular volume, electrolyte abnormalities, metabolic derangements, and need for vasopressor therapy can all lead to arrhythmias. 12 Furthermore, patients with AF/AFL had marked elevations of troponin, BNP, CRP, d ‐dimer, ESR, and ferritin. In some cases, their arrhythmias may have been a manifestation of the inflammatory response with multiorgan system involvement associated with cytokine release syndrome that has been described with severe COVID‐19. 13
In our study, 13.8% of all patients and 26.3% of patients with AF/AFL had evidence of cardiac injury. However, both patients with and without atrial arrhythmias had similar LV ejection fraction. The association between COVID‐19‐associated myocardial injury and atrial arrhythmias may reflect any combination of systemic inflammatory response, microvascular injury, stress cardiomyopathy, acute coronary syndrome, hypoxia with supply‐demand mismatch, and direct viral cardiac injury. 14
4.3. Clinical implications
This study has implications for treatment and management of COVID‐19. First, AF was the most frequent arrhythmia seen in the patients in our study. Stroke or TIA was seen in 6% of AF patients, with 80% of those events occurring while the patients were not on therapeutically dosed anticoagulation. In the context of the high rate of thromboembolic events that have been described with COVID‐19, 15 therapeutic anticoagulation may be considered in patients with newly detected AF and COVID‐19 if there is no evidence of severe coagulopathy or active bleeding, even if the CHA2DS2VASc score is low. Finally, given the significantly elevated inflammatory markers seen in patients with arrhythmias, effective treatment of COVID‐19‐associated cytokine release syndrome may help reduce arrhythmia occurrence. Several clinical trials investigating anti‐inflammatory therapy, such as steroids, interleukin‐1 (IL‐1) and IL‐6 inhibitors, and other therapies for the treatment of severe COVID‐19 are in progress.
4.4. Study limitations
This is a retrospective study with data obtained via chart abstraction and review, which may be subject to error or interpretation. However, the process utilized in this study has previously shown excellent reliability (mean Cohen's kappa, 0.92; mean intraclass coefficient, 0.94). 10 We did not examine timing of diagnosis of atrial arrhythmias in our cohort which would have allowed exploration of the temporal relationship between arrhythmia onset and in‐hospital clinical events, such as intubation or admission to ICUs. Diagnostic testing including ECG and laboratory assessment (including biomarkers) was not uniform among all patients. Considerations of the risks versus benefits of testing in critically ill patients with COVID‐19 limited the degree and frequency of echocardiographic and cardiac magnetic resonance imaging that could have better characterized the cardiac function of the study patients. Indeed, due to these considerations, no electrophysiology studies, catheter ablations, or elective cardioversions were performed on any patient in this study cohort. As such, electrophysiologic studies to determine mechanisms of AFL was not possible. Due to the lack of a prospective protocol to determine rate versus rhythm control of atrial arrhythmias, we did not examine the impact of AF/AFL treatment strategy on outcomes. Next, while all patients in the study cohort were placed on telemetry, there were variations in telemetry monitoring systems across hospital units may have led to possible under‐detection of arrhythmias in some cases. For example, not all telemetry systems had full disclosure to permit thorough retrospective review for arrhythmias. We also did not have postdischarge follow‐up to assess for the occurrence of atrial arrhythmias after hospitalization. Next, because 13.9% of the study patients remained hospitalized at the time of closure of study data collection, there was incomplete mortality data. Nonetheless, there was at least 30‐day follow‐up for all hospitalized patients. However, for patients who were discharged in less than 30 days, sudden death occurring out‐of‐hospital could have been missed. Finally, we did not adjudicate cause of death in our patients who experienced mortality.
5. CONCLUSION
Atrial arrhythmias are common among patients hospitalized with COVID‐19, with AF being most prevalent. The presence of AF/AFL is associated with increased clinical manifestations of severe COVID‐19 and elevated markers of inflammation and cardiac injury. Atrial arrhythmias in COVID‐19 are independently associated with in‐hospital mortality.
DISCLOSURES
Dr. Cheung has received consulting fees from Abbott, Biosense Webster, Biotronik and Boston Scientific and fellowship grant support from Abbott, Biosense Webster, Biotronik, Boston Scientific, and Medtronic. Dr. Safford has received research grant support from Amgen. The other authors report no relevant disclosures.
ACKNOWLEDGMENTS
The authors would like to thank the following Weill Cornell Medicine medical students for their contributions to the COVID‐19 registry through medical chart abstraction: Zara Adamou, Haneen Aljayyousi, Mark N. Alshak (student leader), Bryan Ang, Elena Beideck, Orrin S. Belden, Anthony F. Blackburn, Joshua W. Bliss, Kimberly A. Bogardus, Chelsea D. Boydstun, Clare A. Burchenal, Eric T. Caliendo, John K. Chae, David L. Chang, Frank R. Chen, Kenny Chen, Andrew Cho, Alice Chung, Alisha N. Dua, Andrew Eidelberg, Rahmi S. Elahjji, Mahmoud Eljalby, Emily R. Eruysal, Kimberly N. Forlenza, Rana Khan Fowlkes, Rachel L. Friedlander, Gary George, Shannon Glynn, Leora Haber, Janice Havasy, Alex Huang, Hao Huang, Jennifer H. Huang, Sonia Iosim, Mitali Kini, Rohini V. Kopparam, Jerry Y. Lee, Mark Lee, Aretina K. Leung, Han A. Li (student leader), Bethina Liu, Charalambia Louka, Brienne Lubor, Dianne Lumaquin, Matthew L. Magruder, Ruth Moges, Prithvi M. Mohan, Max F. Morin, Sophie Mou, J. J. Nario, Yuna Oh, Noah Rossen, Emma M. Schatoff, Pooja D. Shah, Sachin P. Shah, Daniel Skaf, Shoran Tamura, Ahmed Toure, Camila M. Villasante, Gal Wald, Graham T. Wehmeyer (student leader), Samuel Williams, Ashley Wu, Andrew L. Yin, and Lisa Zhang. Dr. Goyal is supported by American Heart Association grants 18IPA34170185 and 20CDA35310455. This study received support from NewYork‐Presbyterian Hospital (NYPH) and Weill Cornell Medical College (WCMC), including the Clinical and Translational Science Center (CTSC) (UL1 TR000457) and Joint Clinical Trials Office (JCTO).
Peltzer B, Manocha KK, Ying X, et al. Outcomes and mortality associated with atrial arrhythmias among patients hospitalized with COVID‐19. J Cardiovasc Electrophysiol. 2020;31:3077‐3085. 10.1111/jce.14770
Disclosures: None.
REFERENCES
- 1. Center for Systems Science and Engineering, Johns Hopkins University . COVID dashboard. 2020. https://coronavirus.jhu.edu/map.html. Accessed August 25, 2020.
- 2. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5:811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan China. JAMA Cardiol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yu CM, Wong RS, Wu EB, et al. Cardiovascular complications of severe acute respiratory syndrome. Postgrad Med J. 2006;82:140‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saad M, Omrani AS, Baig K, et al. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single‐center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Colon CMBJ, Chiles JW, McElwee SK, Russell DW, Maddox WR, Kay GN. Atrial arrhythmias in COVID‐19 patients. JACC Clinic Electrophysiol. 2020. [DOI] [PMC free article] [PubMed]
- 8. Bhatla A, Mayer MM, Adusumalli S, et al. COVID‐19 and cardiac arrhythmias. Heart Rhythm. 2020;17:1439‐1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of COVID‐19 in New York City. N Engl J Med. 2020;382:2372‐2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reinelt P, Karth GD, Geppert A, Heinz G. Incidence and type of cardiac arrhythmias in critically ill patients: a single center experience in a medical‐cardiological ICU. Intensive Care Med. 2001;27:1466‐1473. [DOI] [PubMed] [Google Scholar]
- 12. Tracy C, Boushahri A. Managing arrhythmias in the intensive care unit. Crit Care Clin. 2014;30:365‐390. [DOI] [PubMed] [Google Scholar]
- 13. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, HLH Across Speciality Collaboration, UK . COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Atri D, Siddiqi HK, Lang J, Nauffal V, Morrow DA, Bohula EA. COVID‐19 for the cardiologist: a current review of the virology, clinical epidemiology, cardiac, and other clinical manifestations and potential therapeutic strategies. JACC Basic Transl Sci. 2020;5:518‐536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Connors JM, Levy JH. COVID‐19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033‐2040. [DOI] [PMC free article] [PubMed] [Google Scholar]


