Abstract
Background
The use of alcoholic‐based hand rubs (ABHRs) is an important tool for hand hygiene, especially in times of the COVID‐19 pandemic. Possible irritant effects of ABHR may prevent their use by persons at risk of infection.
Methods
This systematic review is based on a PubMed search of articles published between January 2000 and September 2019 in English and German, and a manual search, related to the irritation potential of alcohol‐based disinfectants restricted to n‐propanol (1‐propanol) and its structural isomer isopropanol (isopropyl alcohol, 2‐propanol).
Results
The majority of the included studies show a low irritation potential of n‐propanol alone. However, recent studies provide evidence for significant barrier damage effects of repeated exposure to 60% n‐propanol in healthy, as well as atopic skin in vivo. The synergistic response of combined irritants, (ie, a combination of n‐propanol or isopropanol with detergents such as sodium lauryl sulfate) is greater, compared with a quantitatively identical application of the same irritant alone.
Conclusion
While recent studies indicate a higher risk of skin irritation for n‐propanol and isopropanol than reported in the past, this risk still seems to be lower than that for frequent handwashing with detergents, as recommended by some to prevent COVID‐19 infections.
Keywords: alcohol‐based hand rubs, irritant contact dermatitis, n‐propanol, skin barrier
1. INTRODUCTION
Since the times of Ignaz Semmelweis (1815–1865), hand hygiene has been established as one of the core procedures in the health care services. 1 , 2 While many of these hand hygiene measures are associated with a risk of skin damage, the assessment of the toxicity of the individual interventions differs widely. It is striking that the compliance of correct hand disinfection is suboptimal, mostly below 50%. 3 Such low compliance has many causes, such as the number of available dispensers, 4 workload, and lack of personnel, 5 but also the skin compatibility of the application. 6
Nowadays, compliance can be increased considerably through simple interventions and educational/feedback interventions. 7 The World Health Organization hand hygiene improvement strategy recommends as a first step (system change), in its five‐phase, multimodal hand hygiene improvement strategy, to exchange hand washes with alcohol‐based hand rubs (ABHRs). 8 However, the good study results regarding the skin tolerance of ABHRs are in contrast to the skeptical assessment of nursing staff, 9 which contributes to the overall low compliance. One reason for this is that ABHRs may cause burning sensations. 10 This burning occurs particularly on irritated skin. Reflectively, healthcare workers (HCW) may blame ABHR for this burning sensation and condemn the hand disinfectant as a “harmful product”. With the resulting change to hand washing procedures, further deterioration of the skin condition may occur, possibly progressing from slight irritation to a clinically relevant hand eczema. 10 Consequently, the correct handling of hand hygiene (hand washing, ABHR, skin protection, and skin care) must be ascertained as early as possible to keep the level of irritative skin changes in working life as low as possible.
Today, ABHR have re‐gained popularity and are now widely used for infection control in clinical practice. ABHRs were found to be a suitable alternative to traditional hand washing as they require less time, act faster, are less irritating to the skin, and contribute to significantly lower infection rates. 11 Currently, hand disinfectants are the most important prevention measure after face masks in the global SARS‐CoV‐2 pandemic. Among other things, virus containment and transmission reduction are of highest priority. Recently, it was proven that a mixture of isopropanol 12 led to complete viral inactivation without cytotoxic activity, at a minimal concentration of 30%. 13
Irritant contact dermatitis (ICD) is the most common form of occupational skin disease with a prevalence of approx. 21%–75% in occupational groups with high exposure to wet‐work. 14 , 15 , 16 , 17 Therefore, HCW with higher frequencies of hand washing and use of disinfectants are severely affected. 18 Several field studies have elucidated that ABHRs (short‐chain aliphatic alcohols such as n‐propanol or isopropyl alcohol, so called “rub‐ins”) have a low irritation potential compared to detergents. 19 , 20 , 21 This systematic review evaluated the clinical evidence of the irritation potential of n‐propanol and isopropanol as components of ABHRs.
2. METHODS
This systematic review was based on a search of the PubMed database with the following research criteria: [n‐propanol] AND [irritation], [alcohol‐based hand rubs] AND [detergent], [n‐propanol] AND [skin barrier], [n‐propanol] AND [irritant contact dermatitis], [hand disinfection] AND [irritation], [nonanoic acid] AND [irritation].
The search was limited to English language and German language publications in human study subjects published between the years 2000 and 2019.
We reviewed the reference lists of the full length articles to identify additional articles that met the predefined inclusion criteria (Figure 1).
FIGURE 1.
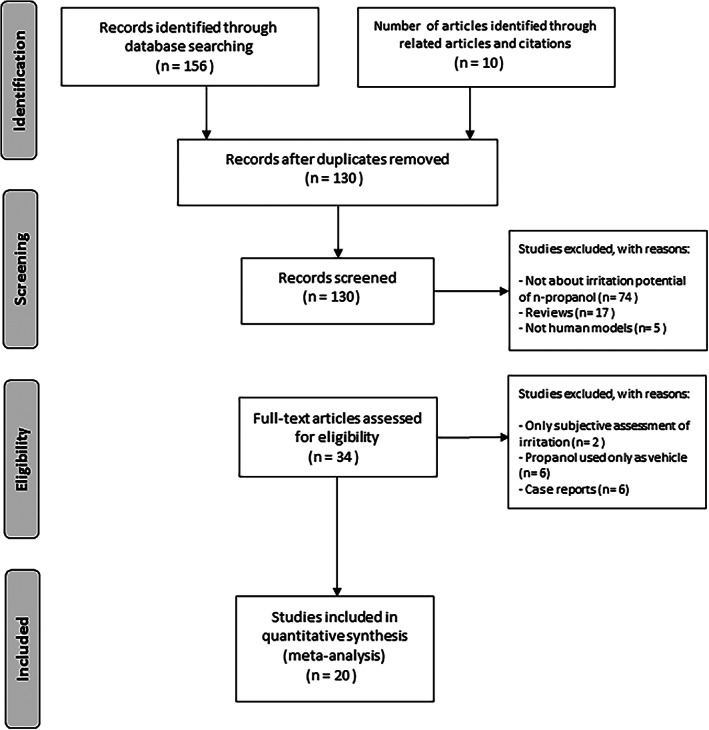
An overview of the literature research and study selection
A total of 156 articles were identified from the initial search and 10 additional articles were found by manual search. After the review of all articles, 166 full text articles were further evaluated. As we focused on the primary literature and avoided double counting, 34 reviews were excluded from our primary analysis. We also excluded articles without the relation to the irritation potential of ABHR and articles dealing with animal models.
Following these exclusion criteria, we finally considered 20 articles. For each study included, we recorded the intervention, substance, population, measurements, author, year, location, and conclusion, shown in Tables 1, 2, 3, 4. We included prospective studies only.
TABLE 1.
Included studies comparing the effect of various concentrations of n‐propanol or isopropanol on previously irritated or non‐irritated skin
| Conclusion | ||||||
|---|---|---|---|---|---|---|
| Intervention | Substance | Population, sex | Measurement | Author/ year/ location | Clinical outcome | Skin physiological measurements |
| Repetitive occlusive patch test (tandem application) and n‐propanol alone |
60% aqueous n‐ propanol 0,5% aqueous SLS |
20 (10F, 10M) healthy |
Visual score Tewameter Chromameter |
Kappes et al/ 2001/ Germany 24 | No irritation with application of n‐propanol (Prop/Prop) alone, compared to alternate use of SLS/Prop and SLS/SLS | Slight TEWL increase with n‐propanol alone. Highest TEWL value with SLS/SLS, followed by SLS/Prop |
|
Patch test Repeated open exposure test |
60% and 100% n‐propanol Water 0,3% SDS |
12 (8F, 4M) healthy |
TEWL Corneometer |
Lübbe et al/2001/ Switzer land 30 |
Pre‐irritated skin sites with 0.3% SDS showed significant increase in TEWL with 100% n‐propanol. No change in TEWL values with lower concentrations of n‐propanol | |
|
Wash test Patch test (tandem application) |
Detergent: aqua, sodium laureth sulfate, sodium laureth‐8 carboxylate + laureth‐7, glycereth‐2‐cocoate, glycerin, PEG‐4 rapeseed amide, lactic acid Alcohol disinfection: 1,3% glycerol, 5% v/v IPA % 78% v/v ethanol 1% aqueous SLS |
15 (7F, 7M) healthy |
Visual score Chromameter Tewameter |
Pedersen et al/ 2005/ Denmark 33 | No fissuring or scaling was found on any of the test sites | No amplification of the irritation potential of ethanol by 5% v/v IPA |
|
Wash test Patch test |
Detergent: aqua, sodium laureth sulfate, sodium laureth‐8 carboxylate + laureth‐7, glycereth‐2‐cocoate, glycerin, PEG‐4 rapeseed amide, lactic acid Alcohol disinfection: 1,3% glycerol, 5% v/v IPA % 78% v/v ethanol 1% aqueous SLS |
19 (10F, 9M) healthy; 3 exclusions during study. |
Visual score Chromameter Tewameter |
Pedersen et al/ 2005/ Denmark 32 | No fissuring or scaling was found on any of the test sites | No amplification of the irritation potential of ethanol by 5% v/v IPA |
|
Repeated occlusive patch test (tandem model) and single application of alcohols |
Part 1: ethanol, 2‐propanol, 2‐propanol (each in concen‐ trations of 60%, 70%, 80%, 90%, 100%) Part 2: 80% ethanol, 60% 1‐propanol, 70% 2‐propanol, 0,5% SLS |
105 (49F, 56M) healthy |
Tewamete Corneometer Chromameter Subjective assessment |
Löffler et al/ 2007/ USA/ Germany 6 |
No increase in water loss with single substance irritation, including n‐propanol or isopropanol with slight decrease in skin hydration Induced skin irritation by SLS was not exacerbated by alcohols |
|
|
Patch test (tandem + single application) Wash test |
0,5% w/v SLS Sterillium (45% w/w 2‐propanol, 30% w/w 1‐propanol & 0,2% w/w mecetronium etilsulfate) Propanol solution (45% w/w 2‐propanol, 30% w/w 1‐propanol) Water |
45 (49F, 56M) healthy |
Tewameter Laser Doppler Flowmetry Corneometer |
Slotosch et al/ 2007/ Germany 23 |
n‐propanol alone showed TEWL values identical to the empty chamber control field. In descending order the highest water loss was seen with SLS/SLS, followed by SLS/Sterillium and Prop/Prop |
|
|
Repeated open application test (ROAT) model |
SLS (0% = sterile water, 0,5%, 1,0%, 2,0%) Nonanoic acid in n‐propanol (0% = 100% neat n‐propanol, 10%, 20%, 30%) |
24 (16F, 8M) healthy |
Visual scoring Tewameter Chromameter Corneometer |
Clemmensen et al/ 2008/ Denmark 25 |
Maximum visual scores as high as 5 were observed with SLS 2.0%, NAA 20% and NAA 30% | Neat n‐propanol has similar irritation level as 1.0% SLS, additional NAA leads to higher water loss and reduced skin capacity |
| Patch test |
80% aq. ethanol 60% aq. 1‐propanol 70% aq. 2‐propanol 100% distilled water 0,25% % 0,5% aq. SLS Softasept Sterillium Sterillium Virugard Desmanol Cutasept |
13F healthy 21 HCW (18F, 3M) |
Interpretation according to DKG patch test guidelines |
Stutz et al/ 2009/ Germany 9 |
Sensitization to alcoholic hand rubs is very low. None of the tested nurses reacted to any alcohol during patch test | |
| Patch test | 100% IPA | 1450 | Interpretation according to DKG patch test guidelines | Garcia‐Gavin et al/ 2011/ Belgium 27 | Positive patch test in 44 patients | |
|
Patch test (in vivo) Forearm controlled application test (FCAT) ➔ 20x/D and 100x/D |
70% of alcohol (ethanol, n‐propanol, isopropanol) | 25F |
Visual score Tewameter Corneometer |
Cartner et al /2016/USA/UK 26 | Maximum visual redness was discovered with n‐propanol | Highest skin barrier impairment and lowest skin hydration was found with n‐propanol, followed by ispopropanol and ethanol, regardless of the application frequency |
|
Occlusive modified tandem repeated irritation test (TRIT) |
60% aq. n‐propanol 0,5% aq. SLS |
25 (16F, 9M) healthy |
Visual score Tewameter Corneometer Colorimeter |
Angelova‐Fischer et al/ 2016/Netherlands 31 | All fields showed higher irritation at D5 compared to D1 |
No significant difference in skin redness, independent of previous occlusion, was found with n‐propanol/n‐propanol TEWL values of n‐propanol/n‐propanol were closest to the control field, compared to tandem application of SLS/n‐propanol and SLS/SLS Decrease in skin capacitance were seen in all tested fields by D5 |
Note: DKG, deutsche kontaktallergie‐gruppe; F, female; IPA, isopropyl aclohol; NAA, nonanoic acid; M, male; PEG, polyethelyne glycol; SDS, sodium dodecyl sulfate; synonymous: SLS, sodium lauryl sulfate; TEWL, transepidermal water loss.
TABLE 2.
Included studies evaluating the influence of an atopic predisposition
| Conclusion | ||||||
|---|---|---|---|---|---|---|
| Intervention | Substance | Population, sex | Measurement | Author/year/location | Clinical outcome | Skin physiological measurements |
| Repetitive semi‐occlusive patch test |
Sterillium (45% 2‐propanol, 30% 1‐propanol, 0,2% mecetronium etylsulfate) Sterillium pure (45% 2‐propanol, 30% 1‐propanol, 0,2% mecetronium etylsulfate) Sterillium Gel (85% ethanol) Sterillium Virugard (95% ethanol) Amphisept E (80% ethanol) |
54 (45F, 9M) 26 of them Atopics with Erlangen Atopy score of 12.1 ±3.1 |
Visual assessment by one investigator Chromameter |
Kampf et al/ 2006/Germany 42 | Mean tolerability with five hand rubs was between 0.01 ±0.03 and 0.02± 0.1 which is identical to the mean tolerability of the negative control (0.02±0.07) | Skin redness was between 0.01±0.1 and 0.28±1.0, similar to the negative control. No difference between atopic and non‐atopic subjects could be made |
| Occlusion‐modified tandem repeated irritation test | n‐propanol (30%, 45%, 60%, 75% aq.) |
20 (16F, 4M) healthy 20 (17F, 3M) atopic dermatitis |
Tewameter Corneometer Colorimetry NMF |
Angelova‐Fischer et al/2020/ Germany, Austria, Netherlands, Croatia 44 |
Cumulative exposure to 30% n‐propanol, applied as a single irritant, was sufficient to induce damage to the epidermal barrier in atopics, whereas the same exposure had no significant effect on healthy skin, unless the barrier function had been previously impaired | |
Note: F, female; M, male; NMF, natural moisturising factor.
TABLE 3.
Included studies demonstrating the irritation effects of n‐propanol and isopropanol on components of the stratum corneum
| Conclusion | ||||||
|---|---|---|---|---|---|---|
| Intervention | Substance | Population, sex | Measurement | Author/year/location | Clinical outcome | Skin physiological measurements |
| NMF analysis |
60% aq. n‐propanol 0,5% aq. SLS |
25 (16F, 9M) healthy | D Squame | Angelova‐Fischer et al/ 2016/Netherlands 31 |
Reduction of NMF levels −55.4% with n‐propanol and −79.2% Pretreatment with occlusion −60.8% and −87.4%, respectively |
|
| Patch test |
0,5% SLS 0,15% sodium hydroxide 60% n‐propanol 2,0% acetic acid |
8 (5F, 3M) healthy |
Tewameter Chromameter Corneometer D Squame AFM (atomic force microscopy) DTI (dermal texture index) |
Soltanipoor et al/ 2017/Netherlands, Germany, Denmark 45 |
55% reduction of NMF levels with n‐propanol, but as high as 75% with repeated exposure of SLS Changes in corneocyte topography with n‐propanol, SLS and NaOH after 96 hours with increase in DTI and circular nano objects |
n‐propanol caused only slight changes in TEWL and erythema, but a significant decrease in skin capacitance after 96 hours |
| Keratinocyte culture |
0%, 0,1%, 0,5%, 1%, 2% – 10% increments of 70% w/w alcohol (ethanol, n‐propanol, isopropanol) IL‐1a DuoSet ELISA development kit and TNF‐a DuoSet development kit |
MTT assay | Cartner et al/2016/USA,UK 26 |
Marked cellular toxicity and significant TNF‐α and IL‐ 1α release by the skin residential cell with n‐propanol, followed by isopropanol and ethanol |
||
| Occlusion‐modified tandem repeated irritation test | n‐propanol (30%, 45%, 60%, 75% aq.) |
20 (16F, 4M) healthy 20 (17F, 3M) atopic dermatitis |
D‐SQUAME (NMF analysis) | Angelova‐Fischer et al/ 2019/Germany, Austria, Netherland, Croatia 44 | Cumulative exposure to n‐propanol reduced significantly the NMF levels in healthy and atopic skin equally | |
Note: ELISA, enzyme‐linked immunosorbent assay; F, female; M, male; MTT assay, cell viability assay; NMF, natural moisturizing factor; SLS, sodium lauryl sulfate.
TABLE 4.
Included studies demonstrating the influence of emollients on the irritation potential of n‐propanol or isopropanol
| Conclusion | ||||||
|---|---|---|---|---|---|---|
| Intervention | Substance | Population, sex | Measurement | Author/year/location | Clinical outcome | Skin physiological measurements |
| Wash test |
Sterillium (45% w/w propan‐2‐ol, 30% w/w propan‐1‐ol % 0,2% w/w ethylhexadecyldimethyl ammonium ethylsulfate) Hibiscrub (4% chlorhexidine digluconate) |
60 healthy |
Skin roughness (profilometry) Tewameter, D‐squames Corneometer, Visual assessment |
Pietsch et al/ 2001/ Denmark 52 |
Higher compliance with Sterillium | Sterillium has the better values for TEWL, skin roughness and skin hydration |
| Wash test |
AHD (ethanol 75%) Desderman (78,2% ethanol & 0,1% 1.4.5.6‐tetrabrom‐o‐cresol) Mucasept A (70% iso‐propanol % 10% ethanol) Manorapid (Poly‐Alkohol & 63,1% iso‐propanol) Spitacid (46& ethanol, 27% isopropanol & 1% benzylalcohol) Sterillium (45% iso‐propanol, 30% n‐propanol & 0,2% mecetronium etilsulphate) |
17 Group 1 no ABHR experience: (3F, 7M) 3 of them Atopics Group 2 with daily experiences: (7 lab workers) |
Tewameter Sebumeter pH‐ meter) Corneometer, Sensorial assessment by HCW only |
Kramer et al/ 2002/ Germany 53 |
Lowest skin dryness after application with Sterillium | No significant change in TEWL, skin hydration and sebum content |
| Repetitive occlusive patch test | Sterillium (45% w/w iso‐propanol, 30% w/w n‐propanol, 0,2% w/w mecetronium etilsulphate) | 55 (46F, 9M) healthy | Visual score |
Kampf et al/ 2003/ Germany,USA 51 |
Sterillium has no clinically relevant potential for dermal irritation and sensitization | |
| Repeated open application test |
2 hand rubs based on 45% propan‐2‐ol, 30% propan‐1‐ol & 0,2% mecetronium etilsulfate. 1 rub contained 0,81% emollients (myristyl alcohol, glycerol, dexpanthenol, levomenol, lanolin alcohol) |
35 (26F, 9M) 21 of them Atopics with Erlangen Atopy score of 11.4 ± 2.2 ➔ 3 exclusions during study. |
Visual score | Kampf et al /2005/Germany 43 | No statistical significant difference was found between atopic and non‐atopic subjects for any of the products | |
| Wash test |
Gel A: 70% ethanol (v/v) + 2% (v/v) glycerine Gel B: 70% ethanol (v/v) + 5% (v/v) glycerine Gel C: 70% ethanol (v/v) + 8% (v/v) glycerine Gel D: 75% ethanol (v/v) + 2% (v/v) glycerine Gel E: 80% ethanol (v/v) + 2% (v/v) glycerine Gel F: 70% IPAl (v/v) + 2% (v/v) glycerine |
13 F healthy +21 HCW (18F, 3M) | Tewameter, Corneometer, pH‐ meter Chromameter. D‐squames. Subjective assessment | Houben et al/ 2006/Belgium 54 |
Increased skin hydration, proportionally to rising glycerine content |
No significant effect on TEWL and increased skin hydration among all gel types |
| Wash test (normal daily application of hand rub) |
Formulation A: 80% v/v ethanol + glycerol Formulation B: 75% v/v IPA + glycerol Formulation C: 75% v/v IPA + isopropyl myristate |
38F healthy |
Visual score (visual scoring of skin scale) Self assessment (7‐point Likert scale) |
Pittet et al/ 2007/ Switzerland 55 |
Lowest tolerance by HCW with formulation C | Skin condition improved with formulation A and B |
Note: ABHR, alcohol‐based hand rubs; AHD, company name of a desinfection product; F, females; HCW, healthcare workers; IPA, isopropyl aclohol; M, males; TEWL, trans epidermal water loss.
3. RESULTS
A total of 166 citations were retrieved. Twenty articles (12.04%) met the inclusion criteria. The majority of the studies (11/20) related to the irritation potential of n‐propanol and isopropanol in different concentrations, five to the effectiveness of added emollients, two to the compliance of patients with an atopic predisposition, and four to the effects of n‐propanol and isopropanol on structural components of the stratum corneum. Because of the overlapping subject matter, several studies were included in different categories for evaluation.
Two options for hand hygiene are generally available in clinical practice: (i) hand washing with some type of detergent and water or (ii) hand disinfection with ABHR. For the purpose of analysis the results are reported in four sections: (i) comparison of the effects of various concentrations of n‐propanol and isopropanol on previously irritated or non‐irritated skin, which is further divided into the irritation potential of n‐propanol and the irritation potential of n‐propanol and isopropanol in combination with detergents in a tandem model, (ii) influence of an atopic predisposition on the irritation capability of n‐propanol and isopropanol, (iii) irritation effects of n‐propanol and isopropanol on components of the stratum corneum and (vi) interaction of emollients on the irritation potential of n‐propanol and isopropanol.
3.1. Comparison of the effects of various concentrations of n‐propanol or isopropanol on previously irritated or non‐irritated skin
3.1.1. The irritation potential of n‐propanol alone
Of the 20 studies, six studies examined the irritation effects of n‐propanol or isopropanol alone while using different application methods, as shown in Table 1.
In a tandem application model with consecutively applied 60% aq. n‐propanol or a propanol mixture (2‐propanol 45% w/w, 1‐propanol 30% w/w) with 0.5% aq. sodium lauryl sulfate (SLS), Kappes et al and Slotosch et al additionally tested the irritation capability of propanol alone. The resulting irritation was assessed by corneometry and transepidermal water loss (TEWL) measurements. The alternating application of n‐propanol (Prop/Prop) showed values identical to water (plain control field) or an empty chamber, which served as a negative control. 22 Biometric measurements demonstrated a significant exponential increase, including water loss and skin irritation after the single application of SLS/SLS. 23
Another study conducted repetitive patch testing with various concentrations of three different alcohols by the consecutive application of the same alcohol. Ethanol, 1‐propanol and 2‐propanol were applied in concentrations ranges from 60%–100%. Evaluated by bioengineering techniques, all three alcohols failed to induce irritation regarding erythema (chromameter values) and skin barrier (TEWL values) at all patches. 6 Clemmensen et al published a study comparing irritation induction by different concentrations of SLS and nonanoic acid (NAA) in two test models. Here, n‐propanol served as a vehicle for NAA but was beyond that tested separately as a pure solution. The authors demonstrated that 100% n‐propanol had the same irritancy level as 1% SLS, but did not differ statistically significantly from NAA concentrations in the repeated open application model. However, in the wash test model, n‐propanol was less irritating than SLS in all concentrations. 24
In a forearm controlled application test on 35 volunteers, using ethanol, n‐propanol, and isopropanol in various concentrations from 0%–10%, the volunteers were randomized for a standard frequency application (20x) or high frequency application (100x), mimicking the in‐use conditions of HCW in hospitals. According to Cartner et al, the highest drop‐out rates were recorded with the use of n‐propanol. By day 10, all treatments of n‐propanol at 100x application were stopped and, equally, >50% of the subjects stopped at the 20x application rate. Moreover, the maximum visual redness score of 5.0 was only seen with n‐propanol. 25 Allergic reactions to ABHR are rarely found in the literature. It has been proposed by García‐Gavín et al that 100% isopropyl alcohol in a patch test caused allergic reactions and that it should be considered as a potent allergen. 26 Stutz et al tested 50 volunteer nurses, who thought they were allergic to ABHR. A total of 80% aq. ethanol, 60% aq. 1‐propanol and 70% aq. 2‐propanol, as well as five conventional disinfectants were analyzed using patch tests. A delayed type sensitization to an ABHR could be excluded in all 50 nurses. 9
3.1.2. The irritation potential of n‐propanol or isopropanol in combination with detergents in a tandem model
A valid method for the sequential application of two irritants is known as the tandem repeated irritation test (TRIT), which has been well established over time. 27 , 28 Simultaneous or alternate application of detergent and an ABHR in combination has been reported to produce an additional irritation response, compared to single alcohol application. In total, five studies described the irritation capability of n‐propanol when combined with SLS, which is summarized in Table 1. Prior to the application of alcohol, previous irritation of the skin was induced by SLS applied under occlusive conditions for several minutes up to several hours.
In a short term repeated tandem application of 60% aq. n‐propanol and 0.5% aq. SLS, Kappes et al found, that the exposure of n‐propanol after 30 minutes occlusive exposure to SLS, slightly enhanced the cumulative irritation potential. All bioengineering parameters showed a significant difference of n‐propanol applied alone and SLS/Prop in a tandem application, compared to single SLS/SLS exposure. 23
Another study conducted a repeated open exposure test to three concentrations of n‐propanol (100%, 60%, 0%) on pre‐irritated (sodium dodecyl sulfate [SDS] or water) and non‐irritated skin. The authors showed that 60% n‐propanol, which corresponds to the concentration of alcohol‐based disinfectants, did not induce any irritation on healthy skin, with results comparable to n‐propanol 0% (water). Pre‐irritated skin sites with 14 hours of 0.3% SDS showed a significant increase in TEWL after application of n‐propanol in all concentrations. On the the other hand, previously water‐occluded sites did not induce TEWL changes. 29 Two other studies carried out repetitive patch testing and tandem application of n‐propanol and 2‐propanol with a detergent, which remained on the skin for 24 hours. Löffler et al tested the alternating application of 60% n‐propanol and 70% isopropanol, mimicking concentrations of commercially available hand rubs, with previously irritated skin by 0.5% SLS and in reverse sequence, with the detergent being applied first.. The results showed no significant alteration in skin barrier disruption or erythema, induced by the alcohols in the patch test, not even when applied after the SLS solution. Skin hydration decreased more with ethanol and 1‐propanol compared to 2‐propanol. Additionally, they discovered that skin hydration was considerably lower with the higher concentrations of ethanol and 1‐propanol. 6 Conversely, repeated exposure to n‐propanol and/or SLS in an occlusion‐modified irritation test by Angelova‐Fischer et al, showed that preceding occlusion with water enhances the irritant‐induced barrier damaging effects. However, the application of n‐propanol/n‐propanol did not induce skin erythema and presented the closest values to the negative control field with regard to TEWL measurements. 30
The other study combining application of alcohol and detergent was performed by Slotosch et al. Here, 0.5% w/v SLS was tandemly applied with Sterillium (Hartmann International, Hamburg, Germany) (2‐propanol 45% w/w, 1‐propanol 30% w/w and mecetronium etilsulfate [MES] 0.2%) and with a propanol solution, composed as Sterillium, but without MES, in a patch test and wash test model.
Evaluated by TEWL, subpapillary dermal blood flow and corneometry, both application methods showed similar results. It was found that there was a significant higher TEWL and increased blood flow using the detergent alone compared to the combined use of detergent/Sterillium and detergent/propanol solution. After the wash test, skin hydration parameters showed comparable results between the hand rub and water application. Slotosch et al highlighted that the irritative effect of SLS on the skin is reduced after tandem application with the propanol solution or the hand rub. 22 Pedersen et al evaluated the short term effects of the alternate use of detergent and disinfection on skin. Although they used an alcohol solution consisting of 75% v/v ethanol, 1.3% glycerol, and 5% v/v isopropyl alcohol, we considered this study as useful in order to give an overview of whether 5% v/v isopropyl alcohol enhances the irritation potential of ethanol or not. It is a very low concentration compared to the corresponding concentrations used in commercially available disinfectants. Nevertheless, Pedersen et al found that the alternate use of detergent and disinfectant caused less irritation than hand treatment with detergent alone. 31 , 32 Compared to studies that evaluated the irritation potential of ethanol, 6 , 33 5% v/v isopropyl alcohol does not seem to amplify the irritation potential of ethanol. 31 , 32
3.2. Influence of an atopic predisposition to the irritation capability of n‐propanol or isopropanol
It has been reported that hospital employees with an atopic predisposition are at higher risk of developing occupational contact dermatitis than non‐atopic individuals. 15 , 34 , 35 , 36 This raises the question 15 , 37 , 38 , 39 , 40 Of the 20 studies, two studies examined the irritation risk of propanol‐based hand rubs in patients with an atopic predisposition (Table 2.).
In a study with 54 volunteers, half of whom have had an atopic predisposition, a patch test with five commercially available disinfectants under a repetitive semi‐occlusive condition was performed. This study was conducted by Kampf et al, who showed that both healthy and atopic subjects tolerated all five hand disinfectants well. Evaluated on the basis of skin redness, the experiment was controlled with de‐mineralized water (negative control) and 2% SLS (positive control). Skin redness values for ABHRs were in the same range as for the negative control site (0.15 ±0.8), whereas the positive control was as high as 1.35 ±1.6. 41
Recently, another study demonstrated enhanced barrier impairment and local erythema after repetitive application of n‐propanol on previously damaged atopic and healthy skin, when preceded by exposure to water and occlusion. Hereby, the cumulative effect of repeated exposure to n‐propanol (30%, 45%, 60%, and 75%) in atopic and healthy subjects, with or without preceded occlusion with water, was evaluated. Repeated exposure to water enhanced the irritant‐induced effects of n‐propanol. The lowest concentration of n‐propanol was sufficient to induce barrier impairment in atopic skin without previous trauma. 43
3.3. Irritation effects of n‐propanol or isopropanol on components of the stratum corneum
In a repetitive occlusive exposure to n‐propanol 60% aq. and SLS 0.5% aq., Angelova‐Fischer et al found that previously occluded skin areas showed a significantly higher losses of natural moisturising factor (NMF) than test fields without pretreated occlusion. The relative reduction of NMF after exposure to Pro/Pro was −55.4% and after SLS −79.2%. In contrast, pretreatment with occlusion showed much higher losses, of −60.8% and − 87.4%, respectively. 30 Subsequently, an extended study was conducted by the same authors with the discovery of a significant decrease in NMF levels after cumulative exposure to various concentrations of n‐propanol (30%, 45%, 60%, 75%) in healthy and atopic skin groups. Here, the relative NMF reduction in the healthy skin group was lower than in the atopic skin group, independent of previous barrier damage by occlusion. Occlusion with water alone had no impact on NMF levels in both groups. 43
This result is consistent with findings of Soltanipoor et al, which confirmed that 60% n‐propanol reduces NMF levels in the stratum corneum (SC). Furthermore, the authors showed that n‐propanol caused remarkable changes in corneocyte surface topography under repeated occlusive conditions and that this effect is strongly associated with a decrease in NMF and SC hydration. The reduction of NMF was inversely correlated with the increase in circular nano objects (CNO) and the dermal texture index (DTI) detected with atomic force microscopy (AFM). N‐propanol showed significant changes in skin capacitance, but not in TEWL parameters, leading the authors to suggest that the decrease in skin hydration depends on the decrease of NMF rather than the effect of n‐propanol on the corneocyte lipid bilayers. 44
In a prospective in vitro and in vivo study by Cartner et al, the cellular toxicity of three alcohol solutions was analyzed using neonatal human epidermal keratinocytes by evaluating the production of inflammatory cytokines. Ethanol, n‐propanol, and isopropanol in various concentration ranges from 0% to 10% were used. It was found that n‐propanol distinctly increased the expression of TNF‐ α and IL‐1 α , and to a lesser extent isopropanol and ethanol. 25 The findings of this section are summarized in Table 3.
3.4. Interaction of emollients on the irritation potential of n‐propanol or isopropanol
The benefits of adding emollients to a propanol‐based hand rub supports the regeneration of the skin barrier and may minimize the risk of developing the sensation of skin dryness. 45 The application of moisturizers after repeated irritation with water and detergents improves skin hydration. 46 As early as 1995, MES was found to have protective properties in Sterillium and to reduce skin roughness. 47 Furthermore, glycerol, a well‐known moisturizing substance used in commonly available hand disinfectants, increases the skin water content. 48 The drying effect of alcohol can be reduced or eliminated by adding emollients such as 1% to 3% glycerol or other skin‐conditioning agents to alcohol‐based formulations. 49
Of the 20 studies, six studies examined the beneficial effects of emollients in customary disinfecting agents and are shown in Table 4. This category deliberately includes studies that investigate the irritation potential of Sterillium, a globally known disinfectant with additive humectants.
Kampf et al published a study of 53 volunteers repetitively patch tested in two phases with Sterillium (2‐propanol 45% w/w, 1‐propanol 30% w/w and MES 0,2%) under occlusive conditions. In the first phase, the induction phase, Sterillium exerted a barely perceptible, minimalerythema in one of the nine included patients. In general, none of the remaining participants showed any skin changes at any time. During the second phase (the challenge phase), 72 hours after the application of the disinfectant, none of the subjects showed skin reactions. 50
Pietsch et al, who tested Sterillium (45% w/w propan‐2‐ol, 30% w/w propan‐1‐ol % 0.2% w/w ethylhexadecyldimethyl ammonium ethylsulfate) and the water‐based handwashing antiseptic Hibiscrub (4% chlorhexidine digluconate) in a long‐term application form, came to the same conclusion. All biophysical parameters indicated a significantly higher compliance towards Sterillium than Hibiscrub. 51
Kramer et al proved the emollient effect of Sterillium in a clinical trial on the dermal tolerance of six commercially available ABHRs with up to 20 applications per day, mimicking daily the routine use of HCW in hospitals. Subjective assessment of the products resulted in the lowest skin dryness after the use of Sterillium. Furthermore, there was no significant change in TEWL, skin hydration, or sebum content. This achievement was interpreted as the emollient effect of MES and glycerol contained in the hand rub. 52 However, it is not stated whether the use of emollients was restricted throughout the study or not.
Using a repeated open application test, Kampf et al demonstrated the emollient effect in propanol‐based hand rubs. Thirty‐five volunteers, half of them having an atopic predisposition, were tested with two hand rubs, one product containing 0.81% (w/w) emollients, a mixture of myristyl alcohol, glycerol, dexpanthenol, levomenol and lanolin. Assessment by visual scoring for erythema and dryness showed that the addition of emollients to a propanol‐based hand rub can significantly decrease ICD under frequent‐use conditions. The overall mean sum score for ICD among the 35 volunteers was 0.8 (± 2.4) (hand rub with emollients) and 1.5 (±3.5) (hand rub without emollient mixture). 53
Houben et al examined skin tolerance to six alcohol‐based hand gels (Gel A – Gel F) and alterations in skin condition depending on the concentration of glycerol by repetitive applications on volar forearm sites of non‐professional volunteers and HCW without visible skin pathologies, mimicking in‐use conditions (18 applications in 6 hours for 3 weeks).
Gels A – C contained 70% ethanol with glycerine concentrations ranging from 2.0% to 8.0% v/v. Gel D contained 75% ethanol and 2.0% v/v glycerine and gel E contained 80% ethanol and 2.0% v/v glycerine. Gel F contained 70% isopropanol and 2% v/v glycerin. Skin parameters revealed an unchanged TEWL and increased skin hydration after 7 hours, which persisted until 24 hours in non‐HCW workers for all gel types. A slight scaly skin was seen in gels containing higher concentrations of ethanol, leading to the suggestion that 70% ethanol or isopropanol are preferable. Noteworthy is that the hydrating effects were more striking for the gels with an elevated glycerine concentration. In contrast to the biophysical measurements, the sensorial assessment of the professional volunteers revealed lower acceptance to gel F (isopropanol mixture), due to a worse smell and drying properties. 53
This result is consistent with Pittet et al, who tested the skin tolerability and user acceptability of three alcohol based formulations on 38 nurses with previous hand hygiene actions of up to 10 times per hour. There was a higher tolerance and skin condition improvement with formulation A (80% v/v ethanol + glycerol) and formulation B (75% v/v isopropyl alcohol + glycerol), while formulation C (75% v/v isopropyl alcohol + isopropyl myristate) caused more dryness and irritation. 54
4. DISCUSSION
Hands are an important route of transmission for all kinds of pathogens. Therefore, hand disinfection is of crucial importance in the prevention of chains of infections. Hand hygiene with ABHR should balance the two goals of keeping the skin from acquiring or transmitting nosocomial pathogens and protecting the skin barrier. Despite the proven efficacy of alcohol‐based products, 55 they lack acceptance, and low compliance is found in HCW, as repeated use of alcohol may lead to excessive drying and a stinging sensation. 10 , 53 However, in general, ABHR cause significantly less skin damage than hand washing with detergents or antiseptic soaps.
Based on the findings of this systematic search, hand disinfectants should be continued as standard hygiene procedures in healthcare centers where ABHR are used routinely many times a day. The tolerability of n‐propanol and isopropanol with or without additives has been established in various studies. 6 , 9 , 22 , 23 , 24 , 50 , 51 , 52 , 53 , 54
The current systematic review shows that hand disinfectants with n‐propanol concentrations of 60%, or certain combinations of propan‐1‐ol and propan‐2‐ol showed little to no irritation in intact skin and previously irritated skin. 6 , 22 , 23 , 29 Bioengineering measurements demonstrated a significant exponential increase, including water loss and skin erythema, after consecutive application of SLS compared to repetitive application of Pro/SLS or propanol alone. In all cases, regardless of the sequence, tandem application of one alcohol or the combined use of an alcohol‐containing disinfectant and a detergent induced less damage to the skin compared to the application of SLS alone. Even on experimentally pre‐irritated skin, n‐propanol only induced minor skin damage. These findings play a decisive role in terms of user compliance of disinfection procedures, especially during the current COVID‐19 pandemic.
In most of the included studies, occlusion was the method of choice to create a milieu similar to wearing gloves. Occlusion increases the penetration of substances and leads to the development of a moist environment on the skin and, thus, to the swelling of keratinocyte layers. This disturbance results in a lower sensitivity threshold to harmful noxious agents. The same conditions can be observed in atopic skin with a compromised skin barrier. There, risk factors may cause a higher susceptibility for ICD. Furthermore, the repetitive nature of the irritant exposure does not allow the skin to recover, leading to persistent dermatitis. These additional aggravating factors play a critical role in the degree of irritation. Atopic dermatitis has often, but not invariably, been associated with an increased response to irritant exposure. 34 , 38 , 39 , 40 , 56 , 57 However, Kampf et al found that the exposure to n‐propanol or propanol‐based hand disinfectants in atopic skin did not enhance the development of hand eczema. The authors selected the atopic population through the Erlangen atopy score. This score allows a standardized assessment and evaluation of a probable atopic skin diathesis, but does not establish a definitive diagnosis of atopic dermatitis. In contrast, Angelova Fischer et al demonstrated significant differences in the severity of the barrier function impairment, assessed by in vivo and in vitro methods, after exposure to different concentrations of n‐propanol between an atopic dermatitis population in the stage of remission and healthy controls. 43 The same authors described the correlation between lipid depletion, primarily NMF, and the consequential skin dryness after repetitive occlusive exposure to n‐propanol in various concentrations. These findings prove an irritating potency of ABHR due to their lipid‐dissolving property.
Therefore, restrictions on application of alcohol‐based disinfectant should be considered in persons with a distinctive barrier defect (eg in atopic eczema), as trials have shown the significant skin damaging results in an atopic population compared to a non‐atopic group. 43 Clearly, complex in vitro methods provide a more comprehensive assessment of pathophysiological responses (release of cytokines and inflammatory mediators) and physiochemical interactions of skin irritation processes compared to bioengineering methods. 58 With regard to clinical relevance, the detection of in vitro irritation does not always correlate with a change in superficial skin morphology. Nevertheless, these recent results show that irritant effects of short‐chain alcohols are undoubtedly not harmless and provide enough evidence to raise critical questions on how to evaluate the irritancy of different classes of irritants (in this case, alcohols).
Since the only two options for hand hygiene procedures are hand washing with antiseptic soaps or ABHR, it is necessary to consider and compare the existing evidences of the irritation potential of both hygiene modalities. With respect to our reviewed publications, SLS showed greater irritability in in vivo as well as in in vitro tests compared to n‐propanol or isopropanol. While recent studies indicate a higher risk of skin irritation for n‐propanol and isopropanol than reported in the past, this risk still seems to be lower than that for frequent handwashing with detergents.
In conclusion, it is extremely important to recall that alcohol‐based formulations for hand disinfections (whether isopropyl alcohol or n‐propanol in 60%–90% vol/vol) are less irritant on skin than most antiseptic or non‐antiseptic detergents and that alcohol‐base formulations, with the addition of appropriate humectants, are at least as tolerable and efficacious as detergents. Commercial ABHR (with only few exceptions) contain hydrating agents 59 that have re‐fatting properties and provide moisture to the skin. It has been proved that humectants promote skin hydration and minimize the incidence of irritant dermatitis. 45 , 46 , 60 , 61 , 62 , 63 Besides glycerol, which increases the skin water content and accelerates the recovery of the skin barrier function, 64 MES proved to protect the skin, even when the alcohol‐based solution is applied regularly. In occupations where the repeated application of disinfectants is necessary in the long term, additional moisturizers in alcohol‐based hand rubs is a benefit and may promote tolerability and compliance.
5. CONFLICT OF INTEREST
The authors have declared no conflicts of interest.
AUTHOR CONTRIBUTIONS
Ramona Tasar: Conceptualization; methodology; writing‐original draft; writing‐review and editing. Cornelia Wiegand: Conceptualization; writing‐review and editing. Peter Elsner: Conceptualization; investigation; project administration.
Tasar R, Wiegand C, Elsner P. How irritant are n‐propanol and isopropanol? – A systematic review. Contact Dermatitis. 2021;84:1–14. 10.1111/cod.13722
DATA AVAILABILITY STATEMENT
Data sharing not applicable ‐ no new data generated.
REFERENCES
- 1. Kampf G. Hände‐Hygiene im Gesundheitswesen. Springer‐Verlag Berlin Heidelberg; 2003. [Google Scholar]
- 2. Pittet D, Boyce JM. Hand hygiene and patient care: pursuing the Semmelweis legacy. Lancet Infect Dis. 2001;1(Supplement 1):9‐20.11871420 [Google Scholar]
- 3. Pittet D. Improving compliance with hand hygiene in hospitals. Infect Control Hosp Epidemiol. 2000;21(6):381‐386. [DOI] [PubMed] [Google Scholar]
- 4. Bischoff WE, Reynolds TM, Sessler CN, Edmond MB, Wenzel RP. Handwashing compliance by health care workers: the impact of introducing an accessible, alcohol‐based hand antiseptic. Arch Intern Med. 2000;160(7):1017‐1021. [DOI] [PubMed] [Google Scholar]
- 5. Vicca AF. Nursing staff workload as a determinant of methicillin‐resistant Staphylococcus aureus spread in an adult intensive therapy unit. J Hosp Infect. 1999;43(2):109‐113. [DOI] [PubMed] [Google Scholar]
- 6. Löffler H, Kampf G, Schmermund D, Maibach HI. How irritant is alcohol? Br J Dermatol. 2007;157(1):74‐81. [DOI] [PubMed] [Google Scholar]
- 7. Keller J, Wolfensberger A, Clack L, et al. Do wearable alcohol‐based handrub dispensers increase hand hygiene compliance? ‐ a mixed‐methods study. Antimicrob Resist Infect Control. 2018;7(1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. WHO Guidelines Approved by the Guidelines Review Committee . WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care Is Safer Care. Geneva, Switzerland: World Health Organization; 2009. [PubMed] [Google Scholar]
- 9. Stutz N, Becker D, Jappe U, et al. Nurses' perceptions of the benefits and adverse effects of hand disinfection: alcohol‐based hand rubs vs. hygienic handwashing: a multicentre questionnaire study with additional patch testing by the German Contact Dermatitis Research Group. Br J Dermatol. 2009;160(3):565‐572. [DOI] [PubMed] [Google Scholar]
- 10. Lubbe J, Ruffieux C, Perrenoud D. A stinging cause for preventive skin care. Lancet. 2000;356(9231):768‐769. [DOI] [PubMed] [Google Scholar]
- 11. Voss A, Widmer AF. No time for handwashing!? Handwashing versus alcoholic rub: can we afford 100% compliance? Infect Control Hosp Epidemiol. 1997;18(3):205‐208. [DOI] [PubMed] [Google Scholar]
- 12. WHO . WHO‐recommended handrub formulations. WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care Is Safer Care; 2009. https://www.ncbi.nlm.nih.gov/books/NBK144054/ [PubMed] [Google Scholar]
- 13. Kratzel A, Todt D, V'kovski P, et al. Efficient inactivation of SARS‐CoV‐2 by WHO‐recommended hand rub formulations and alcohols. bioRxiv. 2020. 10.1101/2020.03.10.986711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Malik M, English J. Irritant hand dermatitis in health care workers. Occup Med (Lond). 2015;65(6):474‐476. [DOI] [PubMed] [Google Scholar]
- 15. Nilsson E, Back O. The importance of anamnestic information of atopy, metal dermatitis and earlier hand eczema for the development of hand dermatitis in women in wet hospital work. Acta Derm Venereol. 1986;66(1):45‐50. [PubMed] [Google Scholar]
- 16. Smit HA, Burdorf A, Coenraads PJ. Prevalence of hand dermatitis in different occupations. Int J Epidemiol. 1993;22(2):288‐293. [DOI] [PubMed] [Google Scholar]
- 17. Stingeni L, Lapomarda V, Lisi P. Occupational hand dermatitis in hospital environments. Contact Dermatitis. 1995;33(3):172‐176. [DOI] [PubMed] [Google Scholar]
- 18. Flyvholm MA, Bach B, Rose M, Jepsen KF. Self‐reported hand eczema in a hospital population. Contact Dermatitis. 2007;57(2):110‐115. [DOI] [PubMed] [Google Scholar]
- 19. Boyce JM, Kelliher S, Vallande N. Skin irritation and dryness associated with two hand‐hygiene regimens: soap‐and‐water hand washing versus hand antisepsis with an alcoholic hand gel. Infect Control Hosp Epidemiol. 2000;21(7):442‐448. [DOI] [PubMed] [Google Scholar]
- 20. Rotter ML, Simpson RA, Koller W. Surgical hand disinfection with alcohols at various concentrations: parallel experiments using the new proposed European standards method. Infect Control Hosp Epidemiol. 1998;19(10):778‐781. [DOI] [PubMed] [Google Scholar]
- 21. Winnefeld M, Richard MA, Drancourt M, Grob JJ. Skin tolerance and effectiveness of two hand decontamination procedures in everyday hospital use. Br J Dermatol. 2000;143(3):546‐550. [DOI] [PubMed] [Google Scholar]
- 22. Slotosch CM, Kampf G, Löffler H. Effects of disinfectants and detergents on skin irritation. Contact Dermatitis. 2007;57(4):235‐241. [DOI] [PubMed] [Google Scholar]
- 23. Kappes UP, Goritz N, Wigger‐Alberti W, Heinemann C, Elsner P. Tandem application of sodium lauryl sulfate and n‐propanol does not lead to enhancement of cumulative skin irritation. Acta Derm Venereol. 2001;81(6):403‐405. [DOI] [PubMed] [Google Scholar]
- 24. Clemmensen A, Andersen F, Petersen TK, Kalden H, Melgaard A, Andersen KE. The irritant potential of n‐propanol (nonanoic acid vehicle) in cumulative skin irritation: a validation study of two different human in vivo test models. Skin Res Technol. 2008;14(3):277‐286. [DOI] [PubMed] [Google Scholar]
- 25. Cartner T, Brand N, Tian K, et al. Effect of different alcohols on stratum corneum kallikrein 5 and phospholipase A2 together with epidermal keratinocytes and skin irritation. Int J Cosmet Sci. 2017;39(2):188‐196. [DOI] [PubMed] [Google Scholar]
- 26. García‐Gavín J, Lissens R, Timmermans A, Goossens A. Allergic contact dermatitis caused by isopropyl alcohol: a missed allergen? Contact Dermatitis. 2011;65(2):101‐106. [DOI] [PubMed] [Google Scholar]
- 27. Fluhr JW, Akengin A, Bornkessel A, et al. Additive impairment of the barrier function by mechanical irritation, occlusion and sodium lauryl sulphate in vivo. Br J Dermatol. 2005;153(1):125‐131. [DOI] [PubMed] [Google Scholar]
- 28. Wigger‐Alberti W, Spoo J, Schliemann‐Willers S, Klotz A, Elsner P. The tandem repeated irritation test: a new method to assess prevention of irritant combination damage to the skin. Acta Derm Venereol. 2002;82(2):94‐97. [DOI] [PubMed] [Google Scholar]
- 29. Lubbe J, Ruffieux C, van Melle G, Perrenoud D. Irritancy of the skin disinfectant n‐propanol. Contact Dermatitis. 2001;45(4):226‐231. [DOI] [PubMed] [Google Scholar]
- 30. Angelova‐Fischer I, Stilla T, Kezic S, Fischer TW, Zillikens D. Barrier function and natural moisturizing factor levels after cumulative exposure to short‐chain aliphatic alcohols and detergents: results of occlusion‐modified tandem repeated irritation test. Acta Derm Venereol. 2016;96(7):880‐884. [DOI] [PubMed] [Google Scholar]
- 31. Pedersen LK, Held E, Johansen JD, Agner T. Less skin irritation from alcohol‐based disinfectant than from detergent used for hand disinfection. Br J Dermatol. 2005;153(6):1142‐1146. [DOI] [PubMed] [Google Scholar]
- 32. Pedersen LK, Held E, Johansen JD, Agner T. Short‐term effects of alcohol‐based disinfectant and detergent on skin irritation. Contact Dermatitis. 2005;52(2):82‐87. [DOI] [PubMed] [Google Scholar]
- 33. Haddock NF, Wilkin JK. Cutaneous reactions to lower aliphatic alcohols before and during disulfiram therapy. Arch Dermatol. 1982;118(3):157‐159. [PubMed] [Google Scholar]
- 34. Coenraads PJ, Diepgen TL. Risk for hand eczema in employees with past or present atopic dermatitis. Int Arch Occup Environ Health. 1998;71(1):7‐13. [DOI] [PubMed] [Google Scholar]
- 35. Kavli G, Angell E, Moseng D. Hospital employees and skin problems. Contact Dermatitis. 1987;17(3):156‐158. [DOI] [PubMed] [Google Scholar]
- 36. Nilsson E. Individual and environmental risk factors for hand eczema in hospital workers. Acta Derm Venereol Suppl. 1986;128:1‐63. [PubMed] [Google Scholar]
- 37. Behroozy A, Keegel TG. Wet‐work exposure: a main risk factor for occupational hand dermatitis. Saf Health Work. 2014;5(4):175‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hamnerius N, Svedman C, Bergendorff O, Björk J, Bruze M, Pontén A. Wet work exposure and hand eczema among healthcare workers: a cross‐sectional study. Br J Dermatol. 2018;178(2):452‐461. [DOI] [PubMed] [Google Scholar]
- 39. Ibler KS, Jemec GB, Agner T. Exposures related to hand eczema: a study of healthcare workers. Contact Dermatitis. 2012;66(5):247‐253. [DOI] [PubMed] [Google Scholar]
- 40. Lammintausta K, Kalimo K. Atopy and hand dermatitis in hospital wet work. Contact Dermatitis. 1981;7(6):301‐308. [DOI] [PubMed] [Google Scholar]
- 41. Kampf G, Wigger‐Alberti W, Wilhelm KP. Do atopics tolerate alcohol‐based hand rubs? A prospective, controlled, randomized double‐blind clinical trial. Acta Derm Venereol. 2006;86(2):140‐143. [DOI] [PubMed] [Google Scholar]
- 42. Kampf G, Wigger‐Alberti W, Schoder V, Wilhelm KP. Emollients in a propanol‐based hand rub can significantly decrease irritant contact dermatitis. Contact Dermatitis. 2005;53(6):344‐349. [DOI] [PubMed] [Google Scholar]
- 43. Angelova‐Fischer I, Soltanipoor M, Stilla T, Fischer TW, Kezic S, Jakasa I. Barrier damaging effects of n‐propanol in occlusion‐modified tandem repeated irritation test: modulation by exposure factors and atopic skin disease. Contact Dermatitis. 2020;82(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 44. Soltanipoor M, Stilla T, Riethmüller C, et al. Specific barrier response profiles after experimentally induced skin irritation in vivo. Contact Dermatitis. 2018;79(2):59‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kampf G, Löffler H. Dermatological aspects of a successful introduction and continuation of alcohol‐based hand rubs for hygienic hand disinfection. J Hosp Infect. 2003;55(1):1‐7. [DOI] [PubMed] [Google Scholar]
- 46. Halkier‐Sörensen L, Thestrup‐Pedersen K. The efficacy of a moisturizer (Locobase) among cleaners and kitchen assistants during everyday exposure to water and detergents. Contact Dermatitis. 1993;29(5):266‐271. [DOI] [PubMed] [Google Scholar]
- 47. Proske OC, Sauermann G Pietsch H., Rohde B. Die Hautverträglichkeit von Mecetroniumetilsulfat in einem Händedesinfektionsmittel ‐ eine klinische Studie. 1995. https://www.bode-science-center.de/science/studien/article/studie-die-hautvertraeglichkeit-von-mecetroniumetilsulfat-in-einem-haendedesinfektionsmittel-ei.html. [Google Scholar]
- 48. Li F, Conroy E, Visscher M, Wickett RR. The ability of electrical measurements to predict skin moisturization. I. Effects of NaCl and glycerin on short‐term measurements. J Cosmet Sci. 2001;52(1):13‐22. [PubMed] [Google Scholar]
- 49. Rotter ML. Arguments for alcoholic hand disinfection. J Hosp Infect. 2001;48(Supplement A):S4‐S8. [DOI] [PubMed] [Google Scholar]
- 50. Kampf G, Muscatiello M. Dermal tolerance of sterillium, a propanol‐based hand rub. J Hosp Infect. 2003;55(4):295‐298. [DOI] [PubMed] [Google Scholar]
- 51. Pietsch H. Hand antiseptics: rubs versus scrubs, alcoholic solutions versus alcoholic gels. J Hosp Infect. 2001;48(Suppl A):S33‐S36. [DOI] [PubMed] [Google Scholar]
- 52. Kramer A, Bernig T, Kampf G. Clinical double‐blind trial on the dermal tolerance and user acceptability of six alcohol‐based hand disinfectants for hygienic hand disinfection. J Hosp Infect. 2002;51(2):114‐120. [DOI] [PubMed] [Google Scholar]
- 53. Houben E, De Paepe K, Rogiers V. Skin condition associated with intensive use of alcoholic gels for hand disinfection: a combination of biophysical and sensorial data. Contact Dermatitis. 2006;54(5):261‐267. [DOI] [PubMed] [Google Scholar]
- 54. Pittet D, Allegranzi B, Sax H, Chraiti MN, Griffiths W, Richet H. Double‐blind, randomized, crossover trial of 3 hand rub formulations: fast‐track evaluation of tolerability and acceptability. Infect Control Hosp Epidemiol. 2007;28(12):1344‐1351. [DOI] [PubMed] [Google Scholar]
- 55. Picheansathian W. A systematic review on the effectiveness of alcohol‐based solutions for hand hygiene. Int J Nurs Pract. 2004;10(1):3‐9. [DOI] [PubMed] [Google Scholar]
- 56. Dickel H, Bruckner TM, Schmidt A, Diepgen TL. Impact of atopic skin diathesis on occupational skin disease incidence in a working population. J Invest Dermatol. 2003;121(1):37‐40. [DOI] [PubMed] [Google Scholar]
- 57. Thyssen JP, Johansen JD, Linneberg A, Menné T. The epidemiology of hand eczema in the general population–prevalence and main findings. Contact Dermatitis. 2010;62(2):75‐87. [DOI] [PubMed] [Google Scholar]
- 58. Boelsma E, Tanojo H, Boddé HE, Ponec M. Assessment of the potential irritancy of oleic acid on human skin: evaluation in vitro and in vivo. Toxicol In Vitro. 1996;10(6):729‐742. [DOI] [PubMed] [Google Scholar]
- 59. Rotter ML, Koller W, Neumann R. The influence of cosmetic additives on the acceptability of alcohol‐based hand disinfectants. J Hosp Infect. 1991;18(Suppl B):57‐63. [DOI] [PubMed] [Google Scholar]
- 60. Menegueti M, Laus A, Aparecida Ciol M, et al. Glycerol content within the WHO ethanol‐based handrub formulation: balancing tolerability with antimicrobial efficacy. Antimicrob Resist Infect Control. 2019;8(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Purnamawati S, Indrastuti N, Danarti R, Saefudin T. The role of moisturizers in addressing various kinds of dermatitis: a review. Clin Med Res. 2017;15(3–4):75‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wigger‐Alberti W, Caduff L, Burg G, Elsner P. Experimentally induced chronic irritant contact dermatitis to evaluate the efficacy of protective creams in vivo. J Am Acad Dermatol. 1999;40(4):590‐596. [DOI] [PubMed] [Google Scholar]
- 63. Williams C, Wilkinson SM, McShane P, et al. A double‐blind, randomized study to assess the effectiveness of different moisturizers in preventing dermatitis induced by hand washing to simulate healthcare use. Br J Dermatol. 2010;162(5):1088‐1092. [DOI] [PubMed] [Google Scholar]
- 64. Fluhr JW, Gloor M, Lehmann L, Lazzerini S, Distante F, Berardesca E. Glycerol accelerates recovery of barrier function in vivo. Acta Derm Venereol. 1999;79(6):418‐421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable ‐ no new data generated.


