Abstract
Background
Monitoring of cardiac implantable electronic devices was highly impacted by the COVID‐19 pandemic considering the high volume of in‐person visits for regular follow‐up. Recent recommendations highlight the important role of remote monitoring to prevent exposure to the virus. This study compared remote monitoring of implantable cardioverter defibrillators (ICDs) in patients whose in‐person annual visit was substituted for a remote monitoring session with patients who were already scheduled for a remote monitoring session.
Methods
This was a cross‐sectional observational study of 329 consecutive patients between 20 March and 24 April 2020. Group 1 included 131 patients whose in‐person annual visit was substituted for a remote monitoring session. Group 2 included 198 patients who underwent a remote monitoring session as scheduled in their usual device follow‐up. The time interval since the last in‐person visit was 13.3 ± 3.2 months in group 1 and 5.9 ± 1.7 months in group 2 (P < .01).
Results
In group 1, 15 patients (11.5%) experienced a clinical event compared to 15 patients (7.6%) in group 2 (P = .25). Nineteen patients (14.5%) required a physician intervention in group 1 compared to 19 patients (9.6%) in group 2 (P = .22). Two patients (1.5%) in group 1 and four patients (2.0%) in group 2 required an early in‐person follow‐up visit during the pandemic (P > .99).
Conclusion
Remote monitoring of ICDs is useful to identify clinical events and allows physicians to treat patients appropriately during the COVID‐19 pandemic regardless of the time interval since their last in‐person visit. It reduces significantly in‐person visit for regular follow‐up.
Keywords: COVID‐19, implantable cardioverter defibrillators, remote monitoring
1. INTRODUCTION
The COVID‐19 (SARS [Severe acute respiratory syndrom]‐CoV‐2 virus) pandemic significantly impacted healthcare systems around the world since its outbreak in December 2019. The management of several diseases was modified in order to provide optimal care for patients and healthcare workers in a safe environment. Monitoring of cardiac implantable electronic devices (CIEDs) was highly impacted considering the high volume of in‐person visits for routine follow‐up. The uptake of remote monitoring had to be accelerated in the pandemic context to reduce in‐person visits for CIED monitoring. 1 , 2 It was even more important since patients with known cardiovascular disease had worse outcomes with COVID‐19. 3
Remote monitoring for CIED is a class 1 recommendation for routine use combined with annual in‐person visits. 4 With modern technology and with additional clinical data, the role of remote monitoring and eventually remote programming may become more adopted in the future. However, many barriers related to patients and healthcare systems limit its widespread utilization in clinical practice. 5 Moreover, to our knowledge, no study has evaluated exclusive remote monitoring of implantable cardioverter defibrillators (ICDs) without annual in‐person visits. Recommendations for remote monitoring in the context of the COVID‐19 pandemic are supported by expert consensus.
Thus, the primary objective of this study was to compare the findings of remote only ICD monitoring in patients whose in‐person annual visit was substituted for a remote monitoring session because of the pandemic restrictions with patients who were already scheduled for a remote monitoring session, having been seen at the device clinic within the past 6 months. We also evaluated the utility of remote monitoring in order to reduce patients’ potential exposure to the virus in a hospital environment.
2. METHODS
This was a cross‐sectional observational study of 329 consecutive patients who underwent remote monitoring of their ICD between 20 March and 24 April 2020 at a single tertiary care centre. The study was initially started for quality assurance. Patients were divided in two groups. The pandemic‐related remote monitoring group (group 1) included 131 patients whose in‐person annual visit was canceled because of the COVID‐19 pandemic restrictions and who underwent remote monitoring instead. Group 2 included 198 patients who underwent remote monitoring as scheduled in their usual device follow‐up schedule since their last annual in‐person visit was in the past 6 months (the usual schedule is one in‐person visit per year and one remote follow‐up per year at 6 months intervals). We did not include nonscheduled remote monitoring sessions. We compared the prevalence of clinical events and physicians’ interventions in each group. We hypothesized that the prevalence of clinical events and physician interventions would be similar in both groups.
Remote monitoring sessions consisted of analysing data collected since last device follow‐up and did not include systematically a communication to patients by physicians. Remote‐monitoring data provided by all manufacturers included lead parameters, battery status, programming parameters, arrhythmia logbook with intracardiac electrograms, heart rhythm statistics and patient activity level. Alternate electrophysiologists revised these sessions without blinding of patients’ groups.
Clinical events were defined as clinically significant arrhythmia, antitachycardia pacing, shock, inappropriate antitachycardia pacing or shock, battery issues, and device/lead anomalies. Clinically significant arrhythmias were defined as new‐onset atrial fibrillation/atrial flutter, sustained ventricular tachycardia, or any arrhythmia leading to a therapeutic change. Battery issues referred to low battery voltage or elective replacement indicator (ERI) requiring early follow‐up or generator change, and device/lead anomalies referred to impedance, sensing, threshold, or LV pacing anomalies leading to early follow‐up, programming change, or any intervention.
Physicians’ interventions were categorized as completely remote interventions, interventions requiring early in‐person visit during the pandemic, and interventions postponed to the next in‐person visit. Remote interventions included phone call to patient to enquire about symptoms, change in medication, recommendation to the treating physician concerning medication or investigations, and early remote monitoring follow‐up. Interventions requiring early in‐person visit included early in‐person follow‐up, planned generator change, or any other planned procedure. Visits or remote monitoring follow‐up before the next scheduled visit was considered 'early'. Interventions postponed to the next in‐person visit referred to suggested nonurgent programming changes.
3. STATISTICAL ANALYSIS
Categorical variables are expressed as percentage, and continuous variables are expressed as mean ± SD. The Fisher's exact test was used for comparisons of categorical variables, including clinical events and physicians’ interventions. Continuous variables were all in a non‐normal distribution and were therefore analysed with the Mann‐Whitney U test. A two‐sided P value of <.05 was considered statistically significant.
4. RESULTS
4.1. Baseline characteristics
A total of 329 consecutive patients (76% male) with a mean age of 67 ± 12 years were included in the study (Table 1). No statistically significant difference was observed between both groups except for the interval since last in‐person visit (13.3 ± 3.2 months in group 1 vs 5.9 ± 1.7 months in group 2, P < .01). Mean time since implantation was 48.8 ± 31.2 months. Most patients were not pacing‐dependent (93.9%) and were implanted for primary prevention (62.6%). Mean left ventricular ejection fraction was 37.8 ± 13.7%. One hundred eighteen patients (35.9%) had dual‐chamber ICDs, 113 (34.3%) had cardiac resynchronisation therapy‐defibrillator (CRT‐D)/ICDs, 94 (28.6%) had single‐chamber ICDs, and four (1.2%) had subcutaneous defibrillators.
TABLE 1.
Baseline characteristics
| Group 1 | Group 2 | ||
|---|---|---|---|
| N = 131 | N = 198 | P value | |
| Age (years) | 66.0 ± 12.0 | 67.4 ± 11.7 | P = .29 |
| Male | 99 (75.6%) | 157 (79.3%) | P = .50 |
| Nondependent | 123 (93.9%) | 186 (93.9%) | |
| Primary prevention | 79 (60.3%) | 127 (64.1%) | P = .49 |
| LVEF (%) | 37.7 ± 12.7 | 36.4 ± 13.5 | P = .32 |
| Months since implantation (mean) | 49.4 ± 29.4 | 48.3 ± 32.3 | P = .54 |
| Months since last interrogation (mean) | 13.3 ± 3.2 | 5.9 ± 1.7 | P < .01 |
| Dual‐chamber ICDs | 50 (38.2%) | 68 (34.3%) | P = .28 |
| Single‐chamber ICDs | 39 (29.8%) | 55 (27.8%) | |
| CRT ICDs | 39 (29.8%) | 74 (37.4%) | |
| Subcutaneous ICDs | 3 (2.3%) | 1 (0.5%) |
Abbreviations: CRT, cardiac resynchronisation therapy; ICDs, implantable cardiac defibrillators; LVEF, left ventricular ejection fraction.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
4.2. Clinical events
Clinical events were reported in fifteen patients (11.5%) in group 1 and 15 patients (7.6%) in group 2 (P = .25) (Table 2). In group 1, clinically significant arrhythmias occurred in seven patients: four had atrial arrhythmias (new‐onset atrial fibrillation or atrial tachycardia), and three had sustained ventricular tachycardias. One patient had a successful antitachycardia pacing to convert a sustained ventricular tachycardia. Two ventricular tachycardia events were analysed as ventricular tachycardia below the detection threshold after revision of the intracardiac Electrogram (EGM) by the physician. All battery issues consisted of low battery voltages without ERI alert encountered. Five device/lead anomalies were identified: two were ventricular farfield on the atrial lead, one was atrial noise without evidence of lead fracture or any lead integrity concern, one was low LV pacing percentage, and one was ineffective ventricular autocapture threshold (Table 3).
TABLE 2.
Proportion of patients with clinical events and physicians’ interventions
| Group 1N = 131 | Group 2N = 198 | P value | |
|---|---|---|---|
| Clinical events 1 | 15 (11.5%) | 15 (7.6%) | P = .25 |
| Total interventions | 19 (14.5%) | 19 (9.6%) | P = .22 |
| Completely remote interventions | 8 (6.1%) | 8 (4.0%) | P = .14 |
| Interventions requiring early in‐person visit | 2 (1.5%) | 4 (2.0%) | P > .99 |
| Interventions postponed to next visit | 9 (6.9%) | 7 (3.5%) | P = .20 |
One patient in each group had two clinical events.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
TABLE 3.
Distribution of clinical events
| Group 1 N = 131 | Group 2 N = 198 | P value | |
|---|---|---|---|
| Clinically significant arrhythmia, n (%) | 7 (5.3%) | 8 (4.0%) | P = .60 |
| Appropriate antitachycardia pacing, n (%) | 1 (0.8%) | 0 | P = .40 |
| Appropriate shock, n (%) | 0 | 0 | P > .99 |
| Inappropriate antitacycardia pacing or shock, n (%) | 0 | 1 (0.5%) | P > .99 |
| Low battery voltage or ERI, n (%) | 3 (2.3%) | 5 (2.5%) | P > .99 |
| Device/lead anomalies, n (%) | 5 (3.8%) | 2 (1.0%) | P = .12 |
Abbreviation: ERI, elective replacement indicator.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
In group 2, eight clinically significant arrhythmias occurred; all were atrial arrhythmias (new‐onset atrial fibrillation, atrial flutter, and atrial tachycardia). One patient had an inappropriate antitachycardia pacing in response to an atrial tachycardia. Five battery issues consisting of low battery voltages were reported, requiring early follow‐up. Two lead anomalies were noted: one was a transient high left ventricular lead impedance, while the other consisted of persistent high atrial and ventricular thresholds (Table 3).
4.3. Physicians’ interventions
Nineteen patients in each group required a physician intervention (14.5% in group 1 vs 9.6% in group 2, P = .22). All interventions were in response to clinical events identified by remote monitoring. Interventions were performed remotely in eight patients in both groups (6.1% in group 1 vs 4.0% in group 2, P = .14). Early in‐person visit was organized in two patients in group 1 and in four patients in group 2 (1.5% vs 2.0%, P > .99). Nine patients in group 1 and seven patients in group 2 had an intervention postponed to their next in‐person visit, all nonurgent programming changes (6.9% vs 3.5%, P = .20) (Table 2).
Overall, 90% of interventions in group 1 and 80% in group 2 were managed without requiring an in‐person visit (Figure 1).
FIGURE 1.
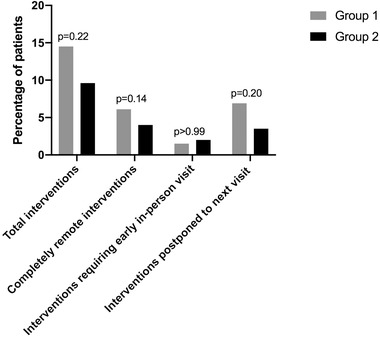
Physicians’ interventions in response to clinical events
Physicians’ interventions in each group are listed in Table 4. In both groups, changes in medication were related to clinically significant arrhythmias, and early remote monitoring follow‐up was organized for low battery voltages. Recommendations to the primary physician were to suggest a change in medication, except for two recommendations in group 1 for ambulatory cardiac monitoring to exclude frequent premature ventricular beats.
TABLE 4.
Distribution of physicians’ interventions
| Group 1 N = 131 | Group 2N = 198 | P value | |
|---|---|---|---|
| Phone call to patient, n (%) | 3 (2.3%) | 6 (3.0%) | P > .99 |
| Change in medication, n (%) | 1 (0.8%) | 3 (1.5%) | P > .99 |
| Recommendation to the primary physician, n (%) | 4 (3.0%) | 1 (0.5%) | P = .08 |
| Early in‐patient follow‐up, n (%) | 2 (1.5%) | 0 | P = .16 |
| Early remote device monitoring follow‐up, n (%) | 2 (1.5%) | 4 (2.0%) | P > .99 |
| Planned device replacement, n (%) | 0 | 1 (0.5%) | P > .99 |
| Planned procedure (ablation, cardioversion), n (%) | 0 | 3 (1.5%) | P = .28 |
| Suggested programming change at the next visit, n (%) | 10 (7.6%) | 7 (3.5%) | P = .13 |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Interventions requiring early in‐person visit during the pandemic were rare. In group 1, one early in‐person visit was scheduled for a patient who had sustained ventricular tachycardia successfully treated with antitachycardia therapy after remote assessment with a phone call and a medication change. Another in‐person visit was organized for a patient with low battery voltage and programming changes suggested to update ventricular tachycardia detection zones as recommended in primary prevention by current guidelines. In group 2, elective cardioversion was organized during the pandemic for one patient with persistent atrial flutter and in another patient with persistent atrial fibrillation. Atrioventricular node ablation was planned for one patient with rapid atrial fibrillation and intolerance to medication. Finally, one patient was scheduled for lead extraction (debulking), reimplantation, and generator change due to low battery voltage and elevated atrial and ventricular thresholds.
Nonurgent changes of device programming were recommended for the next in‐person visit in five patients in both groups to update ventricular tachycardia detection zones as recommended in primary prevention by current guidelines. In group 1, a suggestion was made in one patient to lower ventricular tachycardia detection zone to ensure good ventricular tachycardia detection. It was suggested to prolong post ventricular atrial blanking (PVAB) in one patient to reduce farfield on the atrial lead and in another patient, a suggestion to enable AV search mode to reduce ventricular pacing was made. Finally, it was suggested to disable ventricular autocapture in one patient due to device incapacity to perform ventricular threshold. In group 2, a suggestion was made in one patient to modify antitachycardia therapies in the ventricular tachycardia zone. It was also suggested in one patient to modify pacing mode since he developed permanent atrial fibrillation.
An inappropriate antitachycardia pacing was reported in a patient with recurrent atrial tachycardias in group 2. No intervention was made since subsequent atrial tachycardias were well discriminated by the device.
5. DISCUSSION
This is the first study to report real world data on remote monitoring during the COVID‐19 pandemic. In 131 patients whose in‐person annual visit was substituted for a remote monitoring session because of the COVID‐19 pandemic restrictions (group 1), 15 patients (11.5%) experienced a clinical event and 19 patients (14.5%) required an intervention, compared to 15 (7.6%) and 19 (9.6%) in patients already scheduled for a remote monitoring session (group 2). Ninety percent of these interventions were managed without in‐person visit in group 1, compared to 80% in group 2. Only two patients (1.5%) in group 1 and four patients (2.0%) in group 2 required an in‐person visit during the pandemic period. No statistically significant difference was observed between groups, even if patients in group 1 had not been seen in person for twice as long as patients in group 2 (Figure 2).
FIGURE 2.
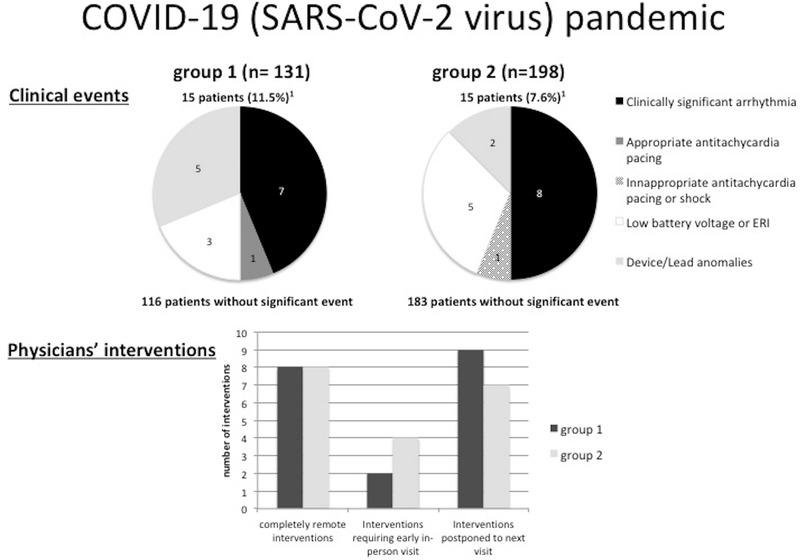
Central illustration. Clinical events and physicians’ interventions during remote monitoring of implantable cardioverter defibrillators in the COVID‐19 pandemic. Group 1 shows patients whose in‐person annual visit was substituted for a remote monitoring session; group 2 shows patients who underwent remote monitoring sessions as scheduled in their usual device follow‐up. 1One patient in each group had two clinical events
The study shows that remote monitoring is useful to detect clinical events in patients with ICDs during the COVID‐19 pandemic and reduces significantly patients’ potential exposure to the virus. Physicians can manage remotely the majority of patients, which is of high interest for patients with chronic cardiovascular disease at higher risk of infection and viral complications with SARS‐CoV‐2 virus. 3
Remote monitoring is recognised as a safe alternative to in‐person visit for the follow‐up of CIED combined with an annual in‐person visit and is recommended by current guidelines. 4 Randomised clinical trials and a meta‐analysis showed similar survival and safety outcomes between remote monitoring and conventional in‐person visit follow‐up for ICDs. 6 , 7 , 8 , 9 , 10 Interestingly, large real‐world registries demonstrated a clear mortality benefit of remote monitoring over conventional in‐person follow‐up, 5 , 11 enhancing the important role of remote monitoring for the follow‐up of CIEDs. Also, remote monitoring leads to more rapid clinical event detection and a reduction in inappropriate shocks. 10 Patient satisfaction and reduced healthcare utilization/costs are other arguments in favour of remote monitoring for ICDs. 6 , 12 , 13 , 14 , 15
While remote monitoring is a class 1 recommendation for routine follow‐up of CIEDs, an annual in‐person visit is still proposed due to the lack of evidence on safety with longer interval between visits. 4 Thus, recommendations during the pandemic in favour of remote monitoring for every patient irrespective of the delay since their last in‐person visit are only supported by expert consensus. 1 The low burden of clinical events was observed in our study, and the relevant physicians’ interventions reassured us on the management of our patients with exclusive remote monitoring during the pandemic. Also, the absence of significant difference between groups suggests that remote monitoring with longer in‐person visit interval is comparable to the recommended ICD follow‐up. Nevertheless, we should keep in mind that remote monitoring session does not replace clinical follow‐up by a cardiologist for management of heart failure and other conditions.
A recent randomised clinical trial investigated the safety of reducing in‐person visit to once every 2 years in patients with permanent pacemaker with remote follow‐up sessions every 6 months and daily automatic home monitoring. 16 Exclusive pacemaker remote monitoring for 2 years did not increase major cardiovascular events and significantly reduced resources utilisation. 16 While this study evaluated only permanent pacemaker without ICDs, it demonstrated the safety of longer interval between in‐person visits to pacemaker clinics for CIEDs.
Our study has potential limitations. First, it was a single‐center study with a modest sample size. The event rate was low, and events were rarely severe in both groups. The cross‐sectional design does not provide follow‐up data. No strict definitions of clinical events and physicians’ intervention were established before device interrogation. Therefore, physicians’ interpretation may have influenced the results. Our cohort includes only ICDs. It would have been interesting to evaluate all CIEDs, but remote monitoring at our institution is currently mostly limited to ICDs/CRT‐Ds. If there was no problem identified, no communication was made to the patient. It may have lead to insecurity in some patients. Remote programming changes were not possible, and suggested nonurgent programming changes were postponed to the next in‐person visit. Finally, our cohort consisted mainly of 'chronic' implants and did not address the issue of wound healing and pocket complications in the first months following implant.
6. CONCLUSION
Remote monitoring is useful to identify clinical events in patients with ICDs whose in‐person annual visit was canceled because of the COVID‐19 pandemic restrictions. This approach allows physicians to modify patients’ treatment appropriately regardless of the time interval since their last in‐person visit, except for programming changes. It also reduces significantly in‐person visit to the device clinics, which is particularly relevant for patients with cardiovascular disease at higher risk of viral complications with SARS‐CoV‐2 virus. Our study highlights the need for a large systematic longitudinal study to evaluate remote monitoring of CIED during the COVID‐19 pandemic and for a randomised clinical trial to evaluate the safety of longer interval or even absence of in‐person visits for the routine follow‐up of ICDs.
AUTHOR CONTRIBUTIONS
Study concept and design: Hugo De Larochellière and Isabelle Nault. Analysis and interpretation of data: Hugo De Larochellière and Isabelle Nault. Drafting of the manuscript: Hugo De Larochellière and Isabelle Nault. Critical revision of the manuscript for important intellectual content: Jean Champagne, Jean‐François Sarrazin, Karine Roy, Christian Steinberg, François Philippon, Louis Blier, Benoit Plourde, Gilles O'Hara, and Franck Molin. Statistical analysis: Hugo De Larochellière and Isabelle Nault. Data collection: Hugo De Larochellière and Isabelle Nault. Approval the final version of the article: Hugo De Larochellière, Jean Champagne, Jean‐François Sarrazin, Christian Steinberg, François Philippon, Karine Roy, Franck Molin, Gilles O'Hara, Benoit Plourde, Louis Blier, and Isabelle Nault.
CONFLICT OF INTEREST
Authors have not reported any potential conflict of interest with respect to the content of this paper to disclose
De Larochellière H, Champagne J, Sarrazin J‐F, et al. Findings of remote monitoring of implantable cardioverter defibrillators during the COVID‐19 pandemic. Pacing Clin Electrophysiol. 2020;43:1366–1372. 10.1111/pace.14086
REFERENCES
- 1. Lakkireddy DR, Chung MK, Gopinathannair R, et al. Guidance for cardiac electrophysiology during the coronavirus (COVID‐19) pandemic from the Heart Rhythm Society COVID‐19 task force; electrophysiology section of the American College of Cardiology; and the Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology, American Heart Association. Circulation. 2020;17(9):e233‐e241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Varma N, Marrouche NF, Aguinaga L, et al. HRS/EHRA/APHRS/LAHRS/ACC/AHA worldwide practice update for telehealth and arrhythmia monitoring during and after a pandemic. EP Europace. 2020. 1‐14. 10.1093/europace/euaa187. [DOI] [Google Scholar]
- 3. Driggin E, Madhavan MV, Bikdeli B, et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID‐19 pandemic. J Am Coll Cardiol. 2020;75(18):2352‐2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Slotwiner D, Varma N, Akar JG, et al. HRS expert consensus statement on remote interrogation and monitoring for cardiovascular implantable electronic devices. Heart Rhythm. 2015;12(7):e69‐e100. [DOI] [PubMed] [Google Scholar]
- 5. Varma N, Piccini JP, Snell J, Fischer A, Dalal N, Mittal S. The relationship between level of adherence to automatic wireless remote monitoring and survival in pacemaker and defibrillator patients. J Am Coll Cardiol. 2015;65(24):2601‐2610. [DOI] [PubMed] [Google Scholar]
- 6. Varma N, Epstein AE, Irimpen A, Schweikert R, Love C, Investigators T. Efficacy and safety of automatic remote monitoring for implantable cardioverter‐defibrillator follow‐up: the lumos‐t safely reduces routine office device follow‐up (TRUST) trial. Circulation. 2010;122(4):325‐332. [DOI] [PubMed] [Google Scholar]
- 7. Guedon‐Moreau L, Lacroix D, Sadoul N, et al. A randomized study of remote follow‐up of implantable cardioverter defibrillators: safety and efficacy report of the ECOST trial. Eur Heart J. 2013;34(8):605‐614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hindricks G, Taborsky M, Glikson M, et al. Implant‐based multiparameter telemonitoring of patients with heart failure (IN‐TIME): a randomised controlled trial. Lancet. 2014;384(9943):583‐590. [DOI] [PubMed] [Google Scholar]
- 9. Crossley GH, Boyle A, Vitense H, Chang Y, Mead RH, Investigators C. The CONNECT (clinical evaluation of remote notification to reduce time to clinical decision) trial: the value of wireless remote monitoring with automatic clinician alerts. J Am Coll Cardiol. 2011;57(10):1181‐1189. [DOI] [PubMed] [Google Scholar]
- 10. Parthiban N, Esterman A, Mahajan R, et al. Remote monitoring of implantable cardioverter‐defibrillators: a systematic review and meta‐analysis of clinical outcomes. J Am Coll Cardiol. 2015;65(24):2591‐2600. [DOI] [PubMed] [Google Scholar]
- 11. Saxon LA, Hayes DL, Gilliam FR, et al. Long‐term outcome after ICD and CRT implantation and influence of remote device follow‐up: the ALTITUDE survival study. Circulation. 2010;122(23):2359‐2367. [DOI] [PubMed] [Google Scholar]
- 12. Timmermans I, Meine M, Szendey I, et al. Remote monitoring of implantable cardioverter defibrillators: patient experiences and preferences for follow‐up. Pacing Clin Electrophysiol. 2019;42(2):120‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guedon‐Moreau L, Lacroix D, Sadoul N, et al. Costs of remote monitoring vs. ambulatory follow‐ups of implanted cardioverter defibrillators in the randomized ECOST study. Europace . Europace. 2014;16(8):1181‐1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ladapo JA, Turakhia MP, Ryan MP, Mollenkopf SA, Reynolds MR. Health care utilization and expenditures associated with remote monitoring in patients with implantable cardiac devices. Am J Cardiol. 2016;117(9):1455‐1462. [DOI] [PubMed] [Google Scholar]
- 15. Piccini JP, Mittal S, Snell J, Prillinger JB, Dalal N, Varma N. Impact of remote monitoring on clinical events and associated health care utilization: a nationwide assessment. Heart Rhythm. 2016;13(12):2279‐2286. [DOI] [PubMed] [Google Scholar]
- 16. Watanabe E, Yamazaki F, Goto T, et al. Remote management of pacemaker patients with biennial in‐clinic evaluation: continuous home monitoring in the Japanese at home study ‐ a randomized clinical trial. Circ Arrhythm Electrophysiol. 2020;13(5):e007734. [DOI] [PMC free article] [PubMed] [Google Scholar]


