Abstract
To explore the clinical characteristics and prognosis of COVID‐19 patients with cerebral stroke. A total of 2,474 COVID‐19 patients from February 10th to March 24th, 2020 were admitted and treated in two branches (Optic Valley and Sino‐French New City branch) of the Tongji Hospital. Data on the clinical characteristics, laboratory parameters and prognosis of COVID‐19 patients with or without cerebral stroke were collected and comparatively analysed. Of the 2,474 COVID‐19 patients, 113 (4.7%) patients had cerebral stroke and 25 (1.0%) patients had new‐onset stroke. Eighty‐eight (77.9%) patients in the previous‐stroke group had cerebral ischaemia, while 25 (22.1%) patients in the new‐onset stroke group had cerebral ischaemia. Most COVID‐19 patients with stroke were elderly with more comorbidities such as hypertension, diabetes and heart diseases than patients without stroke. Laboratory examinations showed hypercoagulation and elevated serum parameters such as IL‐6, cTnI, NT pro‐BNP and BUN. Consciousness disorders, a long disease course and poor prognosis were also more commonly observed in stroke patients. The mortality rate of stroke patients was almost double (12.4% vs. 6.9%) that of patients without stroke. In addition, age, male sex and hypertension were independent predictors for new cerebral stroke in COVID‐19 patients. In conclusion, the high risk of new‐onset stroke must be taken into consideration when treating COVID‐19 patients with an elderly age combined with a history of hypertension. These patients are more vulnerable to multiorgan dysfunction and an overactivated inflammatory response, in turn leading to an unfavourable outcome and higher mortality rate.
Keywords: clinical characteristics, COVID‐19, prognosis, SARS‐CoV‐2, stroke
Of the 2,474 COVID‐19 patients in the Tongji Hospital, 113 (4.7%) patients had cerebral stroke, and 25 (1.0%) patients had new‐onset stroke. In 113 (4.7%) stroke patients, 88 (77.9%) patients in the previous‐stroke, while 25 (22.1%) patients in the new‐onset. Most COVID‐19 patients with stroke were elderly with more comorbidities such as hypertension, diabetes and heart diseases. Laboratory examinations showed hypercoagulation and elevated serum parameters such as IL‐6, cTnI. The mortality rate of stroke patients was almost double that of patients without stroke. In conclusion, COVID‐19 patients with an elderly age combined with a history of hypertension were more likely happening new‐onset stroke. These patients are more vulnerable to multiorgan dysfunction and an overactivated inflammatory response, in turn leading to a higher mortality rate.

Abbreviations
- ALT
alanine aminotransferase
- APTT
activated partial thromboplastin time
- AST
aspartate aminotransferase
- AT, A
thrombin activity
- BUN
blood urea nitrogen
- CI
confidence interval
- CK‐MB
creatine kinase‐mb isoenzymes
- COVID‐19
coronavirus disease 2019
- Cr
creatinine
- cTnI
cardiac troponin I
- FDP
fibrinogen degradation products
- Fgb
fibrinogen
- Hgb
haemoglobin
- hs‐CRP
hypersensitive c‐reactive protein
- IL
interleukin
- MG
myohaemoglobin
- NT pro‐BNP
N terminal pro‐brain natriuretic peptide
- OR
odd ratio
- Plt
platelet
- PT
prothrombin time
- PTA
prothrombin activity
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- TNF
tumour necrosis factor
- TT
thrombin time
- WBC
white blood cell count
Key Points
Question
What are the clinical characteristics and prognosis of COVID‐19 patients with cerebral stroke?
Findings
Based on the data from 2,474 cases, the presence of cerebral stroke in COVID‐19 patients was 5.9% (113/2474) and the incidence of new‐onset stroke was 1.6%(25/2474). COVID‐19 patients with stroke showed older age, multiple symptoms and complicated previous medical history. Meanwhile, they were more likely to have abnormalities in heart, kidney, liver and immune function. Age and hypertension comorbidity were independent predictors for new stroke occurrence under SARS‐CoV‐2 infection.
Meaning
COVID‐19 patients with stroke were elderly with a complicated medical history and more likely to have multi‐organ dysfunction and over‐activated inflammatory response, resulting in poor prognosis and higher mortality. Closer observation and early intervention should be given to COVID‐19 patients with a history or high‐risk factor of stroke, to avoid serious complications and high mortality.
1. INTRODUCTION
In late December 2019, viral pneumonia caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) emerged and caused an epidemic outbreak all over the world in the following few months. Although the source of this virus is still unclear, this illness has led to the death of a large number of infected patients, especially elderly patients with underlying diseases. Multiple studies have confirmed that hypertension, diabetes and cardiovascular disease are the main risk factors associated with acute respiratory distress syndrome and death from coronavirus disease 2019 (COVID‐19; Mao et al., 2019). However, little is known about the impact of cerebral stroke as a common senile disease on the clinical characteristics and prognosis of patients with COVID‐19.
Stroke, also known as an acute cerebrovascular disease, has various vascular causes (including ischaemic and haemorrhagic stroke) with neurological dysfunction. COVID‐19 patients with stroke can present with disturbance of consciousness and difficulties performing physical activity, which may result in central respiratory depression and exacerbate the lung infection (Liu et al., 2020). Moreover, severe COVID‐19 cases are often accompanied by hypoxia, impaired cardiac function and abnormal blood coagulation, which are high‐risk factors for stroke (Guan et al., 2017; Wang et al., 2017; World Health Organization, 2020). Considering the different clinical characteristics and prognosis of ischaemic and haemorrhagic stroke and the elevated incidence of ischaemic stroke, we explored the interacting effects between ischaemic stroke and SARS‐CoV‐2 infection; this information will be of great benefit to better prevent and treat stroke‐related clinical symptoms in COVID‐19 patients.
In the present study, we separately reported the clinical characteristics and prognosis of COVID‐19 patients with previous or new‐onset stroke. The risk factors for new‐onset stroke were also investigated. Our study revealed the effects of cerebral stroke on the clinical features and prognosis of COVID‐19 patients and provided novel insights into the spectrum of this disease. We hope our study may provide a reference to improve the treatment of COVID‐19 patients with stroke.
2. METHODS
2.1. Study design and participants
All patients were admitted and treated in two branches (Optic Valley branch and Sino‐French New City branch) of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology in Wuhan. These two comprehensive clinics are designated hospitals for the treatment of severely and critically ill patients with COVID‐19. The clinical data of 2,474 patients who were diagnosed with COVID‐19 according to the World Health Organization interim guidance were retrospectively collected from February 10th to March 24th, 2020 (World Health Organization, 2020). Laboratory confirmation of SARS‐CoV‐2 infection was performed for all patients on admission to our hospitals. This study was approved by the Medical Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology in accordance with the Helsinki declaration. The requirement for written informed consent was waived because of the urgent need to collect data.
2.2. Data collection
The clinical data, including basic information (age and sex), past medical history (hypertension, diabetes, heart disease and others), typical symptoms (fever, cough, shortness of breath, muscle ache and diarrhoea), neurological symptoms (headache, dizziness and consciousness disorders), medical course, laboratory findings (blood cell count, serum inflammation factors, serum biochemical analysis data, serum electrolytes and blood coagulation factors), chest and brain computed tomography (CT) scans, treatment and outcomes, were retrospectively collected by carefully reviewing the medical records of patients with laboratory‐confirmed SARS‐CoV‐2 infection. Symptoms were assessed by direct communication with conscious patients or with relatives of unconscious persons. Additionally, consciousness disorders were exclusively evaluated by experienced neurologists. Laboratory examinations were performed on admission and when necessary in our hospital. The highest value of each laboratory parameter for the patients during hospitalisation was collected for statistical analysis. We defined a composite endpoint as the date of patient discharge, death or March 24th, 2020. Accordingly, we evaluated two medical courses for each patient, including days from onset to the end of observation as well as days from hospital admission to the end of observation.
Stroke is a severe cerebrovascular disease and is divided into two types: cerebral ischaemic infarction and cerebral haemorrhage (Guan et al., 2017; Wang et al., 2017). In the present study, we enrolled all patients with only ischaemic infarction. In accordance with the period of stroke occurrence, we further categorised COVID‐19 patients with stroke into two groups. One was the previous‐stroke group, in which the patients had a stroke at least one month prior to the confirmation of COVID‐19. The other group was the new‐stroke group, in which a stroke occurred after the COVID‐19 diagnosis. The occurrence of a previous stroke was identified from personal medical records. New strokes in COVID‐19 patients were identified by brain CT or MRI. The diagnosis of stroke was carefully confirmed by at least two trained neurologists.
All patients received personal systematic treatments in accordance with the WHO interim guidelines (World Health Organization, 2020), including antiviral therapy, oxygen support, secondary infection control, multiorgan monitoring and support and so on. Oxygen support was one of the most important approaches and was consistently provided through normal or high‐flow nasal cannulas, non‐invasive ventilation, invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO; Liu et al., 2020). For patients with ischaemic stroke, anti‐coagulation, high brain perfusion and brain protection therapy were administered. To treat patients with haemorrhagic stroke, haemostatic drugs, control of brain oedema and swelling and brain‐protective agents were applied (Chinese Society of Neurology, 2018). In our patient series, none of the stroke patients underwent neurosurgical interventions. In light of the endpoint of observation, the outcomes of COVID‐19 patients included discharge, hospitalisation and death.
2.3. Statistical analyses
All statistical analyses were performed using SPSS 17.0 statistical software. For continuous data, the mean ± standard deviation (SD) and median with range are reported for normal and abnormal distribution data, respectively. One‐way ANOVA was used to analyse the variance of multiple groups. Student's t test was used to compare data with normal distribution between the two groups and nonparametric analysis and the Mann–Whitney U test was used to compare data with a non‐normal distribution between the two groups. Qualitative data are expressed as counts and percentages, and the chi‐square test was used for comparisons. The significant predictors in the univariate analysis were included in multivariate analysis, and the enter method was applied for multivariate regression analysis to determine the risk factors for new‐onset stroke in COVID‐19 patients. The distribution of overall survival of each group was estimated by the Kaplan‐Meier method and compared by the log‐rank test. p < .05 was statistically significant.
3. RESULTS
3.1. Clinical characteristics of COVID‐19 patients with or without stroke
The essential clinical information of COVID‐19 patients is summarised in Table 1. A total of 2,474 patients were included in this study, with a mean age of 61.0 ± 15.7 years. The mean age of patients in the stroke group was significantly older than that of the patients in the non‐stroke group (p < .001). Specifically, patients older than 60 years were more frequently observed in the stroke group than in the non‐stroke group (p < .001). However, there was no difference in sex between groups (p = .558). Comorbidities (hypertension, diabetes and heart disease) were more common amongst patients with stroke (all p < .001). Moreover, the stroke patients showed a higher incidence of consciousness disorders than non‐stroke patients (p < .001), while the other common symptoms, such as cough, shortness of breath and diarrhoea, were similar. Stroke patients were more likely to have low fingertip oxygen saturation than non‐stroke patients (p < .001).
TABLE 1.
Demographic features and clinical characteristics of COVID‐19 patients
| Variables | Total (n = 2,474) | Non‐stroke (n = 2,361) | Stroke | p c | ||
|---|---|---|---|---|---|---|
| Previous (n = 88) | New (n = 25) | p b | ||||
| Mean age (years, mean ± SD) | 61.0 ± 15.7 | 57.8 ± 15.6 | 70.69 ± 10.85 a | 74.2 ± 10.6 a | 0.150 | <.001 |
| Age subgroups | a | a | ||||
| <40 year | 359 (14.5) | 359 (15.2) | 0 (0) | 0 (0) | 0.515 | <.001 |
| 40–60 year | 775 (31.3) | 760 (32.2) | 13 (14.77) | 2 (8.0) | ||
| ≥60 year | 1,340 (54.2) | 1,242 (52.6) | 75 (85.23) | 23 (92.0) | ||
| Sex, n (%) | ||||||
| Male | 1,235 (49.9) | 1,172 (49.6) | 47 (53.41) | 16 (64.0) | 0.260 | .558 |
| Female | 1,239 (50.1) | 1,189 (50.4) | 41 (46.59) | 9 (36.0) | ||
| Comorbidities, n (%) | ||||||
| Hypertension | 745 (30.1) | 665 (28.2) | 63 (71.59) a | 17 (68.0) a | 0.728 | <.001 |
| Diabetes | 355 (14.3) | 321 (13.6) | 27 (30.68) a | 7 (28.0) a | 0.796 | <.001 |
| Heart disease | 202 (8.2) | 173 (7.3) | 22 (25.00) a | 7 (28.0) a | 0.762 | <.001 |
| Others disease | 931 (37.1) | 879 (37.2) | 32 (29.6) | 20 (51.3) | 0.019 | .725 |
| Prognosis, n (%) | a | a | ||||
| Discharge | 1,744 (70.5) | 1,687 (71.5) | 49 (82.95) | 8 (32.0) | 0.111 | <.001 |
| Hospitalisation | 551 (22.3) | 509 (21.5) | 29 (5.68) | 13 (52.0) | ||
| Death | 179 (7.2) | 165 (6.9) | 10 (11.36) | 4 (16.0) | ||
| Days from onset to end point of observation (days, mean ± SD) | 39.6 ± 14.0 | 39.5 ± 14.0 | 44.16 ± 13.06 a | 46.2 ± 14.4 | <0.001 | .002 |
| Days from admission to end point of observation (days, mean ± SD) | 21.2 ± 12.2 | 21.2 ± 12.1 | 23.69 ± 13.42 | 18.7 ± 8.4 a | <0.001 | .699 |
| Symptoms and signs at the onset of COVID‐19 | ||||||
| Cough | 1,680 (67.9) | 1,601 (67.8) | 63 (71.59) | 16 (64.0) | 0.465 | .640 |
| Shortness of breath | 983 (39.7) | 933 (39.5) | 44 (50.00) | 6 (24.0) | 0.021 | .315 |
| Diarrhoea | 540 (21.8) | 519 (22.0) | 16 (18.18) | 5 (20.0) | 0.837 | .393 |
| Headache or dizziness | 545 (22.0) | 518 (21.9) | 16 (18.18) | 11 (44.0) a | 0.008 | .624 |
| Muscle ache | 511 (20.7) | 483 (20.4) | 19 (21.59) | 9 (36.0) | 0.141 | .268 |
| consciousness disorder | 66 (2.7) | 50 (2.1) | 7 (8.14) a | 9 (36.0) a | <0.001 | <.001 |
| Fever (°C) | ||||||
| <37.3 | 656 (26.5) | 614 (26.0) | 32 (36.36) | 10 (40.0) | 0.980 | .030 |
| 37.3–38 | 617 (24.9) | 589 (24.9) | 22 (25.00) | 6 (24.0) | ||
| 38.1–39 | 645 (26.1) | 618 (26.2) | 21 (23.86) | 6 (24.0) | ||
| ≥39 | 556 (22.5) | 540 (22.9) | 13 (14.77) | 3 (12.0) | ||
| Fingertip oxygen saturation (<93%) | 251 (10.1) | 229 (11.5) | 18 (23.07) a | 4 (16.0) | 0.620 | .012 |
Values are numbers (%) unless stated otherwise
One‐way ANOVA was used to analyse the difference amongst the non‐stroke, previous and new; p < .05.
Previous versus new.
Stroke versus non‐stroke.
The stroke patients were further divided into two subgroups according to the time point of stroke onset: patients with previous stroke and patients with new stroke. The detailed clinical characteristics of the patients in different subgroups are listed in Table 1. Generally, there were 88 patients with previous stroke and 25 patients with newly identified stroke. In comparison to non‐stroke patients, both previous‐ and new‐stroke patients showed similar incidences of common symptoms, such as cough, shortness of breath, diarrhoea and fever, but a higher incidence of consciousness disorders (all p < .001). The proportion of patients with headache or dizziness (44.4% vs. 18.0%, p = .01) was higher amongst new‐stroke patients than amongst non‐stroke patients. Previous‐stroke patients were more likely to have low fingertip oxygen saturation (<93%) than non‐stroke patients (23.1% vs. 11.5%, p < .001).
3.2. 2 Laboratory parameters of COVID‐19 patients with and without stroke
Table 2 shows the laboratory findings of patients with and without stroke. Previous‐ and new‐stroke patients exhibited more neutrophilia (33.0% and 48.0% vs. 21.1%, p < .001, respectively), lymphocytopenia (58% and 76% vs. 31.2%, p < .001, respectively) and anaemia (25% and 28% vs. 8.5%, p < .001, respectively) than the non‐stroke group. The levels of cytokines and infection‐related factors in the blood, including IL‐1β, TNF‐α, IL‐8, IL‐2R, IL‐10 and hsCRP, were detected in a certain proportion of patients with or without stroke. An abnormal concentration of IL‐6 was more frequently found in previous‐ and new‐stroke patients than in non‐stroke patients (68.3% and 87.0% vs. 42.4%, p < .001, respectively). More previous‐ and new‐stroke patients had markedly elevated concentrations of myohaemoglobin (MG, 30.4% and 45% vs. 12.7%, p < .001, respectively), N terminal pro‐brain natriuretic peptide (NT pro‐BNP, 78.2% and 91.3% vs. 50.5%, p < .001, respectively), blood urea nitrogen (BUN, 44.8% and 44% vs. 16.5%, p < .001, respectively) and creatinine (Cr, 44.8% and 44% vs. 13%, p < .001, respectively) than non‐stroke patients. In addition, elevated concentrations of cardiac troponin I (cTnI, 26.4% vs. 11.3%, p < .001) and creatine kinase‐myoglobin (CK‐MB, 10.1% vs. 4.7%, p = .028) and decreased levels of albumin (42.5% vs. 19.8%, p < .001) were more frequently found in previous‐stroke patients than in non‐stroke patients. A disturbance in electrolytes, including potassium and sodium, was more likely to occur in both previous‐ and new‐stroke patients than in non‐stroke patients. In terms of coagulation‐related indexes, significantly increased D‐dimer (80.5% and 84% vs. 58.8%, p < .001, respectively) and fibrinogen (Fgb, 75.6% and 80.8% vs. 60.6%, p = .005 and p = .001) levels were more prevalent in previous‐ and new‐stroke patients than in non‐stroke patients. Additionally, a prolonged activated partial thromboplastin time (APTT, 47.7% vs. 33.8%, p = .008), more fibrinogen degradation products (FDP, 58.9% vs. 32.6%, p < .001) and higher incidence of decreased prothrombin activity (PTA, 1.1% vs. 11.6%, p < .001) and thrombocytopenia (39.8% vs. 19.4%, p < .001) were more prevalent amongst previous‐stroke patients than in non‐stroke patients.
TABLE 2.
Laboratory findings of patients with COVID‐19
| Total (n = 2,474) | Non‐stroke (n = 2,361) | Stroke | p a | p b | p c | ||
|---|---|---|---|---|---|---|---|
| Previous (n = 88) | New (n = 25) | ||||||
| Blood routine examination | |||||||
| WBC, ×109/L (3.5–9.5) | 6.9 (5.4–9.6) | 8.34 (5.3–9.3) | 8.12 (5.1–11.8) | 9.84 (6.6–13.6) | |||
| <4 | 227/2,454 (9.3) | 216/2,341 (9.2) | 10/88 (11.3) | 1/25 (4.0) | .016 | .005 | .285 |
| 4–10 | 1,691/2,454 (68.9) | 1,630/2,341 (69.6) | 49/88 (55.7) | 12/25 (48.0) | |||
| >10 | 536/2,454 (21.8) | 495/2,341 (21.1) | 29/88 (33.0) | 12/25 (48.0) | |||
| Neutrophils,×109/L (1.8–6.3) | 4.6 (3.3–7.3) | 6.2 (3.2–7.1) | 5.2 (3.3–8.4) | 7.0 (4.7–10.8) | |||
| >6.3 | 748/2,454 (30.5) | 696/2,341 (29.7) | 38/88 (43.2) | 14/25 (56.0) | .007 | .004 | .256 |
| Lymphocytes,×109/L (1.1–3.2) | 1.7 (0.9–2.4) | 4.6 (0.9–2.5) | 1.0 (0.5–1.5) | 0.7 (0.5–1.0) | |||
| <1.1 | 799/2,452 (32.6) | 729/2,339 (31.2) | 51/88 (58.0) | 19/25 (76.0) | <.001 | <.001 | .101 |
| Hgb, g/L (120–150) | 121 (106.0–135.0) | 119.35 (107.0–135.0) | 105.0 (88.0–130.0) | 98.0 (87.5–125.0) | |||
| ≥120 | 1,315/2,457 (53.3) | 1,281/2,344 (54.7) | 28/88 (31.8) | 6/25 (24.0) | <.001 | <.001 | .735 |
| 90–120 | 912/2,457 (37.1) | 863/2,344 (36.8) | 37/88 (42.0) | 12/25 (48.0) | |||
| <90 | 229/2,457 (9.3) | 200/2,344 (8.5) | 22/88 (25.0) | 7/25 (28.0) | |||
| Cytokines and infection‐related factors | |||||||
| IL‐1β (≥5pg/ml) | 469/2,078 (22.6) | 452/1,974 (22.9) | 13/81 (16.0) | 4/23 (17.4) | .149 | .532 | .878 |
| TNF‐α, pg/ml (<8.1) | 8.4 (6.3–11.4) | 11.2 (6.3–11.4) | 8.5 (6.1–10.8) | 10.7 (8.6–12.8) | |||
| ≥8.1 | 1,151/2,075 (54.7) | 1,072/1,971 (54.4) | 44/81 (54.3) | 20/23 (87.0) | .99 | .002 | .005 |
| IL−6, pg/ml (<7) | 5.4 (2.0–23.3) | 74.4 (1.9–21.3) | 19.4 (4.7–71.5) | 18.1 (7.6–31.7) | |||
| ≥7 | 917/2,078 (44.1) | 841/1,982 (42.4) | 56/82 (68.3) | 20/23 (87.0) | <.001 | <.001 | .077 |
| IL−8, pg/ml (<62) | 11.2 (6.5–21.8) | 60.9 (6.4–21.7) | 11.1 (7.0–23.0) | 15.3 (11.9–25.2) | |||
| ≥62 | 162/2,078 (7.8) | 158/1,974 (8.0) | 2/81 (2.5) | 2/23 (8.7) | .068 | .903 | .171 |
| IL−2R, U/ml (223–710) | 521.0 (329.0–820.3) | 676.4 (321.0–808.0) | 588.5 (384.0–913.3) | 642.0 (473.5–861.0) | |||
| ≥710 | 670/2,071 (32.4) | 619/1,964 (31.5) | 32/84 (38.1) | 19/23 (39.1) | .130 | <.001 | .928 |
| IL−10, pg/ml (<9.1) | 5.0 (5.0–6.6) | 12.7 (5.0–6.7) | 5.0 (5.0–5.1) | 5.0 (5.0–6.3) | |||
| ≥9.1 | 367/2,067 (17.8) | 353/1,963 (18.0) | 11/81 (13.6) | 3/23 (13.0) | .310 | .539 | .947 |
| hsCRP, mg/L (<1) | 18.2 (2.2–73.5) | 47.5 (2.1–70.5) | 49.4 (9.3–125.4) | 33.8 (11.8–94.6) | |||
| ≥1 | 2,055/2,393 (85.9) | 1,953/2,281 (85.6) | 78/87 (90.0) | 24/25 (96.0) | .290 | .140 | .327 |
| Serum biochemical index | |||||||
| Albumin, g/L (≥35) | 35.9 (30.8–40.2) | 35.6 (31.1–40.3) | 30.8 (27.9–38.0) | 35.0 (28.0–37.5) | |||
| ≥35 | 1,357/2,451 (55.3) | 1,305/2,339 (55.8) | 38/87 (43.7) | 14/25 (56.0) | <.001 | .179 | .544 |
| ≥30, <35 | 587/2,451 (23.9) | 572/2,339 (24.5) | 12/87 (13.8) | 3/25 (12.0) | |||
| <30 g/L | 507/2,451 (20.7) | 462/2,339 (19.8) | 37/87 (42.5) | 8/25 (32.0) | |||
| cTnI, pg/ml (<34.2) | 4.1 (1.9–12.3) | 198.8 (1.9–12.3) | 9.8 (4.1–36.6) | 10.3 (4.3–31.9) | |||
| ≥34.2 | 264/2,202 (12.0) | 236/2,091 (11.3) | 23/87 (26.4) | 5/24 (20.8) | <.001 | .143 | .576 |
| MG, ng/ml (<154.9) | 39.0 (27.7–74.4) | 113.5 (27.4–66.1) | 76.6 (42.5–200.6) | 133.9 (58.7–246.0) | |||
| ≥154.9 | 251/1,812 (13.9) | 218/1,713 (12.7) | 24/79 (30.4) | 9/20 (45.0) | <.001 | <.001 | .215 |
| CK‐MB, ng/ml (<7.2) | 0.8 (0.5–1.4) | 2.1 (0.4–1.3) | 1.5 (0.7–2.9) | 1.3 (0.7–2.9) | |||
| ≥7.2 | 88/1,793 (4.9) | 79/1,694 (4.7) | 8/79 (10.1) | 1/20 (5.0) | .028 | .943 | .476 |
| NT pro‐BNP, pg/ml (<116) | 126.0 (44.0–470.0) | 1.8 (41.0–421.8) | 707.5 (128.0–2,806.8) | 595.0 (290.0–2,055.0) | |||
| ≥116 | 1,071/2,059 (52.0) | 989/1,958 (50.5) | 61/78 (78.2) | 21/23 (91.3) | <.001 | <.001 | .158 |
| BUN, mmol/L (3.1–8.0) | 5.0 (3.7–6.7) | 6.6 (3.7–8.6) | 7.2 (5.0–12.5) | 6.7 (4.6–10.7) | |||
| >8.0 | 436/2,455 (17.8) | 386/2,343 (16.5) | 39/87 (44.8) | 11/25 (44.0) | <.001 | <.001 | .942 |
| Cr, umol/L (59–104) | 73.0 (56.0–89.0) | 90.5 (57.0–88.3) | 79.0 (60.0–100.0) | 68.0 (43.0–88.0) | |||
| >104 | 354/2,455 (14.4) | 304/2,343 (13.0) | 39/87 (44.8) | 11/25 (44.0) | .007 | <.001 | .942 |
| ALT, U/L (<41) | 31.0 (19.0–57.0) | 54.6 (19.0–57.0) | 24.0 (15.0–59.0) | 24.0 (15.5–35.0) | |||
| ≥41 | 928/2,455 (37.8) | 894/2,343 (38.2) | 30/87 (34.5) | 4/25 (16.0) | .488 | .023 | .076 |
| AST, U/L (<40) | 28.0 (20.0–44.0) | 51.2 (20.0–44.0) | 29.0 (20.0–61.0) | 29.5 (21.0–45.8) | |||
| ≥40 | 740/2,451 (30.2) | 698/2,340 (29.8) | 32/87 (36.8) | 10/24 (41.7) | .165 | .208 | .662 |
| Electrolyte disturbance | |||||||
| potassium, mmol/L (3.5–5.1) | 4.5 (4.2–4.9) | 4.5 (4.2–4.9) | 4.7 (4.1–5.3) | 4.6 (3.5–5.1) | |||
| <3.5 | 188/2,452 (7.7) | 166/2,339 (7.1) | 15/88 (17.0) | 7/25 (28.0) | <.001 | <.001 | .277 |
| 3.5–5.1 | 1,853/2,452 (75.6) | 1,792/2,339 (76.6) | 47/88 (53.4) | 14/25 (56.0) | |||
| >5.1 | 411/2,452 (16.7) | 381/2,339 (16.3) | 26/88 (29.6) | 4/25 (16.0) | |||
| Sodium, mmol/L (136–145) | 140.6 (138.1–142.3) | 140.6 (138.2–142.3) | 141.4 (135.3–143.9) | 141.4 (135.9–144.5) | |||
| <136 | 421/2,451 (17.2) | 389/2,338 (16.6) | 27/88 (30.7) | 5/25 (20.0) | <.001 | .401 | .514 |
| 136–145 | 1,872/2,451 (76.4) | 1,806/2,338 (77.3) | 49/88 (55.7) | 17/25 (68.0) | |||
| >145 | 158/2,451 (6.9) | 143/2,338 (6.1) | 12/88 (13.6) | 3/25 (12.0) | |||
| Blood coagulation factor | |||||||
| TT, s (14–19) | 16.7 (15.5–17.6) | 18.6 (15.4–17.8) | 17.0 (16.2–19.5) | 16.7 (15.5–17.6) | |||
| >19 | 528/2,351 (22.5) | 503/2,240 (22.5) | 23/86 (26.8) | 2/25 (8.0) | .351 | .084 | .048 |
| PT, s (11.5–14.5) | 14.1 (13.4–15.6) | 15.0 (13.4–15.5) | 14.4 (13.4–16.3) | 14.4 (14.0–15.4) | |||
| >14.5 | 924/2,401 (38.5) | 871/2,289 (38.1) | 41/87 (47.1) | 12/25 (48.0) | .088 | .308 | .939 |
| APTT, s (29–42) | 39.5 (36.7–43.9) | 41.1 (36.5–43.6) | 41.5 (37.8–49.0) | 41.3 (39.3–47.3) | |||
| >42 | 802/2,325 (34.5) | 749/2,214 (33.8) | 41/86 (47.7) | 12/25 (48.0) | .008 | .137 | .939 |
| AT: A, % (80–120) | 96.0 (85.0–105.0) | 94.5 (86.0–105.0) | 91.0 (72.0–100.0) | 87.0 (64.0–98.0) | |||
| >120 | 360/1,823 (19.7) | 355/1,727 (20.6) | 4/75 (5.3) | 1/21 (4.8) | .001 | .074 | .917 |
| D‐Dimer, mg/L (<0.5) | 0.7 (0.3–2.2) | 2.8 (0.3–2.0) | 2.3 (0.8–6.5) | 1.8 (0.8–3.9) | |||
| ≥0.5 | 1,441/2,408 (59.8) | 1,350/2,296 (58.8) | 70/87 (80.5) | 21/25 (84.0) | <.001 | .011 | .689 |
| FDP, mg/L (<5) | 4.0 (4.0–7.7) | 15.4 (4.0–6.8) | 8.5 (4.0–25.5) | 4.9 (4.0–17.4) | |||
| ≥5 | 616/1,822 (33.8) | 562/1,725 (32.6) | 44/76 (58.9) | 10/21 (47.6) | <.001 | .144 | .401 |
| Fgb, mg/L (2–4) | 4.5 (3.4–5.8) | 4.6 (3.4–5.7) | 5.5 (4.1–6.6) | 5.0 (4.1–5.7) | |||
| >4 | 1,433/2,334 (61.4) | 1,348/2,223 (60.6) | 65/86 (75.6) | 20/25 (80.0) | .005 | .001 | .646 |
| PTA, % (75–125) | 96.0 (87.0–104.0) | 94.3 (88.0–104.0) | 94.0 (70.0–103.0) | 91.0 (79.0–97.5) | |||
| >125 | 269/2,420 (11.1) | 268/2,308 (11.6) | 1/87 (1.1) | 0/25 (0) | <.001 | .070 | .590 |
| Plt, ×109/L (150–350) | 239 (170.8–319.0) | 251.4 (174.0–319.0) | 229.5 (104.8–346.8) | 241 (130.0–303.0) | |||
| <150 | 496/2,446 (20.3) | 453/2,333 (19.4) | 35/88 (39.8) | 8/25 (32.0) | <.001 | .115 | .480 |
Continuous variables are presented as median (interquartile range) and categorical variables are numbers (percentages) unless stated otherwise; p a, one‐way ANOVA analysis amongst the non‐stroke, previous and new; p b, new versus non‐stroke; p c, previous versus new.
Abbreviations: ALT, alanine aminotransferase; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; AT, A, thrombin activity; BUN, blood urea nitrogen; CK‐MB, creatine kinase‐mb isoenzymes; Cr, creatinine; cTnI, cardiac troponin I; FDP, fibrinogen degradation products; Fgb, fibrinogen; Hgb, haemoglobin; hs‐CRP, hypersensitive c‐reactive protein; IL, interleukin; MG, myohaemoglobin; NT pro‐BNP, N terminal pro‐brain natriuretic peptide; Plt, platelet; PT, prothrombin time; PTA, prothrombin activity; TNF, tumour necrosis factor; TT, thrombin time; WBC, white blood cell count.
3.3. Prognosis and survival analysis of COVID‐19 patients with and without stroke
The stroke patients had a significantly worse outcome than the patients in the non‐stroke group (p < .001), including a higher mortality rate and longer disease course. Furthermore, the mean duration from symptom onset to the end of observation was longer in the stroke group than in the non‐stroke group (p < .05; Table 1). However, the mean duration from admission to the end of observation between the two groups was similar (Table 1). Kaplan–Meier survival curves showed an improved overall survival (OS) for patients without stroke compared to those with previous stroke and new stroke (all p < .001; Figure 1). However, no significant difference in OS was observed between patients with previous stroke and those with new stroke (Figure 1).
FIGURE 1.
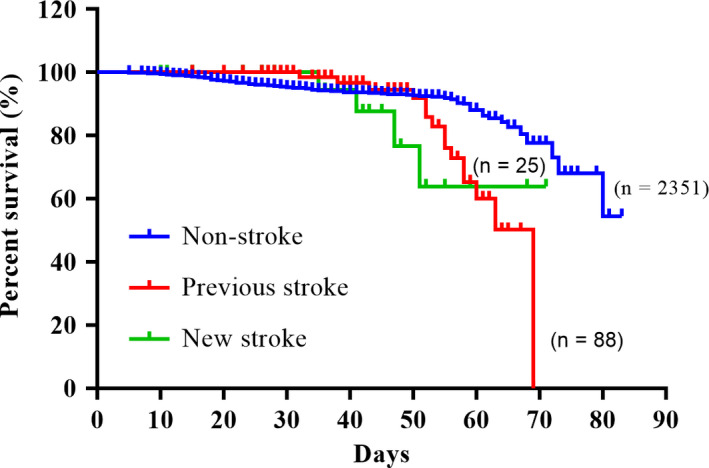
Kaplan–Meier survival curves showing previous and new stroke‐related overall survival (OS) of COVID‐19 patients. Images showing the intergroup comparison of OS between non‐stroke, new stroke and previous stroke group. Ten patients in the non‐stroke group with uncertain onset time were excluded. Non‐stroke versus Previous stroke, p = .006; Non‐stroke versus New stroke, p < .001; Previous stroke versus New stroke, p = .762
3.4. Analysis of predictors for new‐onset stroke in COVID‐19 patients
Data on the clinical variables and log‐rank regression analysis are listed in Table 3. In the analysis, age ≥ 60 years, male sex and hypertension were independent predictors for new stroke occurrence in COVID‐19 patients (p = .01, p = .01 and p < .001, respectively). However, heart disease and diabetes were not independent predictors for new stroke occurrence during SARS‐CoV‐2 infection.
TABLE 3.
Regression analysis for incident risk of new‐onset stroke amongst COVID‐19 patients
| Variable | Controls (n = 2,361) | New stroke (n = 25) | Logistic regression | ||
|---|---|---|---|---|---|
| Adjusted OR | 95% CI | p value | |||
| Age (≥60 years) | 1,242 (52.6) | 24 (96.0) | 0.3 | 0.2–0.5 | <.001 |
| Fingertip oxygen saturation (<93%) | 228 (9.7) | 4 (16.0) | 3.8 | 2.5–5.7 | <.001 |
| consciousness disorder | 50 (2.1) | 9 (36.0) | 0.1 | 0.1–0.2 | <.001 |
Abbreviations: CI, confidence interval; OR, odd ratio.
4. DISCUSSION
In the present study, we retrospectively analysed the effect of cerebral stroke on the clinical features and prognosis of COVID‐19 patients, providing novel insights into the spectrum of this disease. The prevalence of stroke (4.7%) in COVID‐19 patients was markedly higher than that in the population (2.41%) without infection (Wu et al., 2019). One possible reason is that this cohort was predominantly from the designated hospital for severely and critically ill patients. Additionally, SARS‐CoV‐2 infection might also promote cerebral strokes since a certain portion of cases were new onset. If this is the case, treating patients with viral infection and concurrent stroke will be a great burden for the public health system.
COVID‐19 patients share some common characteristics with the normal population in terms of risk factors for stroke, including older age (Chen, et al., 2020) and stroke patients had more comorbidities (Guan et al., 2019), such as hypertension, diabetes and heart disease. However, only hypertension was an independent predictor for new stroke in this study.
As the robustly increased levels of biomarkers (e.g., cTnI, CK‐MB, NT pro‐BNP, Cr, BUN and IL‐6) predict extrapulmonary organ injuries, the presence of multiorgan dysfunction and overactivated systematic inflammation were more common in COVID‐19 patients with stroke than in those without stroke. These changes are important factors contributing to the unfavourable outcomes of patients with stroke and were also widely observed in patients with severe disease (Chen, et al., 2020; Hilker et al., 2003; Qin et al., 2020; Sui & Zhang, 2011; Yeh et al., 2011) and deceased patients (Mao et al., 2019). In addition, due to dysphagia, impaired locomotor function and insufficient nutrition (Quyet et al., 2019), patients with stroke have poor immunity and cardiac function, leading to a huge risk for suffering from hospital‐acquired pneumonia caused by organisms other than viruses (Chen, et al., 2020; Zhu et al., 2020). This is especially critical for stroke patients who lose consciousness and spontaneous respiration, thus requiring mechanical ventilation (Umapathi et al., 2004). Coinfection will in turn aggravate the illness in COVID‐19 patients (Lee et al., 2019; Mao et al., 2019). In this study, the higher incidence of consciousness disorders in stroke patients might be an early sign related to their poor outcomes.
Although the exact mechanisms underlying stroke in COVID‐19 patients are still unknown, we can speculate about the potential links between stroke and SARS‐CoV‐2 infection from the clinical features and cases caused by other coronaviruses, such as severe acute respiratory syndrome coronavirus (SARS‐CoV) and Middle East respiratory syndrome coronavirus (MERS‐CoV), since these viruses share some common characteristics (Liu et al., 2020) and are involved in stroke (Ding et al., 2004). For instance, both ischaemic and haemorrhagic stroke were found to occur in patients with SARS (Ding et al., 2004) and MERS (Grau et al., 2010) infection at high incidences, and SARS‐CoV could even be found in the cerebrum (Maaijwee et al., 2014).
Cytokine storms are widely considered to be a factor leading to the aggravation of pneumonia and extrapulmonary injuries such as cardiac and renal failure (Hilker et al., 2003; Yeh et al., 2011). There might be a correlation between cytokines and stroke. Associated with cerebrovascular risks, increases in cytokines and systemic inflammation‐related factors such as IL‐6, C‐reactive protein and monocyte chemotactic protein 1 in acute respiratory tract infections were triggers for stroke (Corrales‐Medina et al., 2013; Oudit et al., 2009). Since inflammatory responses mediated by cytokines were found to disturb the stability of coronary plaque, which leads to cardiac ischaemia (Oyoo & Espinoza, 2005), we speculate that cytokines might also affect the stability of carotid and cerebrovascular atherosclerosis, resulting in abnormal occlusive thrombus or the rupture of blood vessels. The markedly elevated levels of IL‐6 in new‐stroke patients in this study could help to support this hypothesis. However, the other cytokines were not found to differ between stroke and non‐stroke patients. In addition, viral infection of the vascular endothelium is another possible mechanism. SARS‐CoV‐2 is highly homologous in the genome of SARS‐CoV (Liu et al., 2020) and may also have high affinity for the host angiotensin‐converting enzyme 2 receptor (Ding et al., 2004; Pagliano et al., 2020). This mechanism needs further investigation even though vasculitis caused by viral infection is a risk factor for stroke (Pagliano et al., 2020). Additionally, in the new‐onset stroke group, the incidence of haemorrhagic stroke was markedly increased in comparison with that in the previous‐stroke group. This might be related to impaired blood coagulation, so the administration of haemostatic drugs should be rigorously considered.
As mentioned above, COVID‐19 patients with stroke were more vulnerable to multiorgan dysfunction and an overactivated inflammatory response, which in turn resulted in significantly impaired outcomes. In this study, patients with stroke had nearly double the mortality rate of patients without stroke. The discharge rate of patients with stroke was only half of that of patients without stroke. In comparison to patients without stroke, not only patients with previous stroke but also those with new stroke had a significantly decreased OS.
5. LIMITATIONS
Our study has a few limitations. First, because the outbreak of SARS‐CoV‐2 occurred in a short time and the public health resources were seriously restricted, some patients could not be hospitalised in time, leading to a deviation between the observation point of the disease course and the length of stay in the hospital. This also results in the absence of initial values of laboratory parameters at symptom onset. Second, some tests (for example, cTnI, NT pro‐BNP and arterial blood gas tests) were not performed in all patients, and missing data might lead to bias in the analysis of clinical characteristics. Additionally, considering that this is a single‐centre retrospective study that only enrolled severe and critically ill COVID‐19 patients, mild or asymptomatic COVID‐19 patients were excluded and further studies are warranted to gain a better understanding of the risk factors for and outcomes of COVID‐19 patients combined with stroke.
6. CONCLUSION
To summarise, elderly COVID‐19 patients with hypertension are more likely to develop cerebral stroke, which is associated with severe neurological symptoms, multiorgan injuries, coagulation dysfunction and an overactivated inflammatory response. All these factors will contribute to unfavourable outcomes, including a prolonged disease course, shortened OS and high mortality. Therefore, intensive treatments for both COVID‐19 and stroke must be taken into consideration for patients with risk factors for stroke.
ROLE OF THE FUNDER/SPONSOR
The funding sources had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
Huaqiu Zhang had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Xiaolong Yao, Shengwen Liu, Junwen Wang, Kai Zhao, Xiaobing Long, Xuejun He contributed equally and share the first authorship. Concept and design: Jihong Liu, Wei Wang, Huaqiu Zhang. Acquisition, analysis, or interpretation of data: Xiaolong Yao, Xiaobing Long, Xuejun He. Drafting of the manuscript: Xiaolong Yao, Shengwen Liu, Junwen Wang, Kai Zhao, Xiaobing Long. Critical revision of the manuscript for important intellectual content: Huicong Kang, Kai Shu, Zhouping Tang, Ting Lei. Statistical analysis: Xiaobing Long, Xuejun He, Yiping Yang, Xiaopeng Ma, Pengjie Yue. Obtained funding: Huaqiu Zhang.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1111/ejn.15007.
ACKNOWLEDGEMENTS
We acknowledge Hubei CDC for its efforts and hard works during these cases.
Yao X, Liu S, Wang J, et al. The clinical characteristics and prognosis of COVID‐19 patients with cerebral stroke: A retrospective study of 113 cases from one single‐centre. Eur J Neurosci. 2021;53:1350–1361. 10.1111/ejn.15007
Xiaolong Yao, Shengwen Liu, Junwen Wang, Kai Zhao, Xiaobing Long and Xuejun He contributed equally.
Funding information
This work was supported by the Natural Science Foundation of China (81371381; 81702478).
Edited by Yoland Smith.
Contributor Information
Jihong Liu, Email: jhliu@tjh.tjmu.edu.cn.
Wei Wang, Email: WWang@tjh.tjmu.edu.cn.
Huaqiu Zhang, Email: zhanghq_04@yahoo.com.
DATA AVAILABILITY STATEMENT
The data sets used and analysed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- Chen, G. , Wu, D. , Guo, W. , Cao, Y. , Huang, D. A. , Wang, H. , Wang, T. , Zhang, X. , Chen, H. , Haijing, Y. U. , Zhang, X. , Zhang, M. , Shiji, W. U. , Song, J. , Chen, T. , Han, M. , Li, S. , Luo, X. , Zhao, J. , & Ning, Q. (2020). Clinical and immunologic features in severe and moderate Coronavirus Disease 2019. Journal of Clinical Investigation, 130(5), 2620–2629. 10.1172/JCI137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, N. , Zhou, M. , Dong, X. , Qu, J. , Gong, F. , Han, Y. , Qiu, Y. , Wang, J. , Liu, Y. , Wei, Y. , Xia, J. , Yu, T. , Zhang, X. , & Zhang, L. I. (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet, 395(10223), 507–513. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, T. , Wu, D. , Chen, H. , Yan, W. , Yang, D. , Chen, G. , Ma, K. E. , Xu, D. , Yu, H. , Wang, H. , Wang, T. , Guo, W. , Chen, J. , Ding, C. , Zhang, X. , Huang, J. , Han, M. , Li, S. , Luo, X. , … Ning, Q. (2020). Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ, 368, m1091. 10.1136/bmj.m1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinese Society of Neurology, Chinese Stroke Society . (2018). Chinese guidelines for diagnosis and treatment of acute ischemic stroke 2018. Chinese Journal of Neurology, 51(9), 666–682. [Google Scholar]
- Corrales‐Medina, V. F. , Musher, D. M. , Shachkina, S. , & Chirinos, J. A. (2013). Acute pneumonia and the cardiovascular system. Lancet, 381(9865), 496–505. 10.1016/S0140-6736(12)61266-5 [DOI] [PubMed] [Google Scholar]
- Ding, Y. , He, L. , Zhang, Q. , Huang, Z. , Che, X. , Hou, J. , Wang, H. , Shen, H. , Qiu, L. , Li, Z. , Geng, J. , Cai, J. , Han, H. , Li, X. , Kang, W. , Weng, D. , Liang, P. , & Jiang, S. (2004). Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS‐CoV) in SARS patients: Implications for pathogenesis and virus transmission pathways. The Journal of Pathology, 203(2), 622–630. 10.1002/path.1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau, A. J. , Urbanek, C. , & Palm, F. (2010). Common infections and the risk of stroke. Nature Reviews. Neurology, 6(12), 681–694. 10.1038/nrneurol.2010.163 [DOI] [PubMed] [Google Scholar]
- Guan, T. , Ma, J. , Li, M. , Xue, T. , Lan, Z. , Guo, J. , Shen, Y. , Chao, B. , Tian, G. , Zhang, Q. , Wang, L. , & Liu, Y. (2017). Rapid transitions in the epidemiology of stroke and its risk factors in China from 2002 to 2013. Neurology, 89(1), 53–61. 10.1212/WNL.0000000000004056 [DOI] [PubMed] [Google Scholar]
- Guan, W.‐J. , Ni, Z.‐Y. , Hu, Y. U. , Liang, W.‐H. , Ou, C.‐Q. , He, J.‐X. , Liu, L. , Shan, H. , Lei, C.‐L. , Hui, D. S. C. , Du, B. , Li, L.‐J. , Zeng, G. , Yuen, K.‐Y. , Chen, R.‐C. , Tang, C.‐L. , Wang, T. , Chen, P.‐Y. , Xiang, J. , … Zhong, N.‐S. (2020). Clinical characteristics of Coronavirus Disease 2019 in China. New England Journal of Medicine, 382(18), 1708–1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilker, R. , Poetter, C. , Findeisen, N. , Sobesky, J. , Jacobs, A. , Neveling, M. , & Wolf‐Dieter, H. (2003). Nosocomial pneumonia after acute stroke: Implications for neurological intensive care medicine. Stroke, 34(4), 975–981. 10.1161/01.STR.0000063373.70993.CD [DOI] [PubMed] [Google Scholar]
- Lee, S. Y. , Khang, Y. H. , & Lim, H. K. (2019). Impact of the 2015 middle east respiratory syndrome outbreak on emergency care utilization and mortality in South Korea. Yonsei Medical Journal, 60(8), 796–803. 10.3349/ymj.2019.60.8.796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. W. , Du, X. B. , Chen, J. , Jin, Y. , Peng, L. I. , Wang, H. H. X. , Luo, M. , Chen, L. , & Zhao, Y. (2020). Neutrophil‐to‐lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID‐19. Journal of Infection, 81(1), e6–e12. 10.1016/j.jinf.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maaijwee, N. A. , Rutten‐Jacobs, L. C. , Schaapsmeerders, P. , van Dijk, E. J. , & de Leeuw, F. E. (2014). Ischaemic stroke in young adults: Risk factors and long‐term consequences. Nature Reviews. Neurology, 10(6), 315–325. 10.1038/nrneurol.2014.72 [DOI] [PubMed] [Google Scholar]
- Mao, L. , Jin, H. , Wang, M. , Hu, Y. U. , Chen, S. , He, Q. , Chang, J. , Hong, C. , Zhou, Y. , Wang, D. , Miao, X. , Li, Y. , & Hu, B. O. (2020). Neurologic manifestations of hospitalized patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurology, 77(6), 683. 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudit, G. Y. , Kassiri, Z. , Jiang, C. , Liu, P. P. , Poutanen, S. M. , Penninger, J. M. , & Butany, J. (2009). SARS‐coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. European Journal of Clinical Investigation, 39(7), 618–625. 10.1111/j.1365-2362.2009.02153.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyoo, O. , & Espinoza, L. R. (2005). Infection‐related vasculitis. Current Rheumatology Reports, 7(4), 281–287. 10.1007/s11926-005-0038-3 [DOI] [PubMed] [Google Scholar]
- Pagliano, P. , Spera, A. M. , Ascione, T. , & Esposito, S. (2020). Infections causing stroke or stroke‐like syndromes. Infection, 10.1007/s15010-020-01415-6 [DOI] [PubMed] [Google Scholar]
- Qin, C. , Zhou, L. , Hu, Z. , Zhang, S. , Sheng Yang, Y. U. , Tao, C. X. , Ma, K. E. , Shang, K. E. , Wang, W. , & Tian, D.‐S. (2020). Dysregulation of immune response in patients with COVID‐19 in Wuhan, China. Clinical Infectious Diseases, 71(15), 762–768. 10.1093/cid/ciaa248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quyet, D. , Hien, N. M. , Khan, M. X. , Pham, D. D. , Thuan, D. D. , Dang, D. M. , Hai, N. D. , Nam, B. V. , Huy, P. Q. , Duy Mai, T. , Truong, D. T. , Nga, V. T. , & Dang Phuc, D. (2019). Risk factors for stroke associated pneumonia. Open Access Macedonian Journal of Medical Sciences, 7(24), 4416–4419. 10.3889/oamjms.2019.873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui, R. , & Zhang, L. (2011). Risk factors of stroke‐associated pneumonia in Chinese patients. Neurological Research, 33(5), 508–513. 10.1179/016164111X13007856084205 [DOI] [PubMed] [Google Scholar]
- Umapathi, T. , Kor, A. C. , Venketasubramanian, N. , Lim, C. C. T. , Pang, B. C. , Yeo, T. T. , Lee, C. C. , Lim, P. L. , Ponnudurai, K. , Chuah, K. L. , Tan, P. H. , Tai, D. Y. H. , & Ang, S. P. B. (2004). Large artery ischaemic stroke in severe acute respiratory syndrome (SARS). Journal of Neurology, 251(10), 1227–1231. 10.1007/s00415-004-0519-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W. , Jiang, B. , Sun, H. , Ru, X. , Sun, D. , Wang, L. , Wang, L. , Jiang, Y. , Li, Y. , Wang, Y. , Chen, Z. , Wu, S. , Zhang, Y. , Wang, D. , Wang, Y. , & Feigin, V. L. (2017). Prevalence, incidence, and mortality of stroke in China: Results from a nationwide population‐based survey of 480 687 adults. Circulation, 135(8), 759–771. 10.1161/CIRCULATIONAHA.116.025250 [DOI] [PubMed] [Google Scholar]
- World HealthOrganization . (2020). Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected, Interim guidance. https://www.who.int/publications‐detail/clinical‐management‐of‐severe‐acute‐respiratory‐infection‐when‐novel‐coronavirus‐(ncov)‐infection‐is‐suspected
- Wu, S. , Wu, B. , Liu, M. , Chen, Z. , Wang, W. , Anderson, C. S. , Sandercock, P. , Wang, Y. , Huang, Y. , Cui, L. , Pu, C. , Jia, J. , Zhang, T. , Liu, X. , Zhang, S. , Xie, P. , Fan, D. , Ji, X. , Wong, K.‐S. , … Zhang, S. (2019). Stroke in China: Advances and challenges in epidemiology, prevention, and management. The Lancet Neurology, 18(4), 394–405. 10.1016/S1474-4422(18)30500-3 [DOI] [PubMed] [Google Scholar]
- Yeh, S. J. , Huang, K. Y. , Wang, T. G. , Chen, Y.‐C. , Chen, C.‐H. , Tang, S.‐C. , Tsai, L.‐K. , Yip, P.‐K. , & Jeng, J.‐S. (2011). Dysphagia screening decreases pneumonia in acute stroke patients admitted to the stroke intensive care unit. Journal of the Neurological Sciences, 306(1–2), 38–41. 10.1016/j.jns.2011.04.001 [DOI] [PubMed] [Google Scholar]
- Zhu, N. , Zhang, D. , Wang, W. , Li, X. , Yang, B. O. , Song, J. , Zhao, X. , Huang, B. , Shi, W. , Lu, R. , Niu, P. , Zhan, F. , Ma, X. , Wang, D. , Xu, W. , Wu, G. , Gao, G. F. , & Tan, W. (2020). A novel coronavirus from patients with pneumonia in China, 2019. New England Journal of Medicine, 382(8), 727–733. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets used and analysed during the current study are available from the corresponding author on reasonable request.


