Abstract
This study aimed to determine the effect of yoga stretching on salivary stress hormones and cardiac autonomic nervous system. To our knowledge, this study is the first to investigate changes in cardiac autonomic nervous system after yoga stretching. In this crossover design study, 10 adult men (age, 26.3 ± 2.5 years) without yoga experience participated in the rest and yoga trials for 90 min. Measurements were carried out before (pre), immediately (post), 60 min, and 120 min after rest or yoga stretching. Saliva samples were collected by chewing a sterile cotton ball at a frequency of 60 cycles per minute. Salivary cortisol and testosterone concentrations were measured using an enzyme-linked immunosorbent assay. With the subjects in the sitting position, heart rate variability was measured using pulse analyzer plus for 150 seconds. As regards rate changes, salivary testosterone level tended to increase (p = 0.088), testosterone/cortisol ratio significantly increased (p < 0.05), and cortisol level significantly decreased (p < 0.05) at 120 min after yoga stretching. The square root of the mean-squared differences of successive normal-to-normal intervals and natural logarithm high-frequency component, which are indicators of parasympathetic nerve activity, increased at 60 min (p < 0.05) and 120 min (p < 0.05) in the yoga trial, respectively. In conclusion, yoga stretching can enhance parasympathetic nerve activity and improve stress hormones. Therefore, yoga stretching may be useful to compensate for physical inactivity and increase life expectancy in the general population.
Key points.
We confirmed that salivary cortisol concentration decreased and testosterone/cortisol ratio increased after yoga stretching.
As major finding of this study, yoga stretching for 90 min is associated with a significant enhancement in the parasympathetic nerve activity.
Yoga stretching, which is a low-intensity exercise aimed at relaxation, may be useful for recovery after high-intensity training and athlete’s conditioning.
Key words: Relaxation, mental state, autonomic nervous system, recovery, testosterone, cortisol
Introduction
Stress is a state of threatened homeostasis casued by extrinsic or intrinsic stressors, which activate the hypothalamic–pituitary–adrenal axis and the autonomic nervous system (Ader et al., 1995; Chrousos and Gold, 1992). An extreme amount of stress and imbalance in the autonomic nervous system are known to adversely affect the immune, cardiovascular, and neuroendocrine systems and cause depressive psychosis and lifestyle-related diseases (Anderson, 1998; Rosengren et al., 2004; Won and Kim, 2016). These factors are also associated with overreaching and overtraining syndrome in athletes (Djaoui et al., 2017; Garet et al., 2004; Kellmann, 2010; Maso et al., 2004).
Cortisol and testosterone are steroid hormones that change in response to psychological and physical stress (Papacosta and Nassis, 2011). Additionally, the testosterone/cortisol (T/C) ratio has been used to more clearly emphasize the variations in these two hormones (Elloumi et al., 2003; Passelergue and Lac, 1999). The T/C ratio is considered valuable in the assessment of tiredness and prevention of overtraining syndrome (Maso et al., 2004). Abnormalities in the cardiac autonomic nervous system have been suggested as potential mechanisms linking depression and poor cardiovascular health in the general population (Grippo and Johnson, 2009). Additionally, the cardiac autonomic nervous system is also useful to assess the condition and performance of athletes (Djaoui et al., 2017; Garet et al., 2004; Kellmann, 2010). A previous study in swimmers has reported that a decrease in parasympathetic nerve activity during intensive training is associated with the loss in performance (Garet et al., 2004). Additionally, the same study showed a correlation between the rebound in parasympathetic nerve activity during a tapering period and the gain in performance (Garet et al., 2004). Therefore, the maintaining a better balance in the cardiac autonomic nervous system is important for public health and athlete’s conditioning.
Previous studies found beneficial effects of yoga on physical fitness, cognitive function, psychological disorders, cardiovascular conditions, and immune function (Field, 2011). Yoga is an integrated mind–body practice, and some of its styles are Hatha yoga (a relaxing, restorative form that is sometimes used as an overarching term for all forms of yoga), Ashtanga (strenuous series of poses sometimes referred to as “power yoga”), Iyengar (holding poses longer and some very strenuous positions), and others (Field, 2011). In recent years, yoga has been gradually investigated and utilized for athlete’s conditioning and rehabilitation (Bühlmayer et al., 2017; Evans et al., 2018). However, these yoga styles are often difficult for beginners because of pranayama (yoga breathing) and meditation.
On the contrary, yoga stretching, which involves holding relaxed poses with a natural breathing rhythm without pranayama or meditation, can be performed comfortably and easily by beginners. Previous studies reported that a single bout of yoga stretching could enhance salivary immune functions and improve stress hormones and mental states (Eda et al., 2013; 2018). It is possible that a relaxation effect of yoga stretching is associated with cardiac autonomic nervous system, especially parasympathetic nerve activity. However, to our knowledge, no study has investigated this speculation. Prior to investigating athletes, it is necessary to assess the beneficial effects of yoga stretching in non-athletes. Thus, this study aimed to determine the effect of yoga stretching on salivary stress hormones and cardiac autonomic nervous system in adult men who are not athletes. Therefore, our hypothesis is that yoga stretching will improve stress hormones and enhance parasympathetic nerve activity, and the beneficial effects will persist even after yoga stretching.
Methods
Subjects
Ten adult men (age, 26.3 ± 2.5 years; height, 1.74 ± 0.06 m; body mass, 68.3 ± 9.3 kg; body mass index, 22.7 ± 2.7 kg·m-2; body fat percentage, 19.4 ± 4.0%) without yoga experience participated in this crossover design study. The sample size was estimated using G*Power 3 (Faul et al., 2007). To detect an enhancement of parasympathetic nerve activity with a power of 80% and alpha level of 5%, a sample size of ≥6 participants was required. All subjects had passed a complete medical examination within the preceding year. No subjects had been treated with any drugs that were known to affect immune function, and none reported allergies or recent infections within the prior 3 months.
Experimental approach to the problem
Subjects rested for 90 min in a seated position in the control trial (CON) and performed yoga for 90 min in the yoga trial (YOG) in random order (Figure 1). Measurements were carried out before (pre), immediately (post), 60 min, and 120 min after rest or yoga. The CON and YOG trials were performed at the same time of day by all subjects. During the rest period, participants were only allowed to read books and were prohibited from sleeping or using a computer. Only mineral water was permitted for drinking during these trials.
Figure 1.
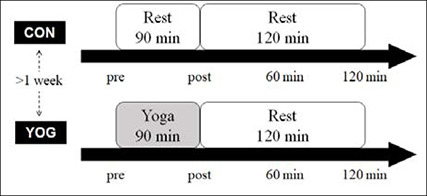
Experimental procedure.
This study was approved by the Ethics Committee on Human Research of Waseda University, and experiments were conducted in accordance with the Declaration of Helsinki. Subjects were given a detailed explanation of the risks, stress, and potential benefits of the study before they signed an institutionally approved informed consent document to participate in the study.
Procedures: Yoga exercise
Subjects engaged in yoga stretching for 90 min using the methods described previously (Eda et al., 2013; 2018). A male instructor taught yoga stretching for all trials. Briefly, they massaged their legs and applied pressure to acupressure points for 20 min, followed by executing various yoga poses, such as the warrior, extended triangle, and downward-facing dog poses, for 65 min. Finally, the corpse pose, which involves lying in a supine position, was held for 5 min.
Salivary sample measurements
Saliva samples were collected as described previously (Akimoto et al. 2003). Subjects sat and rinsed their mouths with distilled water for 30 s three times and then rested for 5 min. Saliva production was stimulated by chewing a sterile cotton ball (Salivette; Sersted, Vümbrecht, Germany) at a frequency of 60 cycles per minute. Obtained saliva samples were separated from the cotton by centrifugation at 3500 rpm for 10 min. The saliva volume secreted by chewing for 1 min was measured and expressed as saliva flow rate (mL/min). After measurement of the sample volume, saliva samples were frozen at –80°C and stored until the end of the study period. Cortisol and testosterone concentrations were measured using enzyme immunoassay kits (Salimetrics, Carlsbad, CA) according to the manufacturer’s protocol. The intra- (coefficient of variation: 3-7% [cortisol] and 2.5-6.7% [testosterone]) and inter-assay (coefficient of variation: 3-11% [cortisol] and 5.6-14.1% [testosterone]) precision and control samples (cortisol: high [1.011 ± 0.253 μg/dL] and low [0.104 ± 0.026 μg/dL]; testosterone: high [215.62 ± 53.91 pg/mL] and low [20.49 ± 8.20 pg/mL]) were provided by Salimetrics. The ELISA results of the present study showed a high (0.914 μg/dL) and low (0.088 μg/dL) control for cortisol, and high (190.27 pg/mL) and low (14.59 pg/mL) control for testosterone. The T/C ratio was obtained by dividing the testosterone concentration by the cortisol concentration.
Heart Rate Variability (HRV)
HRV was measured using pulse analyzer plus (TAS9; YKC, Tokyo, Japan) for 150 s in the sitting position. Previous studies have suggested that HRV analyses over shorter periods are useful for monitoring dynamic changes in autonomic nerve activity (Smith et al., 2013), and that a recording time of at least 120 s may be appropriate (Ahn, 2013). The breathing rate was controlled to 15 times per minute (inspiration in 2 s and expiration in 2 s) during the measurement. The mean heart rate (HR), the standard deviation of normal-to-normal interval (SDNN), and the square root of the mean-squared differences of successive normal-to-normal intervals (RMSSD) were measured by time-domain analysis. Total power (TP, 0–0.40 Hz), high-frequency component (HF, 0.15–0.40 Hz), low-frequency component (LF, 0.04–0.15 Hz), and the ratio of LF to HF (LF/HF) were quantified as frequency domain parameters of HRV. Natural logarithm (Ln) values were calculated for TP, LF, and HF components.
Mental state
Mental state was assessed using the Profile of Mood States (POMS)-Brief Form (Kanekoshobo, Tokyo, Japan) (Yokoyama et al. 1990). The POMS questionnaire rated tension-anxiety (T-A), depression (D), anger-hostility (A-H), vigor (V), fatigue (F), and confusion (C) on a 5-point scale, and the standardized points (t points) were computed. Total mood disturbance (TMD) was calculated by subtracting the mood subscale V from the five mood subscales (T-A, D, A-H, F, and C).
Statistical analyses
Data are presented as mean ± SD values. Statistics were computed using SPSS computer software (Version 25.0; SPSS Japan, Inc., Japan). Analysis of variance for 2 (day; CON and YOG) × 4 (time; pre, post, 60 min, and 120 min) repeated measures was used to determine the effect of yoga stretching. As post hoc analysis, Dunnett test was used to compare specific differences when significance was found. An intraclass correlation (ICC Rs) was calculated for each measurement to assess the reliability of each test. Reliability ICC Rs for the dependent variables was 0.77-0.98. The effect size is estimated as f values. For all analyses, p < 0.05 was set for statistical significance.
Results
The saliva flow rate, testosterone levels, cortisol levels, and T/C ratio are shown in Table 1. Change (%) rates in saliva flow rate, testosterone levels, and cortisol levels showed no significant change in the CON trial (Figure 2). As regards change rates, salivary testosterone level tended to increase (p = 0.088, 95% confidence intervals (CI): -1.2 to 21.2, f = 0.48) and cortisol level significantly decreased (p < 0.05, 95% CI: -86.5 to -8.4, f = 0.61) at 120 min compared with those at pre. In the CON trial, the T/C ratio at 120 min was significantly higher than that at pre (p < 0.01, 95% CI: 37.8 to 207.6, f = 0.72). On the contrary, in the YOG trial, the T/C ratio increased at post (p = 0.071, 95% CI: -8.0 to 235.1, f = 0.69), 60 min (p < 0.01, 95% CI: 37.5 to 280.6), and 120 min (p < 0.05, 95% CI: 18.2 to 261.3). At post, the T/C ratio in the YOG trial tended to be higher than that in the CON trial (p = 0.097, 95% CI: -16.9 to 168.8, f = 0.59).
Table 1.
Changes in saliva flow rate and salivary hormones in the CONTROL and YOGA trials. Data indicated mean [±SD] values.
| CONTROL | YOGA | |||||||
|---|---|---|---|---|---|---|---|---|
| pre | post | 60min | 120min | pre | post | 60min | 120min | |
| Saliva flow rate (mL/min) | 1.56 [0.34] | 1.49 [0.47] | 1.48 [0.36] | 1.39 [0.41] | 1.51 [0.45] | 1.62 [0.42] | 1.53 [0.32] | 1.57 [0.33] |
| Testosterone (pg/mL) | 203.4 [44.7] | 191.9 [41.0] | 206.2 [39.4] | 207.9 [39.2] | 179.7 [44.5] | 178.1 [35.6] | 182.1 [45.3] | 193.7 [44.1] |
| Cortisol (ng/mL) | 1.30 [0.76] | 1.30 [1.33] | 0.90 [0.53] | 0.70 [0.34] | 1.71 [0.76] | 1.06 [0.55] | 1.14 [1.04] | 0.82 [0.35] |
| T/C ratio | 228.1 [177.8] | 296.3 [187.1] | 314.1 [202.4] | 389.1 [304.7] | 124.3 [56.5] | 209.0 [115.6] | 258.5 [184.1] | 261.2 [76.8] |
Figure 2.
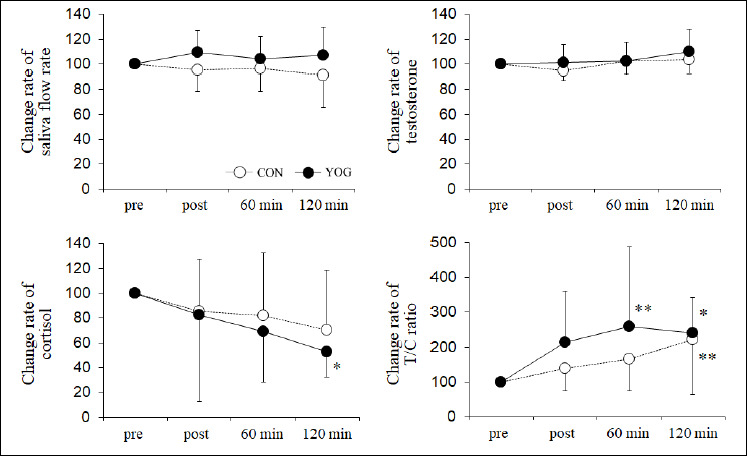
Change (%) rates in saliva flow rate, testosterone levels, cortisol level, and T/C ratio before (pre), immediately (post), 60 min, and 120 min after rest or yoga. Data are expressed as mean ± SD values. * p < 0.05, ** p < 0.01 vs pre. CON, control trial; YOG, yoga trial.
HRV changes are shown in Figure 3. HR decreased at post (p < 0.05), 60 min (p = 0.051), and 120 min (p < 0.05) in the CON trial and at 60 min (p < 0.05, 95% CI: -6.8 to -0.2, f = 0.74) and 120 min (p < 0.01, 95% CI: -7.6 to -1.0) in the YOG trial. Significant interactions were found in the SDNN (p < 0.05, f = 0.68), RMSSD (p < 0.01, f = 0.79), Ln TP (p < 0.05, f = 0.67), and Ln HF (p < 0.01, f = 0.74) between CON and YOG trials. The SDNN at 120 min in the YOG trial was significantly higher than that at pre in the YOG trial (p < 0.05, 95% CI: 2.0 to 30.5, f = 0.66) and that at 120 min in the CON trial (p < 0.05, 95% CI: 3.8 to 23.0, f = 0.33). The RMSSD increased at post in the CON trial (p < 0.05, 95% CI: 1.1 to 21.3, f = 0.55) and at 60 min (p < 0.05, 95% CI: 1.6 to 34.0, f = 0.76) and 120 min (p < 0.05, 95% CI: 2.8 to 35.2) in the YOG trial. The RMSSD values in the CON trial were significantly higher at post (p < 0.05, 95% CI: 0.8 to 14.0, f = 0.60) and lower at 120 min (p < 0.05, 95% CI: -32.3 to -2.5) than those in the YOG trial. Ln TP increased at post (p < 0.05, 95% CI: 0.02 to 0.52, f = 0.63), 60 min (p < 0.05, 95% CI: 0.01 to 0.51), and 120 min (p < 0.05, 95% CI: 0.03 to 0.53) in the CON trial and at 60 min (p < 0.05, 95% CI: 0.07 to 0.75, f = 0.91) and 120 min (p < 0.01, 95% CI: 0.12 to 0.79) in the YOG trial. Ln LF significantly increased at 120 min (p < 0.05, 95% CI: 0.03 to 1.45, f = 0.52) in the CON trial and at 60 min (p < 0.05, 95% CI: 0.07 to 1.55, f = 0.79) in the YOG trial. Ln HF significantly increased at 60 min (p < 0.05, 95% CI: 0.15 to 1.20, f = 0.91) and 120 min (p < 0.05, 95% CI: 0.11 to 1.16) in the YOG trial alone. Additionally, Ln HF values in the YOG trial were significantly higher at 60 min (p < 0.05, 95% CI: 0.1 to 1.1, f = 0.75) and 120 min (p < 0.01, 95% CI: 0.2 to 1.0) than those in the CON trial. Ln LF/HF showed no significant change in both trials.
Figure 3.
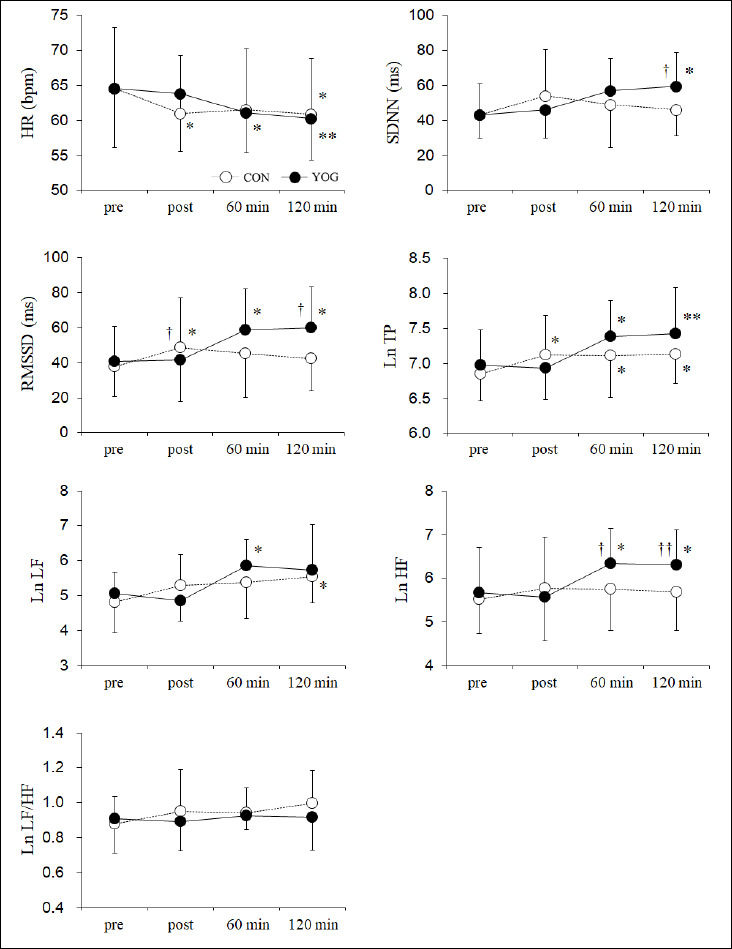
Changes in the mean heart rate (HR), standard deviation of normal-to-normal interval (SDNN), square root of the mean squared differences of successive normal-to-normal intervals (RMSSD), natural logarithm total power (Ln TP), Ln low-frequency component (LF), Ln high-frequency component (HF), and Ln LF/HF before (pre), immediately (post), 60 min, and 120 min after rest or yoga. Data are expressed as mean ± SD values. * p < 0.05, ** p < 0.01 vs pre, † p < 0.05, †† p < 0.01; CON vs YOG. CON, control trial; YOG, yoga trial.
No significant difference was found in the mental state of the participants during the CON trial (Table 2). In the YOG trial, POMS scores of T-A and TMD significantly decreased at post (T-A; p < 0.01, 95% CI: -7.1 to -2.0, f = 1.10, TMD; p < 0.05, 95% CI: -7.23 to -0.37, f = 0.66), 60 min (T-A; p < 0.01, 95% CI: -7.6 to -2.5, TMD; p < 0.05, 95% CI: -7.53 to -0.67), and 120 min (T-A; p < 0.01, 95% CI: -7.4 to -2.3, TMD; p < 0.05, 95% CI: -7.03 to -0.17). Additionally, POMS scores of D (p < 0.05, 95% CI: -5.34 to -0.26, f = 0.56) and A-H (p < 0.05, 95% CI: -5.28 to -0.12, f = 0.53) at 120 min were significantly lower than those at pre. At post, the scores of V (p < 0.01, 95% CI: 2.2 to 10.6, f = 0.67) and F (p < 0.05, 95% CI: 1.3 to -0.26, f = 8.9) in the YOG trial were significantly higher than those in the CON trial. Interactions between rest and yoga stretching were seen in T-A (p = 0.097, f = 0.57) and F (p = 0.067, f = 0.54).
Table 2.
Changes of POMS scores in CON and YOG trials. Data indicated mean [±SD] values.
| CONTROL | YOGA | |||||||
|---|---|---|---|---|---|---|---|---|
| pre | post | 60min | 120min | pre | post | 60min | 120min | |
| Tension-Anxiety | 36.8 [6.2] | 35.0 [3.5] | 35.9 [5.0] | 34.4 [3.0] | 39.9 [7.2] | 35.4 [4.6] ** | 34.9 [4.2] ** | 35.1 [4.1] ** |
| Depression | 41.3 [1.6] | 41.5 [2.8] | 41.5 [2.4] | 42.0 [2.9] | 44.8 [4.9] | 43.3 [5.9] | 42.6 [5.2] | 42.0 [4.2] * |
| Anger-Hostility | 38.1[2.6] | 37.6 [1.3] | 37.6 [1.3] | 37.6 [1.3] | 40.5 [4.2] | 38.7 [3.5] | 38.4 [3.5] | 37.8 [2.5] * |
| Vigor | 37.7 [13.4] | 37.9 [10.7] | 37.7 [11.2] | 37.3 10.2] | 42.1 [12.1] | 44.3 [8.9] †† | 42.2 [10.7] | 38.7 [11.7] |
| Fatigue | 39.7 [5.5] | 38.4 [3.8] | 39.1 [5.5] | 39.0 [4.4] | 41.5 [6.2] | 43.5 [6.4] † | 42.3 [6.6] | 40.9 [5.9] |
| Confusion | 46.9 [5.2] | 45.6 [4.0] | 44.8 [6.0] | 44.1 [2.9] | 47.6 [7.1] | 45.4 [4.1] | 45.3 [3.6] | 45.4 [4.6] |
| Total Mood Disturbance | 4.6 [10.3] | 2.6 [6.9] | 3.1 [8.8] | 2.6 [6.7] | 7.3 [7.8] | 3.5 [8.1] * | 3.2 [6.3] * | 3.7 [6.1] * |
* p < 0.05,
** p < 0.01 vs pre in the YOGA trial.
† p < 0.05,
†† p < 0.01 vs CONTROL trial.
Discussion
In this study, we examined the effect of yoga stretching on salivary stress hormones and the autonomic nervous system. To our knowledge, this is the first to investigate changes in cardiac autonomic nervous system after yoga stretching. We confirmed that salivary cortisol concentration decreased and T/C ratio increased after yoga stretching. As the major finding of this study, yoga stretching for 90 min significantly enhanced parasympathetic nerve activity, and the beneficial effect was observed much later, at approximately 60 min after yoga stretching.
Testosterone, the primary steroid hormone within the androgen family, is regulated by the hypothalamic–pituitary–gonadal axis (Papacosta and Nassis, 2011). Testosterone is produced by the testis in men and the adrenal cortex and ovaries in women, and the level of testosterone in men is higher than that in women (Le Panse et al., 2010). Additionally, changes in testosterone owing to stress are common in both men and women (Lennartsson et al, 2012). In addition, 2–5% of all testosterone in the blood remains free or unbound, and the remaining 95–98% is bound to albumin and sex-binding globulins (Papacosta and Nassis, 2011). Salivary testosterone is free or unbound testosterone and shows biological activity (Papacosta and Nassis, 2011). In men, salivary and blood testosterone concentrations have showed significant correlations (Sannikka et al., 1983; Vittek et al., 1985). Salivary testosterone is known to increase in response to acute high-intensity exercise (Budde et al., 2010a; 2010b), but it remains unchanged after acute low- or moderate-intensity exercise (Budde et al., 2010b). However, in the present study, the testosterone level tended to increase at 120 min after yoga, and this increase in testosterone level was confirmed in a previous study (Eda et al., 2018). Testosterone contributes to an increase in protein synthesis and a decrease in protein degradation and thus enhances muscle growth and strength-related performance (Crewther et al., 2006; Papacosta and Nassis, 2011). On the other hand, testosterone levels are known to decrease with age, and previous studies have reported that lower testosterone levels are associated with a higher risk of metabolic syndrome, cardiovascular disease, type 2 diabetes, and mortality (Haring et al., 2010; Jones, 2010). The increase in the testosterone level in the present study indicates that yoga stretching may promote muscle growth and strength-related performance in athletes and decrease the risks of lifestyle-related diseases and mortality in the general population.
Cortisol is a member of the glucocorticoid family and is secreted from the adrenal cortex via the hypothalamic–pituitary–adrenal axis (Papacosta and Nassis, 2011). The level of cortisol is known to increase in response to mental and physical stress and decrease in response to relaxation. Cortisol concentrations in the blood and saliva have showed significant correlations (Cadore et al., 2008; O’Connor and Corrigan, 1987). In a previous study, African dance for 90 min increased and yoga for 90 min decreased salivary cortisol concentration significantly (West et al., 2004). A previous study also showed that salivary cortisol concentration decreased after yoga stretching (Eda et al., 2018). In the present study, the cortisol level decreased at 120 min compared with that at pre in the YOG trial. This finding is consistent with those in previous studies, and the significant decrease in cortisol after yoga in the present study may demonstrate the relaxing effect of yoga stretching.
The T/C ratio is known to reflect anabolic and catabolic balance and considered useful for an athlete’s conditioning (Elloumi et al., 2008; Passelergue and Lac, 1999). A previous study of powerlifting athletes reported that the T/C ratio decreased at weighing-in and immediately after the competition, which was considered a catabolic phase (Le Panse et al., 2010). A previous study of rugby players reported that the T/C ratio might be useful for assessing the level of tiredness and preventing the development of overtraining syndrome (Maso et al., 2004). Another study reported that a decrease in the T/C ratio could be associated with a reduction in soccer’s performance during the season (Kraemer et al., 2004). A previous study has shown a significant increase in the T/C ratio immediately after yoga stretching (Eda et al., 2018). In the present study, the T/C ratio increased at post, 60 min, and 120 min in the YOG trial. Additionally, at post, the T/C ratio in the YOG trial tended to be higher than that in the CON trial. Therefore, the present study showed the potential for promoting anabolic action after yoga stretching in the general population. These findings indicate that yoga stretching may increase training effects and enhance an athlete’s condition and performance; however, further study in athletes is needed.
In this study, cardiac vagal activity was assessed by measuring HRV. Ln TP and SDNN, which reflected overall activity of the cardiac autonomic nervous system, were higher after yoga stretching than those after rest. The RMSSD and Ln HF increased at 60 min and 120 min after yoga stretching; thus, we suggested that yoga stretching might enhance parasympathetic nerve activity. Parasympathetic nerve activity is known to decrease after an acute exercise such as resistance training (Kingsley and Figueroa, 2016), endurance exercise (Heffernan et al., 2006), and high-intensity interval training (Perkins et al., 2017). On the contrary, acute static stretching exercise was reported to promote parasympathetic nerve activity (Wong and Figueroa, 2019). A previous study suggested three potential mechanisms of the effect of stretching exercise on HRV, namely, an improved baroreflex sensitivity, psychic-physical relaxation response, and a rise in nitric oxide levels (Wong and Figueroa, 2019). Especially, in acute stretching exercise, the psychic–physical relaxation response was speculated to contribute mainly to an enhancement of parasympathetic nerve activity. Previous studies have reported a decrease in parasympathetic nerve activity and activation of sympathetic nerve activity during acute stretching exercises (Farinatti et al., 2011; Wong and Figueroa, 2019); thus, it was suggested that the present study showed a delay in the enhancement of parasympathetic nerve activity due to fact that the effects of exercise on autonomic nerve activity remained even after yoga stretching.
Recent studies have reported both chronic (Hartfiel et al. 2011; Uebelacker et al. 2010) and acute (Eda et al., 2013; 2018; Telles et al. 2009) relaxation effects of yoga. As an example of chronic effects, 60 min of yoga per week for 6 weeks was reported to significantly improve the mental scores in the POMS Bipolar scale and the Inventory of Positive Psychological Attitudes scale (Hartfiel et al. 2011). Previous studies reported acute relaxation effects such as improvement in the POMS scores (T-A, D, A-H, and F) (Eda et al., 2013; 2018) and decrease in tension (Telles et al. 2009) after yoga. In the present study, the POMS scores on the T-A, D, A-H, and TMD were significantly improved after yoga and a relaxation effect of yoga was confirmed. These results are mostly consistent with the results of previous studies (Eda et al., 2013; 2018).
Improvements in stress hormones, autonomic nerve activity, and mood states are effective in preventing depressive psychosis and lifestyle-related diseases. In this study, these parameters improved after yoga; therefore, we suggested that yoga may be useful to compensate for physical inactivity and increase life expectancy in the general population.
This study has some limitations. This study has no active control trial such as general stretching, and athletes or yoga practitioners were not included. However, this study indicates the possibility of yoga’s usefulness for athlete’s conditioning; thus, the next study needs to investigate the effects of yoga on athlete’s recovery after acute high-intensity exercise or during a training camp. Additionally, yogic breathing method was not used and subjects performed a natural breathing style. However, a previous study reported that yogic breathing might be associated with psychic–physical response (Saoji et al., 2019); therefore, it is necessary to investigate the effects of yoga with yogic breathing on athlete’s conditioning and performance.
Conclusion
Yoga stretching for 90 min can enhance parasympathetic nerve activity and improve stress hormones and mental states in healthy adult men without yoga experience. These parameters were reported to be effective in preventing depressive psychosis and lifestyle-related diseases. Therefore, yoga stretching, which can improve these parameters, may be useful to compensate for physical inactivity and increase life expectancy in the general population.
Acknowledgements
We thank all of the subjects for participating in this study. The authors have no conflicts of interests to declare. The experiments comply with the current laws of the country in which they were performed.
Biographies
Nobuhiko EDA
Employment
Japan Institute of Sports Sciences, Tokyo, Japan / Waseda Institute for Sport Sciences, Waseda University, Saitama, Japan
Degree
PhD
Research interests
Exercise Physiology, Exercise Immunology, Conditioning
E-mail: nobuhiko.eda@jpnsport.go.jp
Hironaga ITO
Employment
Japan Sports Science Inc, Shizuoka, Japan / Waseda Institute for Sport Sciences, Waseda University, Saitama, Japan
Degree
PhD
Research interests
Sports Medicine, Exercise Immunology, Conditioning
E-mail: gizatan@gmail.com
Takao AKAMA
Employment
Faculty of Sport Sciences, Waseda University, Saitama, Japan
Degree
MD, PhD
Research interests
Sports Medicine, Exercise Immunology, Conditioning
E-mail: takao-akama@waseda.jp
References
- Ader R., Cohen N., Felten D. (1995) Psychoneuroimmunology: interactions between the nervous system and the immune system. Lancet 345, 99-103. [DOI] [PubMed] [Google Scholar]
- Ahn J.M. (2013) Heart Rate Variability (HRV) of two short-term photoplethysmogram (PPG). Advances in information Sciences and Service Sciences 5, 149-155. [Google Scholar]
- Akimoto T., Kumai Y., Akama T., Hayashi E., Murakami H., Soma R., Kuno S., Kono I. (2003) Effects of 12 months of exercise training on salivary secretory IgA levels in elderly subjects. British Journal of Sports Medicine 37, 76-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson N.B. (1998) Levels of analysis in health science. A framework for integrating sociobehavioral and biomedical research. Annals of the New York Academy of Sciences 840, 563-576. [DOI] [PubMed] [Google Scholar]
- Budde H., Pietrassyk-Kendziorra S., Bohm S., Voelcker-Rehage C. (2010a) Hormonal responses to physical and cognitive stress in a school setting. Neuroscience Letters 474, 131-134. [DOI] [PubMed] [Google Scholar]
- Budde H., Voelcker-Rehage C., Pietrassyk-Kendziorra S, Machado S., Ribeiro P., Arafat A.M. (2010b) Steroid hormones in the saliva of adolescents after different exercise intensities and their influence on working memory in a school setting. Psychoneuroendocrinology 35, 382-391. [DOI] [PubMed] [Google Scholar]
- Bühlmayer L., Birrer D., Röthlin P., Faude O., Donath L. (2017) Effects of Mindfulness Practice on Performance-Relevant Parameters and Performance Outcomes in Sports: A Meta-Analytical Review. Sports Medicine 47, 2309-2321. [DOI] [PubMed] [Google Scholar]
- Cadore E., Lhullier F., Brentano M., Silva E., Ambrosini M., Spinelli R., Silva R., Kruel L. (2008) Correlations between serum and salivary hormonal concentrations in response to resistance exercise. Journal of Sports Sciences 26, 1067-1072. [DOI] [PubMed] [Google Scholar]
- Chrousos G.P., Gold P.W. (1992) The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA 267, 1244-1252. [PubMed] [Google Scholar]
- Crewther B., Keogh J., Cronin J., Cook C. (2006) Possible stimuli for strength and power adaptation: acute hormonal responses. Sports Medicine 36, 215-238. [DOI] [PubMed] [Google Scholar]
- Djaoui L., Haddad M., Chamari K., Dellal A. (2017) Monitoring training load and fatigue in soccer players with physiological markers. Physiology & Behavior 181, 86-94. [DOI] [PubMed] [Google Scholar]
- Eda N., Ito H., Shimizu K., Suzuki S., Lee E., Akama T. (2018) Yoga stretching for improving salivary immune function and mental stress in middle-aged and older adults. Journal of Women & Aging 30, 227-241. [DOI] [PubMed] [Google Scholar]
- Eda N., Shimizu K., Suzuki S., Tanabe Y., Lee E., Akama T. (2013) Effects of yoga exercise on salivary beta-defensin 2. European Journal of Applied Physiology 113, 2621-2627. [DOI] [PubMed] [Google Scholar]
- Elloumi M., Ben Ounis O., Tabka Z., Van Praagh E., Michaux O., Lac G. (2008) Psychoendocrine and physical performance responses in male Tunisian rugby players during an international competitive season. Aggressive Behavior 34, 623-632. [DOI] [PubMed] [Google Scholar]
- Elloumi M., Maso F., Michaux O., Robert A., Lac G. (2003) Behaviour of saliva cortisol [C], testosterone [T] and the T/C ratio during a rugby match and during the post-competition recovery days. European Journal of Applied Physiology 90, 23-28. [DOI] [PubMed] [Google Scholar]
- Evans M.W., Ndetan H., Jr, Ka Sekhon V., Williams R., Oliver B., Jr, Perko M., Woolsey C., Singh K.P. (2018) Adult Use of Complementary and Integrative Approaches to Improve Athletic Performance. Alternative Therapies in Health and Medicine 24, 30-37. [PubMed] [Google Scholar]
- Farinatti P.T., Brandão C., Soares P.P., Duarte A.F. (2011) Acute effects of stretching exercise on the heart rate variability in subjects with low flexibility levels. Journal of Strength and Conditioning Research 25, 1579-1585. [DOI] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Lang A.G., Buchner A. (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods 39, 175-191. [DOI] [PubMed] [Google Scholar]
- Field T. (2011) Yoga clinical research review. Complementary Therapies in Clinical Practice 17, 1-8. [DOI] [PubMed] [Google Scholar]
- Garet M., Tournaire N., Roche F., Laurent R., Lacour J.R., Barthélémy J.C., Pichot V. (2004) Individual Interdependence between nocturnal ANS activity and performance in swimmers. Medicine and Science in Sports and Exercise 36, 2112-2118. [DOI] [PubMed] [Google Scholar]
- Grippo A.J., Johnson A.K. (2009) Stress, depression and cardiovascular dysregulation: a review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress (Amsterdam, Netherlands) 12, 1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haring R., Völzke H., Steveling A., Krebs A., Felix S.B., Schöfl C., Dörr M., Nauck M., Wallaschofski H. (2010) Low serum testosterone levels are associated with increased risk of mortality in a population-based cohort of men aged 20-79. European Heart Journal 31, 1494-1501. [DOI] [PubMed] [Google Scholar]
- Hartfiel N., Havenhand J., Khalsa S.B., Clarke G., Krayer A. (2011) The effectiveness of yoga for the improvement of well-being and resilience to stress in the workplace. Scandinavian Journal of Work, Environment & Health 37, 70-76. [DOI] [PubMed] [Google Scholar]
- Heffernan K.S., Kelly E.E., Collier S.R., Fernhall B. (2006) Cardiac autonomic modulation during recovery from acute endurance versus resistance exercise. The European Journal of Cardiovascular Prevention & Rehabilitation 13, 80-86. [DOI] [PubMed] [Google Scholar]
- Jones T.H. (2010) Testosterone deficiency: a risk factor for cardiovascular disease? Trends in Endocrinology and Metabolism 21, 496-503. [DOI] [PubMed] [Google Scholar]
- Kellmann M. (2010) Preventing overtraining in athletes in high-intensity sports and stress/recovery monitoring. Scandinavian Journal of Medicine & Science in Sports 20, 95-102. [DOI] [PubMed] [Google Scholar]
- Kingsley J.D., Figueroa A. (2016) Acute and training effects of resistance exercise on heart rate variability. Clinical Physiology and Functional Imaging 36, 179-187. [DOI] [PubMed] [Google Scholar]
- Kraemer W. J., French D. N., Paxton N. J., Häkkinen K., Volek J. S., Sebastianelli W. J., Putukian M., Newton R. U., Rubin M. R., Gómez A. L., Vescovi J. D., Ratamess N. A., Fleck S. J., Lynch J. M., Knuttgen H. G. (2004) Changes in exercise performance and hormonal concentrations over a big ten soccer season in starters and nonstarters. Journal of Strength and Conditioning Research 18, 121-128. [DOI] [PubMed] [Google Scholar]
- Lennartsson A.K., Kushnir M.M., Bergquist J., Billig H., Jonsdottir I.H. (2012) Sex steroid levels temporarily increase in response to acute psychosocial stress in healthy men and women. International Journal of Psychophysiology 84, 246-253. [DOI] [PubMed] [Google Scholar]
- Le Panse B., Vibarel-Rebot N., Parage G., Albrings D., Amiot V., De Ceaurriz J., Collomp K. (2010) Cortisol, DHEA, and testosterone concentrations in saliva in response to an international powerlifting competition. Stress 13, 528-532. [DOI] [PubMed] [Google Scholar]
- Maso F., Lac G., Filaire E., Michaux O., Robert A. (2004) Salivary testosterone and cortisol in rugby players: Correlation with psychological overtraining items. British Journal of Sports Medicine 38, 260-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor P.J., Corrigan D.L. (1987) Influence of short-term cycling on salivary cortisol levels. Medicine and Science in Sports and Exercise 19, 224-228. [PubMed] [Google Scholar]
- Papacosta E., Nassis G.P. (2011) Saliva as a tool for monitoring steroid, peptide and immune markers in sport and exercise science. Journal of Science and Medicine in Sport 14, 424-434. [DOI] [PubMed] [Google Scholar]
- Passelergue P., Lac G. (1999) Saliva cortisol, testosterone and T/C ratio variations during a wrestling competition and during the post-competitive recovery period. International Journal of Sports Medicine 20, 109-113. [DOI] [PubMed] [Google Scholar]
- Perkins S.E., Jelinek H.F., Al-Aubaidy H.A., de Jong B. (2017) Immediate and long term effects of endurance and high intensity interval exercise on linear and nonlinear heart rate variability. Journal of Science and Medicine in Sport 20, 312-316. [DOI] [PubMed] [Google Scholar]
- Rosengren A., Hawken S., Ounpuu S., Sliwa K., Zubaid M., Almahmeed W.A., Blackett K.N., Sitthi-amorn C., Sato H., Yusuf S., INTERHEART investigators (2004) Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet 364, 953-962. [DOI] [PubMed] [Google Scholar]
- Sannikka E., Terho P., Suominen J., Santti R. (1983) Testosterone concentrations in human seminal plasma and saliva and its correlation with non-protein-bound and total testosterone levels in serum. International Journal of Andrology 6, 319-330. [DOI] [PubMed] [Google Scholar]
- Saoji A.A., Raghavendra B.R., Manjunath N.K. (2019) Effects of yogic breath regulation: A narrative review of scientific evidence. Journal of Ayurveda and Integrative Medicine 10, 50-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A.L., Owen H., Reynolds K.J. (2013) Heart rate variability indices for very short-term (30 beat) analysis. Part 2: validation. Journal of Clinical Monitoring and Computing 27, 577-585. [DOI] [PubMed] [Google Scholar]
- Telles S., Gaur V., Balkrishna A. (2009) Effect of a yoga practice session and a yoga theory session on state anxiety. Perceptual and Motor Skills 109, 924-930. [DOI] [PubMed] [Google Scholar]
- Uebelacker L.A., Tremont G., Epstein-Lubow G., Gaudiano B.A., Gillette T., Kalibatseva Z., Miller I.W. (2010) Open trial of Vinyasa yoga for persistently depressed individuals: evidence of feasibility and acceptability. Behavior Modification 34, 247-264. [DOI] [PubMed] [Google Scholar]
- Vittek J., L’Hommedieu D.G., Gordon G.G., Rappaport S.C., Southren A.L. (1985) Direct radioimmunoassay (RIA) of salivary testosterone: correlation with free and total serum testosterone. Life Sciences 37, 711-716. [DOI] [PubMed] [Google Scholar]
- West J., Otte C., Geher K., Johnson J., Mohr D.C. (2004) Effects of hatha yoga and African dance on perceived stress, affect, and salivary cortisol. Annals of Behavioral Medicine 28, 114-118. [DOI] [PubMed] [Google Scholar]
- Won E., Kim Y.K. (2016) Stress, the Autonomic Nervous System, and the Immune-kynurenine Pathway in the Etiology of Depression. Current Neuropharmacology 14, 665-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A., Figueroa A. (2019) Effects of Acute Stretching Exercise and Training on Heart Rate Variability: A Review. Journal of Strength and Conditioning Research, Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Yokoyama K., Araki S., Kawakami N., Tkakeshita T. (1990) Production of the Japanese edition of profile of mood states (POMS): assessment of reliability and validity. Nihon Koshu Eisei Zasshi 37, 913-918. (In Japanese: English abstract). [PubMed] [Google Scholar]


