Abstract
The whole world is entangled by the coronavirus disease (COVID‐19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), people are dying in thousands each day, and without an actual medication, it seems not possible for the bringing this global health crisis to a stop. Natural products have been in constant use since ancient times and are proven by time to be effective. Crude extract or pure compounds isolated from medicinal plants and/or herbs such as Artemisia annua, Agastache rugosa, Astragalus membranaceus, Cassia alata, Ecklonia cava, Gymnema sylvestre, Glycyrrhizae uralensis, Houttuynia cordata, Lindera aggregata, Lycoris radiata, Mollugo cerviana, Polygonum multiflorum, Pyrrosia lingua, Saposhnikoviae divaricate, Tinospora cordifolia etc. have shown promising inhibitory effect against coronavirus. Several molecules, including acacetin, amentoflavone, allicin, blancoxanthone, curcumin, daidzein, diosmin, epigallocatechin‐gallate, emodin, hesperidin, herbacetin, hirsutenone, iguesterin, jubanine G, kaempferol, lycorine, pectolinarin, phloroeckol, silvestrol, tanshinone I, taxifolin, rhoifolin, xanthoangelol E, zingerol etc. isolated from plants could also be potential drug candidates against COVID‐19. Moreover, these could also show promising inhibitory effects against influenza‐parainfluenza viruses, respiratory syncytial virus, severe acute respiratory syndrome (SARS), and Middle East respiratory syndrome coronavirus (MERS‐CoV). Here, we have reported 93 antiviral drug candidates which could be a potential area of research in drug discovery.
Keywords: anti‐antiviral activity and COVID‐19, drug candidates, natural products
Abbreviations
- ACE‐2
Angiotensin‐converting enzyme‐2
- ADV
Adenovirus
- AIDS
Acquired immune deficiency syndrome
- CHIKV
Chikungunya virus
- COVID‐19
Coronavirus disease 2019
- CPE
Cytopathic effects
- DENV‐1
Dengue virus serotype 1
- FACS
Fluorescence‐activated cell sorting
- HBV
Hepatitis‐B virus
- HCMV
Human cytomegalovirus
- HCV
Hepatitis‐C virus
- HIV
Human immunodeficiency virus
- HSV
Herpes simplex virus
- IVC
in vitro compartmentalization
- MERS
Middle East respiratory syndrome
- MERS‐CoVs
Middle East respiratory syndrome coronavirus
- RBD
Receptor binding domain
- RdRP
RNA‐dependent RNA polymerase
- RNA
Ribonucleic acid
- RSV
Respiratory syncytial virus
- SARS‐CoVs
Severe acute respiratory syndrome coronaviruses
- SI
Selectivity index
- uHTS
ultra‐High‐throughput screening
1. INTRODUCTION
Coronaviruses are the infectious agents of respiratory tract infections but can also affect the digestive tract and systemically infect both humans and animals (Malik et al., 2020). Coronaviruses are single‐stranded RNA viruses, which are highly diverse and first reported in 1960. Primarily, Coronavirus strains reside in bats and wild birds and can spread to other living beings. The first case of COVID‐19 was reported in Wuhan, China, on December 31, 2019, sometime later, Thailand reported the first case of the disease outside of Mainland China on January 13, 2020 (Hui et al., 2020). The virus that causes COVID‐19 is believed to have originated in bats and then spread to humans, possibly through snakes and pangolins, perhaps by contamination of meat from wild animals and seafood, in meat and seafood markets in China. At present, about 210 countries and territories around the world are affected by COVID‐19. Notably, China, the USA, the UK, Spain, France, and Italy are the most severed because of COVID‐19. As of July 15, 2020, this pandemic has claimed the lives of 586,194 along with 13,683,743 confirmed cases (John Hopkins University, Coronavirus Resource Center). Common symptoms of COVID‐19 include fever, cough, shortness of breath, and diarrhea, but in severe cases, it may cause pneumonia and/or even death (WHO, 2020a). The incubation period maybe 14 days or even longer; the disease may still be infectious up to this latent period and can spread from one person to another via respiratory droplets, close contact, and also via the fomites (CDC, 2020; WHO, 2020b).
SARS‐CoV‐2 is a positive‐sense, single‐stranded RNA beta‐coronavirus with ~28,882 base pairs genome that encodes viral proteins in up to 14 open reading frames (Orfs) (Malik et al., 2020). There are four major viral structural proteins: Spike (S), Envelope (E), Membrane (M), and Nucleocapsid (N). Structural proteins of SARS‐CoV‐2 form the viral capsid that encapsulates the genome and also facilitates the entry process to human cells through the ACE‐2 receptor (Figure 1S in Data S1). Newly emerging viral borne infectious diseases have defied even the survival of humans and other living creatures. Usually, viral diseases are difficult to control and have a more comprehensive public health impact, so viral pathogens like HIV, human influenza, SARS, chikungunya, Ebola, Zika have received more attention and have threatened modern healthcare and pharmaceutical sectors. Many infections and abnormal physiology caused by several viruses belonging to coronavirideae family been reported (Table 1S in Data S1).
Till now, there are no absolute therapies for COVID‐19, but preventive and supportive therapies are employed to control further complications and organ damage (Rodríguez‐Morales, MacGregor, Kanagarajah, Patel, & Schlagenhauf, 2020). Based on the information available key regions of new drug targets are RNA‐dependent RNA polymerase (RdRP) of the virus, cell membrane receptors of SARS‐CoV‐2 (ACE‐2), and spike proteins (S protein), the antiviral mechanism of plant extracts varies as per the structure and replication process of the viruses. Some plants help to increase the inherent antiviral immunity of our body (Webster, Taschereau, Lee, & Jurgens, 2006). Some of the studies have already proven that plant extracts can be used for the preparation of peptides/proteins with the medicinal value, which can be a good source of vaccines and protein/peptides‐based treatment. However, screening of new therapeutic products will take an extended period, and medicinal plants could be an alternative, which is already tested by various ethnic groups since the ancient period. It is believed that medicinal plants are the primary source of healthcare for nearly 85% of the global population (Pešić, 2015), and more than 40% of synthetic drugs available in pharmaceutical markets are derived from plants and microbial‐based natural products (Bauer & Brönstrup, 2014). In order to find the potent antiviral drug of the COVID‐19, research should be directed to emulsion‐based uHTS as well for the screening of hundreds of natural products. Thus, this review is focused on summarizing medicinal plants and/or herbs showing antiviral properties, which could be useful in drug discovery programs.
2. CURRENT TREATMENT APPROACHES FOR VIRAL INFECTIONS
The era of antiviral drug development was begun after the approval of idoxuridine in June 1963, which was the first anti‐herpes antiviral drug that inhibits viral DNA synthesis (De Clercq, 2004). Since then, more than 90 drugs of different functional groups have been authorized for the therapeutic purpose of viral diseases, including HIV, HBV, HCV, and influenza virus infections (De Clercq & Li, 2016). On the other hand, increasing antiviral drug resistance (ADR) like in herpesviruses and HBV is a pressing concern, and ADR could be managed by understanding the mechanism of resistance pattern, formulation of new drugs, optimization of compound delivery, and host components (Manuscript & Implications, ). High costs and adverse side effects of synthetic drugs, along with the emergence of ADR has demanded the safe and novel antiviral drugs. Herbal extracts have become an exceptional alternative for the formulation of antiviral drugs that can inhibit multiple steps of the virus replication cycle. Furthermore, plant‐based antiviral compounds have shown remarkable results even in multiple clinical trials; for instance, anthocyanins (Pour, Fakhri, Asgary, Farzaei, & Echeverría, 2019).
Medicinal plants were investigated for antiviral activity against the different human viruses like HIV, HSV, rabies virus, SARS, MERS, poxvirus, dengue virus, and influenza viruses (Table 2S in Data S1). Nepeta nepetella (150–500 μg/ml), Nepeta coerulea (150–500 μg/ml), Nepeta tuberosa (150–500 μg/ml), Dittrichia viscosa (50–125 μg/ml), and Sanguisorba minor magnolii (50–125 μg/ml), showed an explicit antiviral activity against two different DNA and RNA viruses, that is, HSV‐1 and vesicular stomatitis virus (VSV) (Abad, Guerra, Bermejo, Irurzun, & Carrasco, 2000). Eleutherococcus senticosus roots extract inhibited the replication of RNA viruses such as human rotavirus, RSV, and influenza A virus, but failed to inhibit DNA viruses such as adenovirus and HSV (Glatthaar‐Saalmüller, Sacher, & Esperester, 2001). Azardirachta indica inhibited HSV‐1 infection by blocking the entry of the virus to the receptor (glycoprotein) (Tiwari, Darmani, Yue, & Shukla, 2010). The extracts of Cotoneaster integrifolius and Clinopodium umbrosum, and of Abies spectabilis and Valeriana jatamansi showed moderate activity (IC50 = 16–20 μg/ml) against HSV‐1 and H1N1, respectively (Rajbhandari, Wegner, Jülich, Schöpke, & Mentel, 2001). Macaranga barteri leaves extract showed antiviral activity with IC50 of 0.028 and 0.00017 ng/ml against echovirus E7 and E19, respectively (Ogbole, Akinleye, Segun, Faleye, & Adeniji, 2018). Similarly, Ampelocissus tomentosa (EC50 = 7.79 μg/ml), Kalanchoe pinnata (EC50 = 6.1 μg/ml) and Paris polyphylla (EC50 = 8.74 μg/ml) were active against CHIKV while Clerodendrum serratum (EC50 = 15.9 μg/ml) and Terminalia chebula (EC50 = 10.6 μg/ml) were active against yellow fever virus and enterovirus A71, respectively (Joshi et al., 2020). Amomurn villosum Lour, Melaphis chinensis (Bell) Baker, Sanguisorba officinalis, and Flos caryophylli extracts showed potent inhibitory activity against Neuraminidase (NA) with IC50 ranging from 4.1 to 9. 6 μg/ml (J. Liu et al., 2018).
Gymnema sylvestre R. Br., Pergularia daemia (Forsskal) Chiov., Cassia alata L., Sphaeranthus indicus L., Evolvulus alsinoides L., Clitoria ternatea L., Indigofera tinctoria L., Vitex trifolia L., Clerodendrum inerme (L.) Gaertn, Abutilon indicum G. Don., and Leucas aspera Spr. exhibited anti‐mouse coronavirus (MCV, human SARS surrogate) and anti‐HSV activity at a concentration of 0.4 μg/ml (Vimalanathan, Ignacimuthu, & Hudson, 2009). Furthermore, the extract of Cistus incanus showed antiviral activity against HIV and filoviruses by targeting the glycoproteins of the virus envelope resulting in blockage of attachment to receptors (Rebensburg et al., 2016). Hence, the chemical investigation and mechanistic study on the antiviral activities of the plants are needed to be carried out.
Extracts from medicinal plants such as Dioscorea batatas, Glycyrrhiza radix, Mollugo cerviana, Polygonum multiflorum Thunb., Psoralea corylifolia, Rheum officinale Baill., Salvia miltiorrhiza, and Trichosanthes cucumerina L. were shown active against coronavirus (Alagu Lakshmi, Shafreen, Priya, & Shunmugiah, 2020; Boukhatem & Setzer, 2020; Ho, Wu, Chen, Li, & Hsiang, 2007). In a study, herbal extracts of plants like Tinospora cordifolia have already been urged to be used for the patients infected by SARS‐CoV‐2 (Li et al., 2005). In a study reported by Li et al. (2005), herbal extracts obtained from four different plants Artemisia annua, Lindera aggregata, Pyrrosia lingua, and Lycoris radiata have shown remarkable antiviral activity in a cell‐based stay against the SARS‐CoV where the alkaloid, lycorine (1) (Figure 2a) from L. radiata has shown effective inhibitory activity to stop the infection caused by SARS‐CoV on HepG2 and Vero E6 cells culture (Li et al., 2005). Similarly, plant‐based phenolic compounds have shown anti‐SARS‐CoV activity in the cell‐based study (C.‐W. Lin et al., 2005). Another study reported by Lau et al. (2008) has shown that the extract of Houttuynia cordata is safe and effective for the treatment of pneumonia caused by SARS‐CoV (Lau et al., 2008).
FIGURE 2.
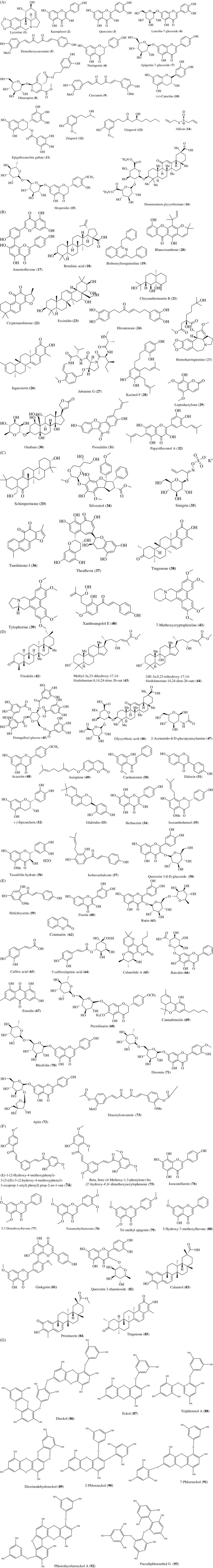
(a) Natural compounds isolated from medicinal plants for antiviral activity: Lycorine (1), Kaempferol (2), Quercetin (3), Luteolin‐7‐glucoside (4), Demethoxycurcumin (5), Naringenin (6), Apigenin‐7‐glucoside (7), Oleuropein (8), Curcumin (9), Catechin (10), Epigallocatechin‐gallate (11), Zingerol (12), Gingerol (13), Allicin (14), Hesperidin (15),Diammonium glycyrrhizinate (16). (b) Natural compounds isolated from medicinal plants for antiviral activity: Amentoflavone (17), Betulinic acid (18), Bisbenzylisoquinoline (19), Blancoxanthone (20), Chrysanthemumin B (21), Cryptotanshinone (22), Escinidin (23), Hirsutenone (24), Homoharringtonine (25), Iguesterin (26), Jubanine G (27), Kazinol F (28), Leptodactylone (29), Ouabain (30), Psoralidin (31), Papyriflavonol A (32). (c) Natural compounds isolated from medicinal plants for antiviral activity: Schimperinone (33), Silvestrol (34), Sinigrin (35), Tanshinone I (36), Theaflavin (37), Tingenone (38), Tylophorine (39), Xanthoangelol E (40), 7‐Methoxycryptopleurine (41). (d) Natural compounds isolated from medicinal plants for antiviral activity: Friedeline (42), Methyl‐3α,23‐dihydroxy‐17,14‐friedolanstan‐8,14,24‐trien‐26‐oat (43), 24E‐3a,9,23‐trihydroxy‐17,14‐ friedolanostan‐14,24‐dien‐26‐oate (44), Pentagalloylglucose (PGG) (45), Glycyrrhizic acid (46), 2‐Acetamido‐β‐D‐glucopyansylamine (47), Acacetin (48), Auraptene (49), Cardamonin (50), Daidzein (51), Epicatechin (52), Glabridin (53), Herbacetin (54), Isoxanthohumol (55), Taxifolin hydrate (56), Isobavachalcone (57), Quercetin 3‐β‐D‐glucoside (58). (e) Natural compounds isolated from medicinal plants for antiviral activity: Helichrysetin (59), Fisetin (60), Rutin (61), Coumarin (62), Caffeic acid (63), 5‐Caffeoylquinic acid (64), Calanolide A (65), Baicalin (66), Emodin (67), Pectolinarin (68), Cannabinoids (69), Rhoifolin (70), Diosmin (71), Apiin (72), Diacetylcurcumin (73). (f) Natural compounds isolated from medicinal plants for antiviral activity: (E)‐1‐(2‐Hydroxy‐4‐methoxyphenyl)‐3‐[3‐[(E)‐3‐(2‐hydroxy‐4‐methoxyphenyl)‐3‐oxoprop‐1‐enyl]phenyl]prop‐2‐en‐1‐one (74), beta,beta′‐(4‐Methoxy‐1,3‐phenylene)bis(2′‐hydroxy‐4′,6′‐dimethoxyacrylophenone (75), Isoscutellarein (76), 5,7‐Dimethoxyflavone (77), Tetramethyllueteonin (78), Tri methyl apigenin (79),5‐Hydroxy‐7‐methoxyflavone (80), Ginkgetin (81), Quercetin 3‐rhamnoside (82), Celastrol (83), Pristimerin (84), Tingenone (85). (g) Natural compounds isolated from medicinal plants for antiviral activity: Dieckol (86), Eckol (87), Triphloretol A (88), Dioxinodehydroeckol (89), 2‐Phloroeckol (90), 7‐Phloroeckol (91), Phlorofucofuroeckol A (92), Fucodiphloroethol G (93)
In another research, Khaerunnisa, Kurniawan, Awaluddin, Suhartati, and Soetjipto (2020) investigated several herbal compounds, which are potent inhibitor of COVID‐19 Mpro (main protease) like kaempferol (2), quercetin (3), luteolin‐7‐glucoside (4), demethoxycurcumin (5), naringenin (6), apigenin‐7‐glucoside (7), oleuropein (8), curcumin (9) catechin (10), epigallocatechin‐gallate (11), zingerol (12), gingerol (13), and allicin (14) (Figure 2a) isolated from some flavonoids of medicinal plants (Khaerunnisa et al., 2020). Utomo, Ikawati, and Meiyanto (2020) shows the likely inhibitory results of Curcuma and Citrus spp. on the replication and progress of the infection, so hesperidin (15) from these species may be used for the development of anti‐SARS‐CoV‐2 drugs as a treatment regime of COVID‐19 (Utomo et al., 2020). Diammonium glycyrrhizinate (16) (Figure 2a), a herbal compound of the sweet root, has been used to constrain the severe symptoms of COVID‐19 while this compound is in clinical use for a long time for the treatment of coughs, digestive problems, and liver infection caused by HBV.
Nevertheless, natural products like amentoflavone (17), betulinic acid (18), bisbenzylisoquinoline (19), blancoxanthone (20), chrysanthemumin B (21), cryptotanshinone (22), escinidin (23), hirsutenone (24), homoharringtonine (25), iguesterin (26), jubanine G (27), kazinol F (28), leptodactylone (29), ouabain (30), psoralidin (31), papyriflavonol A (32), schimperinone (33), silvestrol (34), sinigrin (35), tanshinone I (36), theaflavin (37), tingenone (38), tylophorine (39), xanthoangelol E (40), and 7‐methoxycryptopleurine (41) (Figure 2b,c), with antiviral activity, have been reviewed already as potential drugs candidates against SARS‐CoV‐2 (Islam et al., 2020; Orhan & Senol Deniz, 2020; Xian et al., 2020). In addition to these, different antiviral potent compounds against human viruses have been reviewed (Table 3S, Figure 2Sa,b in Data S1). Hence, the screening and identification of potential anti‐antiviral and anti‐infectious particles/compounds from medicinal plants/herbs extracts are in global demand.
3. ANTIVIRAL DRUG CANDIDATES FROM NATURAL PRODUCTS
3.1. Mechanism
Polyphenols, flavonoids, proanthocyanidins, saponins, monoterpenoids, triterpenoids, glucosides, sesquiterpenes, and alkaloids isolated from various medicinal plants have diverse antiviral activities of which some are listed in Table 1. Antiviral drugs interact with haemagglutinin, which interferes with the entry of the virus. Neuraminidase, an essential glycoprotein required for viral amplification (proliferation and infections), catalyzes the cleavage of α‐(2,3) or α‐(2,6) ketosidic linkage between sialic acid and oligosaccharide of glycoproteins such that viruses release from infectious cells (Muchtaridi, Sugijanto, Mohd Gazzali, & Wahab, 2020). Neuraminidase inhibitors (NAIs) can block the viral enzyme neuraminidase, which prevents the release of viruses from host cells (Liao, Kowal, Cardenas, & Beauchemin, 2017). There is a strong matching between Query sequence H1N1 neurominidase and SARS‐CoV‐2 Orf 3a. Friedeline (42), methyl‐3α,23‐dihydroxy‐17,14‐friedolanstan‐8,14,24‐trien‐26‐oat (43), 24E‐3a,9,23‐trihydroxy‐17,14‐ friedolanostan‐14,24‐dien‐26‐oate (44) (Figure 2d), and catechin (10) (Figure 2d) isolated from Garcinia celebica L. leaves extract areneuraminidase inhibitors, which bind to active amino acid residues of neuraminidase by hydrogen bonds and van der Waals force and interfere virus release from host cells (Muchtaridi et al., 2020).
TABLE 1.
Possible antiviral drug candidates from natural products
| Plant sources | Isolated molecules | Active against virus | References |
|---|---|---|---|
| Scutellaria baicalensis Georgi | Isoscutellarein (76) | Influenza virus | (Nagai, Miyaichi, Tomimori, Suzuki, & Yamada, 1992) |
| Kaempferia parviflora Wall. ex Baker |
5,7‐Dimethoxyflavone (77), Tetramethyllueteonin (78), Tri methyl apigenin (79), 5‐Hydroxy‐7‐methoxyflavone (80), |
Avian influenza virus (H5N1) | (Sornpet, Potha, Tragoolpua, & Pringproa, 2017) |
| Curcuma longa L. | Curcumin(diferuloylmethane) (9) | H5N1 | (Sornpet et al., 2017) |
| Ginkgo biloba L., Cephalotaxus harringtonia K. | Ginkgetin (81) | Influenza virus | (Miki et al., 2007) |
| Houttuynia cordata Thunb. | Quercetin 3‐rhamnoside (82) | Influenza virus | (Choi, Song, Park, & Kwon, 2009) |
| Tripterygium regelii Sprague &Takeda |
Celastrol (83), Pristimerin (84), Tingenone (85), Iguesterin (26) |
SARS‐CoV | (Ryu et al., 2010) |
| Ecklonia cava |
Dieckol (86), Eckol (87), Triphloretol A (88), Dioxinodehydroeckol (89), 2‐Phloroeckol (90), 7‐Phloroeckol (91), Phlorofucofuroeckol A (92), Fucodiphloroethol G (93) |
SARS‐CoV | (Park et al., 2013) |
Note: Catechin (10): immunostimulatory and antimitotic inhibitors; Coumarin (9): Protease Inhibitors For chemical structures of compounds refer to Figure 2f,g.
A polyphenol, pentagalloylglucose (PGG) (45) (Figure 2d) demonstrated anti‐influenza virus activity by reducing virus release, site of virus assembly, and budding (microvillus) and inhibiting hemagglutination induced by the virus (G. Liu et al., 2011). Amplification of the HCV was inhibited by non‐nucleoside inhibitors (benzimidazole scaffold) through inhibition of RdRP, and its mechanism of action was studied (Tomei et al., 2003).
Glycyrrhizic acid (GL) (46) (Figure 2d) inhibited Epstein–Barr virus (EBV) replication with an IC50 value of 0.004 mM by inhibiting viral DNA polymerase GL showed antiviral activity against SARS‐CoVs in Vero cells with a SI of 67 by reducing replication and inhibiting penetration and adsorption of the virus (Cinatl et al., 2003). 2‐Acetamido‐β‐D‐glucopyansylamine (47) (Figure 2d) suppressed the expression of viral antigen (>99%) with an inhibition of 40 μM toward SARS‐CoV replication (Hoever et al., 2005). Pyridine‐N‐oxide derivatives were found active against HIV‐1, HIV‐2, HCMV, and also inhibited SARS‐CoVs by targeting the transcription level of the virus cycle (Balzarini et al., 2006).
Acacetin (48), auraptene (49), cardamonin (50), daidzein (51), epicatechin (52), glabridin (53), herbacetin (54), isoxanthohumol (55), and taxifolin hydrate (56) (Figure 2d) showed antiviral activity by suppressing SARS‐CoV 3C protease (3Cpro) (Jo, Kim, Shin, & Kim, 2020) while herbacetin (54), isobavachalcone (57), quercetin 3‐β‐D‐glucoside (58) (Figure 2d), and helichrysetin (59) inhibited MERS‐CoV 3Cpro activity (Jo, Kim, Kim, Shin, & Kim, 2019).
Different compounds possess antiviral activity by effecting or targeting different stages of their replication life cycle, that is, adsorption, penetration, uncoating, transcription, replication, assembly, and release (De Clercq, 1982).
Antiviral drugs undergo action by (a) acting as blocking agent during adsorption and penetration of virus on receptors; (b) inactivation of virus particles; (c) altering properties of virus membrane; (d) inhibition of viral enzymes (DNA polymerase, RNA polymerase, deoxypyrimidine nucleoside kinase, and thymidine kinase, viral neuraminidase, mRNA guanylyltransferase, and mRNA Methyltransferase); (e) inhibiting reverse transcriptase; (f) inhibiting translational process; (g) decrease in expression level; (h) inhibiting viral assembly; (i) reduced endocytic activity (Calland, Dubuisson, Rouillé, & Séron, 2012; De Clercq, 1982; Ichikawa & Matsuda, 2007).
3.2. Inhibition of viral protease
Protease inhibitors alter or deactivate the configuration of protease involved in the proteolytic activity (events) associated with diseases like AIDS, cancer, thrombosis, hepatitis, and cirrhosis (Mishra, Reddy & Prasad, 2020). Fisetin (60) and rutin (61) (Figure 2e) act as a 3C protease (3Cpro) inhibitors against enterovirus A71 with IC50 of 85 and 110 μM, respectively (Y.‐J. Lin et al., 2012). Some drugs from the primary investigations that are used for COVID‐19 are protease inhibitor lopinavir/ritonavir, nucleoside analogs, neuraminidase inhibitors, remdesivir, umifenovir (arbidol), tenofovir disoproxil (TDF), lamivudine (3TC) and hydroxychloroquine, which blocks virus entry and endocytosis (Lu, 2020) (Figure 1).
FIGURE 1.
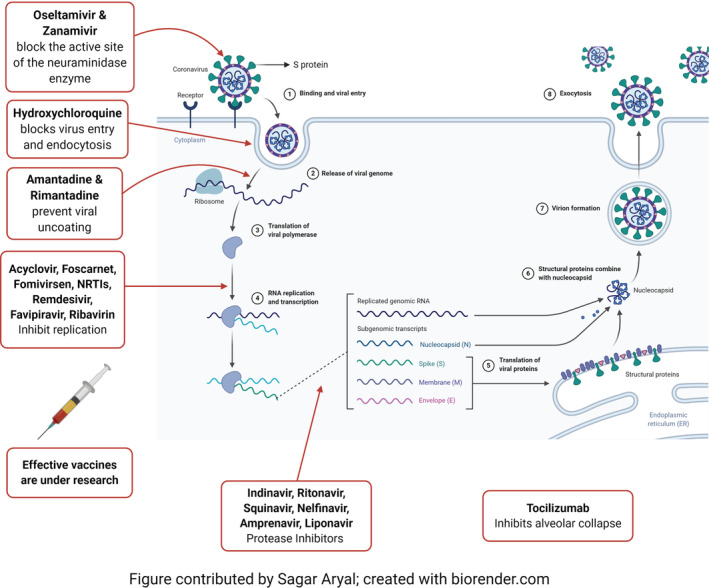
Multliplication stages of virus in a living cell and major antiviral drugs with their mode of action http://biorender.com [Colour figure can be viewed at wileyonlinelibrary.com]
A protease inhibitor, lopinavir/ritonavir, which is commonly used to treat HIV/AIDS patients, could be used for the treatment of COVID‐19‐infected patients. Coumarin (62) (Figure 2e) has a broad spectrum of antiviral activity against hepatitis virus, dengue virus, chikungunya virus, and influenza virus by inhibiting viral proteins required for their entry, replication, and infection, and/or by regulation of cellular and antioxidative pathways (Mishra, Pandey & Manvati, 2020). Caffeic acid (63) and 5‐caffeoylquinic acid (64) (Figure 2e) from Achyrocline satureioides inhibit the protein HIV integrase essential for viral DNA integration into host chromatin (Khaerunnisa et al., 2020; Robinson, Reinecke, Abdel‐Malek, Jia, & Chow, 1996).
3.3. Inhibition of viral replication steps
Coronavirus genome encodes a protein called RNA‐dependent RNA polymerase (RdRP), which allows the viral genome to be transcribed into new RNA copies using the host cell's machinery. Thus, inhibition of RdRP could be a potential target for the control of viral infection. Epigallocatechin‐3‐gallate (11), calanolide A (65), and baicalin (66) (Figure 2e) inhibit virus replication (Kitazato, Wang, & Kobayashi, 2007). Many other nucleoside analogs, including DNA synthesis inhibitors such as tenofovir, disoproxil, lamivudine, and similar other antiviral agents, have the potential to inhibit the SARS‐CoV‐2 multiplication. They are being evaluated through molecular docking studies and testing in laboratory culture infected cells (Khaerunnisa et al., 2020).
3.4. Inhibition of viral spike protein and receptor
Spike glycoprotein of SARS‐CoV‐2 contains a receptor‐binding domain (RBD) that recognizes the target receptor. The receptor angiotensin‐converting enzyme‐2 (ACE‐2) is a preferable receptor for SARS‐CoV‐2 (Peng et al., 2020). Furin blocks a spike protein of mouse hepatitis coronavirus required for attachment and fusion during infection (Bosch, van der Zee, de Haan, & Rottier, 2003; de Haan, Stadler, Godeke, Bosch, & Rottier, 2004). Emodin (67) (Figure 2e) inhibits the spike protein and ACE‐2 interaction preventing coronavirus entry (Ho et al., 2007). Secondary metabolites like hesperidin (15), pectolinarin (68), cannabinoids (69), rhoifolin (70), diosmin (71), apiin (72), diacetylcurcumin (73) (Figure 2e), epigallocatechin gallate (11), (E)‐1‐(2‐Hydroxy‐4‐methoxyphenyl)‐3‐[3‐[(E)‐3‐(2‐hydroxy‐4‐methoxyphenyl)‐3‐oxoprop‐1‐enyl]phenyl]prop‐2‐en‐1‐one (74), and beta,beta′‐(4‐Methoxy‐1,3‐phenylene)bis(2′‐hydroxy‐4′,6′‐dimethoxyacrylophenone (75) (Figure 2f) from medicinal plants are bioactive against SARS‐CoV‐2 main protease and spike glycoprotein so, it is necessary to screen all the available antiviral drugs against COVID‐19 for faster development of therapeutic option to control the pandemic (Adem, Eyupoglu, Sarfraz, Rasul, & Ali, 2020; Peng et al., 2020).
4. SCREENING OF MEDICINAL PLANTS
There are various methods available for in vivo and in vitro screening of extracts of medicinal plants. Notably, this review focused on a little known method in the area of medicinal chemistry, which is mostly known as emulsion‐based in vitro compartmentalization (IVC) technique (Gianella, Snapp, & Levy, 2016). For this purpose, a “water‐in‐oil‐in‐water” emulsion is used in the compartmentalization of droplets for ultrahigh throughput screening (uHTS). Usually, IVC uses aqueous droplets that contain DNA and machinery for protein production within water‐in‐oil emulsions (Rothe, Surjadi, & Power, 2006). In the modified protocol, template viral DNA tagged with a Green fluorescent protein (GFP) could be compartmentalized along with RdRP and plant extracts (or compounds). If RdRP is inhibited due to plant extracts and/or pure compounds, fluorescence switches off, and target droplets could be sorted using fluorescence‐activated cell sorting (FACS) (Bernath et al., 2004). Reprogramming of similar techniques could be explored to study the inhibition of viral protease. Different cell cultures assays, chips‐based methods, animal models, and enzyme inhibition assays have been carried to find potent antiviral compounds from natural sources (Anani et al., 2000; Lloyd, Russell, Blanes, Doble, & Roux, 2013; Mukhtar et al., 2008; Ogbole, Segun, & Adeniji, 2017). Additionally, for a couple of decades, the analysis, process, and development of herbal ingredients have dramatically increased with innovation in laboratory techniques and instrumentation (Fitzgerald, Heinrich, & Booker, 2020).
5. FUTURE PERSPECTIVES
Several synthetic drugs are showing inefficiency for the treatment of patients suffering from SARS‐CoV‐2, so herbal medicines have become a promising option to tackle the ongoing pandemic caused by COVID‐19. Some of the Chinese plant extracts have already been examined for the treatment of COVID‐19 patients in the clinical setting and have shown effective outcomes for the palliation of symptoms (Luo et al., 2020). Prescription of typical herbs at the time of viral infections is in practice in India for the optimum immunity and better physiological status of the patients (Kim et al., 2008). Use of diammonium glycyrrhizinate with vitamin C for the treatment of COVID‐19 associated infections has been approved via a randomized, controlled, and open clinical trial in Zhongnan Hospital of Wuhan University, Hubei, China where safety and efficiency of diammonium glycyrrhizinate with vitamin C for the treatment of COVID‐19 infected patients was investigated but clinical use has not commenced yet. Except earmarking on viral protease, replication, spike protein, and receptor, its protein kinase C and helicase have also been established as potential targets to inhibit SARS‐CoV (Hoever et al., 2005; Jang et al., 2020). Glycyrrhizin has been revealed as a potential inhibitor of SARS CoV protein kinase C and similarly, myricetin and scutellarein as aptitude inhibitor of viral helicase (Xian et al., 2020). A large number of Chinese herbal‐based medicines were used in China for the treatment of patients infected by COVID‐19. The National Health Commission (NHC) of China has recommended a couple of plants originated medicines for the treatment of SARS‐CoV‐2 patients. China used a similar treatment strategy even in 2003 when the SARS‐CoV outbreak was reported. Chinese experts claim that herbal drugs such as Radix isatidis granula, and Huoxiang Zhengqi soft capsules have an excellent function to improve problems like weakness, coughs, anxiety, and digestive disorder. Plant‐based medicines were also deployed in the earlier two outbreaks of coronavirus, which were SARS‐CoV of 2003 and MERS‐CoV of 2012 (“Redeploying Plant Defences,” 2020).
Herbal‐based antiviral medicines have shown promising inhibitory effects, which range from anti‐influenza to anti‐dengue properties (Table 1). In the context of the global burden of COVID‐19, where an absolute medicine is waiting for patients, promptly available plant‐based medicines with studied safety and efficacy can be on the frontline to tackle the ongoing calamity caused by COVID‐19. The problems encountered while using bioactive secondary metabolites like their solubility, stability, and bioavailability need to be addressed to use these metabolites as medicine (Coimbra et al., 2011). Moreover, the application of artificial intelligence tools like molecular docking studies, toxicity analysis, and pharmacological studies would further add significant information regarding potency of the aforementioned secondary metabolites. However, the mutation occurring in targets should be taken into account. Extensive studies and investments are demanding for new drug development strategies using herbal extracts to save sapiens lives on our planet from pandemics like COVID‐19, today, and tomorrow.
6. CONCLUSIONS
There are barely any countries that are left untouched by the COVID‐19 pandemic. It is one of the greatest public health threats to date that needs to be controlled as soon as possible. Still today, there are no vaccines or drugs that have been clinically proven against COVID‐19. Many secondary metabolites with antiviral properties have been isolated from medicinal plants. Various researches have been carried out throughout the world to develop antiviral drugs effective against SARS‐CoV‐2 responsible for COVID‐19. The best way of preventing COVID‐19 infections could be finding the compounds responsible for altering or disturbing any steps of the virus replication cycle. Natural products capable of inhibiting or altering the configuration of structural proteins (spike glycoprotein), non‐structural proteins (3‐chymotrypsin‐like protease, papain‐like protease, helicase, and RdRP) and accessory proteins coded by SARS‐CoV‐2 genome are needed to be explored. Usually, natural products could be a safe, secure, and dependable source to find drugs responsible for controlling the current pandemic. Protease and RNA polymerase inhibitors known to be active against SARS and MERS are also required to be explored. Different compounds (flavonoids, polyphenols, alkaloids, proanthocyanidins, and terpenoids), which are already known to have antiviral activities, need to be rapidly screened for the treatment of SARS‐CoV‐2 infected patients. In the context of not having proven medication of COVID19, patients depend on supportive care and symptomatic therapy. Hence, many herbal extract and natural products may help to treat the symptoms associated with SARS‐CoV‐2 infection.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest regarding the publication of this paper.
AUTHOR CONTRIBUTIONS
Bikash Adhikari, Ganesh Lamichhane, Bibek Raj Bhattarai, Karan Khadayat, Achyut Adhikari, and Niranjan Parajuli: Reviewed the literature of natural products. Achyut Adhikari and Bibek Raj Bhattarai: Drawn chemical structures. Binod Rayamajhee, Bishnu P. Marasini, and Santosh Khanal: Reviewed the literature on coronavirus and COVID‐19.
Supporting information
Data S1. Supporting information.
ACKNOWLEDGEMENTS
We acknowledge Mr. Sagar Aryal for providing the figures showing antiviral drugs and site of action, and genomic organization of SARS‐CoV‐2 through biorender.com.
Adhikari B, Marasini BP, Rayamajhee B, et al. Potential roles of medicinal plants for the treatment of viral diseases focusing on COVID‐19: A review. Phytotherapy Research. 2021;35:1298–1312. 10.1002/ptr.6893
REFERENCES
- Abad, M. J. , Guerra, J. A. , Bermejo, P. , Irurzun, A. , & Carrasco, L. (2000). Search for antiviral activity in higher plant extracts. Phytotherapy Research, 14(8), 604–607. [DOI] [PubMed] [Google Scholar]
- Adem, S. , Eyupoglu, V. , Sarfraz, I. , Rasul, A. , & Ali, M. (2020). Identification of potent COVID‐19 main protease (M pro ) inhibitors from natural polyphenols: An in silico strategy unveils a hope against CORONA. Retrieved from 10.20944/preprints202003.0333.v1 [DOI] [PMC free article] [PubMed]
- Alagu Lakshmi, S. , Shafreen, R. M. B. , Priya, A. , & Shunmugiah, K. P. (2020). Ethnomedicines of Indian origin for combating COVID‐19 infection by hampering the viral replication: Using structure‐based drug discovery approach. Journal of Biomolecular Structure & Dynamics, 1–16. 10.1080/07391102.2020.1778537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anani, K. , Hudson, J. B. , de Souza, C. , Akpagana, K. , Tower, G. H. N. , Arnason, J. T. , & Gbeassor, M. (2000). Investigation of medicinal plants of Togo for antiviral and antimicrobial activities. Pharmaceutical Biology, 38(1), 40–45. 10.1076/1388-0209(200001)3811-BFT040 [DOI] [PubMed] [Google Scholar]
- Balzarini, J. , Keyaerts, E. , Vijgen, L. , Vandermeer, F. , Stevens, M. , De Clercq, E. , … Van Ranst, M. (2006). Pyridine N‐oxide derivatives are inhibitory to the human SARS and feline infectious peritonitis coronavirus in cell culture. Journal of Antimicrobial Chemotherapy, 57(3), 472–481. 10.1093/jac/dki481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer, A. , & Brönstrup, M. (2014). Industrial natural product chemistry for drug discovery and development. Natural Product Reports, 31(1), 35–60. 10.1039/C3NP70058E [DOI] [PubMed] [Google Scholar]
- Bernath, K. , Hai, M. , Mastrobattista, E. , Griffiths, A. D. , Magdassi, S. , & Tawfik, D. S. (2004). In vitro compartmentalization by double emulsions: Sorting and gene enrichment by fluorescence activated cell sorting. Analytical Biochemistry, 325(1), 151–157. 10.1016/j.ab.2003.10.005 [DOI] [PubMed] [Google Scholar]
- Bosch, B. J. , van der Zee, R. , de Haan, C. A. , & Rottier, P. J. (2003). The coronavirus spike protein is a class I virus fusion protein: Structural and functional characterization of the fusion core complex. Journal of Virology, 77(16), 8801–8811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukhatem, M. N. , & Setzer, W. N. (2020). Aromatic herbs, medicinal plant‐derived essential oils, and phytochemical extracts as potential therapies for coronaviruses: Future perspectives. Plants (Basel, Switzerland), 9(6), 800. 10.3390/plants9060800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calland, N. , Dubuisson, J. , Rouillé, Y. , & Séron, K. (2012). Hepatitis C virus and natural compounds: A new antiviral approach? Viruses, 4(10), 2197–2217. 10.3390/v4102197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . (2020, April 17). Coronavirus disease 2019 (COVID‐19) – Symptoms. Centers for Disease Control and Prevention. Retrieved from https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html
- Choi, H. J. , Song, J. H. , Park, K. S. , & Kwon, D. H. (2009). Inhibitory effects of quercetin 3‐rhamnoside on influenza A virus replication. European Journal of Pharmaceutical Sciences, 37(3–4), 329–333. 10.1016/j.ejps.2009.03.002 [DOI] [PubMed] [Google Scholar]
- Cinatl, J. , Morgenstern, B. , Bauer, G. , Chandra, P. , Rabenau, H. , & Doerr, H. (2003). Glycyrrhizin, an active component of liquorice roots, and replication of SARS‐associated coronavirus. The Lancet, 361(9374), 2045–2046. 10.1016/S0140-6736(03)13615-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coimbra, M. , Isacchi, B. , van Bloois, L. , Torano, J. S. , Ket, A. , Wu, X. , … Schiffelers, R. M. (2011). Improving solubility and chemical stability of natural compounds for medicinal use by incorporation into liposomes. International Journal of Pharmaceutics, 416(2), 433–442. 10.1016/j.ijpharm.2011.01.056 [DOI] [PubMed] [Google Scholar]
- De Clercq, E. (1982). Specific targets for antiviral drugs. Biochemical Journal, 205(1), 1–13. 10.1042/bj2050001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq, E. (2004). Antivirals and antiviral strategies. Nat Rev Microbiol, 2, 704–720. 10.1038/nrmicro975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq, E. , & Li, G. (2016). Approved antiviral drugs over the past 50 years, 29(3), 695–747. 10.1128/CMR.00102-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan, C. A. M. , Stadler, K. , Godeke, G.‐J. , Bosch, B. J. , & Rottier, P. J. M. (2004). Cleavage inhibition of the murine coronavirus spike protein by a furin‐like enzyme affects cell‐cell but not virus‐cell fusion. Journal of Virology, 78(11), 6048–6054. 10.1128/JVI.78.11.6048-6054.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald, M. , Heinrich, M. , & Booker, A. (2020). Medicinal plant analysis: A historical and regional discussion of emergent complex techniques. Frontiers in Pharmacology, 10, 1480. 10.3389/fphar.2019.01480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianella, P. , Snapp, E. L. , & Levy, M. (2016). An in vitro compartmentalization‐based method for the selection of bond‐forming enzymes from large libraries. Biotechnology and Bioengineering, 113(8), 1647–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatthaar‐Saalmüller, B. , Sacher, F. , & Esperester, A. (2001). Antiviral activity of an extract derived from roots of Eleutherococcus senticosus . Antiviral Research, 50(3), 223–228. 10.1016/S0166-3542(01)00143-7 [DOI] [PubMed] [Google Scholar]
- Ho, T. , Wu, S. , Chen, J. , Li, C. , & Hsiang, C. (2007). Emodin blocks the SARS coronavirus spike protein and angiotensin‐converting enzyme 2 interaction. Antiviral Research, 74(2), 92–101. 10.1016/j.antiviral.2006.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoever, G. , Baltina, L. , Michaelis, M. , Kondratenko, R. , Baltina, L. , Tolstikov, G. A. , … Cinatl, J. (2005). Antiviral activity of glycyrrhizic acid derivatives against SARS−coronavirus. Journal of Medicinal Chemistry, 48(4), 1256–1259. 10.1021/jm0493008 [DOI] [PubMed] [Google Scholar]
- Hui, D. S. , I Azhar, E. , Madani, T. A. , Ntoumi, F. , Kock, R. , Dar, O. , … Petersen, E. (2020). The continuing 2019‐nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China. International Journal of Infectious Diseases, 91, 264–266. 10.1016/j.ijid.2020.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa, S. , & Matsuda, A. (2007). Nucleoside natural products and related analogs with potential therapeutic properties as antibacterial and antiviral agents. Expert Opinion on Therapeutic Patents, 17(5), 487–498. 10.1517/13543776.17.5.487 [DOI] [Google Scholar]
- Islam, M. T. , Sarkar, C. , El‐Kersh, D. M. , Jamaddar, S. , Uddin, S. J. , Shilpi, J. A. , & Mubarak, M. S. (2020). Natural products and their derivatives against coronavirus: A review of the non‐clinical and pre‐clinical data. Phytotherapy Research. 10.1002/ptr.6700 [DOI] [PubMed] [Google Scholar]
- Jang, K.‐J. , Jeong, S. , Kang, D. Y. , Sp, N. , Yang, Y. M. , & Kim, D.‐E. (2020). A high ATP concentration enhances the cooperative translocation of the SARS coronavirus helicase nsP13 in the unwinding of duplex RNA. Scientific Reports, 10(1), 4481. 10.1038/s41598-020-61432-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo, S. , Kim, H. , Kim, S. , Shin, D. H. , & Kim, M.‐S. (2019). Characteristics of flavonoids as potent MERS‐CoV 3C‐like protease inhibitors. Chemical Biology & Drug Design, 94(6), 2023–2030. 10.1111/cbdd.13604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo, S. , Kim, S. , Shin, D. H. , & Kim, M.‐S. (2020). Inhibition of SARS‐CoV 3CL protease by flavonoids. Journal of Enzyme Inhibition and Medicinal Chemistry, 35(1), 145–151. 10.1080/14756366.2019.1690480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi, B. , Panda, S. K. , Jouneghani, R. S. , Liu, M. , Parajuli, N. , Leyssen, P. , … Luyten, W. (2020). Antibacterial, antifungal, antiviral, and anthelmintic activities of medicinal plants of Nepal selected based on ethnobotanical evidence. Evidence‐Based Complementary and Alternative Medicine, 2020, 1043471. 10.1155/2020/1043471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaerunnisa, S. , Kurniawan, H. , Awaluddin, R. , Suhartati, S. , & Soetjipto, S. (2020). Potential inhibitor of COVID‐19 main protease (M pro ) from several medicinal plant compounds by molecular docking study [preprint]. Medicine & Pharmacology. 10.20944/preprints202003.0226.v1 [DOI] [Google Scholar]
- Kim, H.‐Y. , Shin, H.‐S. , Park, H. , Kim, Y.‐C. , Yun, Y. G. , Park, S. , … Kim, K. (2008). In vitro inhibition of coronavirus replications by the traditionally used medicinal herbal extracts, Cimicifuga rhizoma, Meliae cortex, Coptidis rhizoma, and Phellodendron cortex. Journal of Clinical Virology, 41(2), 122–128. 10.1016/j.jcv.2007.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazato, K. , Wang, Y. , & Kobayashi, N. (2007). Viral infectious disease and natural products with antiviral activity. Drug Discoveries & Therapeutics, 1(1), 14–22. [PubMed] [Google Scholar]
- Lau, K.‐M. , Lee, K.‐M. , Koon, C.‐M. , Cheung, C. S.‐F. , Lau, C.‐P. , Ho, H.‐M. , … Fung, K.‐P. (2008). Immunomodulatory and anti‐SARS activities of Houttuynia cordata . Journal of Ethnopharmacology, 118(1), 79–85. 10.1016/j.jep.2008.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S. , Chen, C. , Zhang, H. , Guo, H. , Wang, H. , Wang, L. , … Xiao, P. (2005). Identification of natural compounds with antiviral activities against SARS‐associated coronavirus. Antiviral Research, 67(1), 18–23. 10.1016/j.antiviral.2005.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, L. E. , Kowal, S. , Cardenas, D. A. , & Beauchemin, C. A. A. (2017). Exploring virus release as a bottleneck for the spread of influenza A virus infection in vitro and the implications for antiviral therapy with neuraminidase inhibitors. PLoS One, 12(8), e0183621. 10.1371/journal.pone.0183621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, C.‐W. , Tsai, F.‐J. , Tsai, C.‐H. , Lai, C.‐C. , Wan, L. , Ho, T.‐Y. , … Chao, P.‐D. L. (2005). Anti‐SARS coronavirus 3C‐like protease effects of Isatis indigotica root and plant‐derived phenolic compounds. Antiviral Research, 68(1), 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Y.‐J. , Chang, Y.‐C. , Hsiao, N.‐W. , Hsieh, J.‐L. , Wang, C.‐Y. , Kung, S.‐H. , … Lin, C.‐W. (2012). Fisetin and rutin as 3C protease inhibitors of enterovirus A71. Journal of Virological Methods, 182(1–2), 93–98. 10.1016/j.jviromet.2012.03.020 [DOI] [PubMed] [Google Scholar]
- Liu, G. , Xiong, S. , Xiang, Y.‐F. , Guo, C.‐W. , Ge, F. , Yang, C.‐R. , … Kitazato, K. (2011). Antiviral activity and possible mechanisms of action of pentagalloylglucose (PGG) against influenza A virus. Archives of Virology, 156(8), 1359–1369. 10.1007/s00705-011-0989-9 [DOI] [PubMed] [Google Scholar]
- Liu, J. , Zu, M. , Chen, K. , Gao, L. , Min, H. , Zhuo, W. , … Liu, A. (2018). Screening of neuraminidase inhibitory activities of some medicinal plants traditionally used in Lingnan Chinese medicines. BMC Complementary and Alternative Medicine, 18(1), 102. 10.1186/s12906-018-2173-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd, A. , Russell, M. , Blanes, L. , Doble, P. , & Roux, C. (2013). Lab‐on‐a‐chip screening of methamphetamine and pseudoephedrine in samples from clandestine laboratories. Forensic Science International, 228(1), 8–14. 10.1016/j.forsciint.2013.01.036 [DOI] [PubMed] [Google Scholar]
- Lu, H. (2020). Drug treatment options for the 2019‐new coronavirus (2019‐nCoV). BioScience Trends, 14(1), 69–71. 10.5582/bst.2020.01020 [DOI] [PubMed] [Google Scholar]
- Luo, H. , Tang, Q. , Shang, Y. , Liang, S. , Yang, M. , Robinson, N. , & Liu, J. (2020). Can Chinese medicine be used for prevention of corona virus disease 2019 (COVID‐19)? A review of historical classics, research evidence and current prevention programs. Chinese Journal of Integrative Medicine, 26(4), 243–250. 10.1007/s11655-020-3192-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik, Y. S. , Sircar, S. , Bhat, S. , Sharun, K. , Dhama, K. , Dadar, M. , … Chaicumpa, W. (2020). Emerging novel coronavirus (2019‐nCoV)—Current scenario, evolutionary perspective based on genome analysis and recent developments. Veterinary Quarterly, 40(1), 68–76. 10.1080/01652176.2020.1727993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki, K. , Nagai, T. , Suzuki, K. , Tsujimura, R. , Koyama, K. , Kinoshita, K. , … Takahashi, K. (2007). Anti‐influenza virus activity of biflavonoids. Bioorganic & Medicinal Chemistry Letters, 17(3), 772–775. 10.1016/j.bmcl.2006.10.075 [DOI] [PubMed] [Google Scholar]
- Mishra, S. , Pandey, A. , & Manvati, S. (2020). Coumarin: An emerging antiviral agent. Heliyon, 6(1), e03217. 10.1016/j.heliyon.2020.e03217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra, U. N. , Reddy, M. V. , & Prasad, D. T. (2020). Plant serine protease inhibitor (SPI): A potent player with bactericidal, fungicidal, nematicidal and antiviral properties. International Journal of Chemical Studies, 8(1), 2985–2993. 10.22271/chemi.2020.v8.i1at.8724 [DOI] [Google Scholar]
- Muchtaridi, M. , Sugijanto, M. , Mohd Gazzali, A. , & Wahab, H. A. (2020). Anti‐neuraminidase bioactives from Manggis Hutan (Garcinia celebica L.) leaves: Partial purification and molecular characterization. Molecules, 25(4), 821. 10.3390/molecules25040821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhtar, M. , Arshad, M. , Ahmad, M. , Pomerantz, R. J. , Wigdahl, B. , & Parveen, Z. (2008). Antiviral potentials of medicinal plants. Virus Research, 131(2), 111–120. 10.1016/j.virusres.2007.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai, T. , Miyaichi, Y. , Tomimori, T. , Suzuki, Y. , & Yamada, H. (1992). In vivo anti‐influenza virus activity of plant flavonoids possessing inhibitory activity for influenza virus sialidase. Antiviral Research, 19(3), 207–217. 10.1016/0166-3542(92)90080-O [DOI] [PubMed] [Google Scholar]
- Ogbole, O. O. , Akinleye, T. E. , Segun, P. A. , Faleye, T. C. , & Adeniji, A. J. (2018). In vitro antiviral activity of twenty‐seven medicinal plant extracts from Southwest Nigeria against three serotypes of echoviruses. Virology Journal, 15(1), 110. 10.1186/s12985-018-1022-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogbole, O. O. , Segun, P. A. , & Adeniji, A. J. (2017). In vitro cytotoxic activity of medicinal plants from Nigeria ethnomedicine on Rhabdomyosarcoma cancer cell line and HPLC analysis of active extracts. BMC Complementary and Alternative Medicine, 17(1), 494. 10.1186/s12906-017-2005-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orhan, I. E. , & Senol Deniz, F. S. (2020). Natural products as potential leads against coronaviruses: Could they be encouraging structural models against SARS‐CoV‐2? Natural Products and Bioprospecting, 10, 171–186. 10.1007/s13659-020-00250-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J.‐Y. , Kim, J. H. , Kwon, J. M. , Kwon, H.‐J. , Jeong, H. J. , Kim, Y. M. , … Ryu, Y. B. (2013). Dieckol, a SARS‐CoV 3CLpro inhibitor, isolated from the edible brown algae Ecklonia cava. Bioorganic & Medicinal Chemistry, 21(13), 3730–3737. 10.1016/j.bmc.2013.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, C. , Zhu, Z. , Shi, Y. , Wang, X. , Mu, K. , Yang, Y. , … Zhu, Weiliang . (2020). Exploring the binding mechanism and accessible angle of SARS‐CoV‐2 spike and ACE2 by molecular dynamics simulation and free energy calculation. Retrieved from 10.26434/chemrxiv.11877492.v1 [DOI]
- Pešić, M. (2015). Development of natural product drugs in a sustainable manner. Brief for GSDR 2015. Retrieved from https://sustainabledevelopment.un.org/content/documents/6544118_Pesic_Development%20of%20natural%20product%20drugs%20in%20a%20%20sustainable%20manner.pdf
- Pour, P. M. , Fakhri, S. , Asgary, S. , Farzaei, M. H. , & Echeverría, J. (2019). The signaling pathways, and therapeutic targets of antiviral agents: Focusing on the antiviral approaches and clinical perspectives of Anthocyanins in the Management of Viral Diseases. Frontiers in Pharmacology, 10, 1–23. 10.3389/fphar.2019.01207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajbhandari, M. , Wegner, U. , Jülich, M. , Schöpke, T. , & Mentel, R. (2001). Screening of Nepalese medicinal plants for antiviral activity. Journal of Ethnopharmacology, 74(3), 251–255. 10.1016/S0378-8741(00)00374-3 [DOI] [PubMed] [Google Scholar]
- Rebensburg, S. , Helfer, M. , Schneider, M. , Koppensteiner, H. , Eberle, J. , Schindler, M. , … Brack‐Werner, R. (2016). Potent in vitro antiviral activity of Cistus incanus extract against HIV and Filoviruses targets viral envelope proteins. Scientific Reports, 6(1), 20394. 10.1038/srep20394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redeploying plant defences. (2020). Nature Plants, 6(3), 177. 10.1038/s41477-020-0628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, W. E. , Reinecke, M. G. , Abdel‐Malek, S. , Jia, Q. , & Chow, S. A. (1996). Inhibitors of HIV‐1 replication that inhibit HIV integrase. Proceedings of the National Academy of Sciences of the United States of America, 93(13), 6326–6331. 10.1073/pnas.93.13.6326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez‐Morales, A. J. , MacGregor, K. , Kanagarajah, S. , Patel, D. , & Schlagenhauf, P. (2020). Going global – Travel and the 2019 novel coronavirus. Travel Medicine and Infectious Disease, 33, 101578. 10.1016/j.tmaid.2020.101578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe, A. , Surjadi, R. N. , & Power, B. E. (2006). Novel proteins in emulsions using in vitro compartmentalization. Trends in Biotechnology, 24(12), 587–592. 10.1016/j.tibtech.2006.10.007 [DOI] [PubMed] [Google Scholar]
- Ryu, Y. B. , Park, S.‐J. , Kim, Y. M. , Lee, J.‐Y. , Seo, W. D. , Chang, J. S. , … Lee, W. S. (2010). SARS‐CoV 3CLpro inhibitory effects of quinone‐methide triterpenes from Tripterygium regelii. Bioorganic & Medicinal Chemistry Letters, 20(6), 1873–1876. 10.1016/j.bmcl.2010.01.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sornpet, B. , Potha, T. , Tragoolpua, Y. , & Pringproa, K. (2017). Antiviral activity of five Asian medicinal pant crude extracts against highly pathogenic H5N1 avian influenza virus. Asian Pacific Journal of Tropical Medicine, 10(9), 871–876. 10.1016/j.apjtm.2017.08.010 [DOI] [PubMed] [Google Scholar]
- Tiwari, V. , Darmani, N. A. , Yue, B. Y. J. T. , & Shukla, D. (2010). In vitro antiviral activity of neem (Azardirachta indica L.) bark extract against herpes simplex virus type‐1 infection. Phytotherapy Research, 24(8), 1132–1140. 10.1002/ptr.3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomei, L. , Altamura, S. , Bartholomew, L. , Biroccio, A. , Ceccacci, A. , Pacini, L. , … Migliaccio, G. (2003). Mechanism of action and antiviral activity of benzimidazole‐based allosteric inhibitors of the hepatitis C virus RNA‐dependent RNA polymerase. Journal of Virology, 77(24), 13225–13231. 10.1128/JVI.77.24.13225-13231.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utomo, R. Y. , Ikawati, M. , & Meiyanto, E. (2020). Revealing the potency of citrus and galangal constituents to halt SARS‐CoV‐2 infection. Retrieved from 10.20944/preprints202003.0214.v1 [DOI]
- Vimalanathan, S. , Ignacimuthu, S. , & Hudson, J. B. (2009). Medicinal plants of Tamil Nadu (Southern India) are a rich source of antiviral activities. Pharmaceutical Biology, 47(5), 422–429. 10.1080/13880200902800196 [DOI] [Google Scholar]
- Webster, D. , Taschereau, P. , Lee, T. , & Jurgens, T. (2006). Immunostimulant properties of Heracleum maximum Bartr. Journal of Ethnopharmacology, 106(3), 360–363. 10.1016/j.jep.2006.01.018 [DOI] [PubMed] [Google Scholar]
- WHO . (2020a). Advice for public. Retrieved from https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public
- WHO . (2020b). Report of the WHO‐China Joint Mission on Coronavirus Disease 2019 (COVID‐19). Retrieved from https://www.who.int/publications-detail/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19)
- Xian, Y. , Zhang, J. , Bian, Z. , Zhou, H. , Zhang, Z. , Lin, Z. , & Xu, H. (2020). Bioactive natural compounds against human coronaviruses: A review and perspective. Acta Pharmaceutica Sinica B, 10, 1163–1174. 10.1016/j.apsb.2020.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting information.


