Abstract
Aims/Introduction
This study aimed to explore the association between glycemic control before admission with severity and mortality of coronavirus disease 2019, and tried to reveal the mechanism.
Materials and Methods
A total of 77 inpatients were grouped into sufficient control group (glycated hemoglobin [HbA1c] <6.5%, n = 49) and insufficient control group (HbA1c ≥6.5%, n = 28). Regression models were used to analyze the clinical data.
Results
Compared with patients with HbA1c <6.5, patients with HbA1c ≥6.5 showed higher heart rate (101 vs 89 b.p.m., P = 0.012), lower percutaneous oxygen saturation (93 vs 97%, P = 0.001), higher levels of multiple indicators of inflammation, such as white blood cell count (7.9 vs 5.9 × 109/L, P = 0.019), neutrophil count (6.5 vs 4.1 × 109/L, P = 0.001), high‐sensitivity C‐reactive protein (52 vs 30 mg/L, P = 0.025) and serum ferritin (1,287 vs 716 μg/L, P = 0.023), as well as lower levels of lymphocyte count (0.7 vs 0.8 × 109/L, P = 0.049) at hospital admission. Thus, patients with HbA1c ≥6.5 were more likely to develop secondary respiratory infections (25 [89%] vs 33 [67%], P = 0.032) and acute respiratory distress syndrome (17 [61%] vs 14 [29%], P = 0.006) than patients with HbA1c <6.5, resulting in a higher proportion of critically ill patients (19 [68%] vs 18 [37%], P = 0.009) and non‐survivors (13 [46%] vs 11 [22%], P = 0.029). After adjustment for potential risk factors, HbA1c was independently associated with in‐hospital death.
Conclusion
HbA1c was an independent risk factor for poor outcomes in coronavirus disease 2019 patients. Severe pulmonary infection and consequent acute respiratory distress syndrome might be the primary causes of death in insufficient glycemic control patients.
Keywords: Coronavirus disease 2019, Glycemic control, Prognosis
Hemoglobin A1c was a significant independent risk factor associated with in‐hospital death of patients with coronavirus disease 2019. Severe pulmonary infection and consequent acute respiratory distress syndrome, but not extrapulmonary organ injuries, might be the primary cause of death in patients with insufficient glycemic control before admission.

INTRODUCTION
At the end of 2019, a kind of novel coronavirus pneumonia caused by a newly identified betacoronavirus (severe acute respiratory syndrome coronavirus 2 [SARS‐CoV‐2]) emerged in Wuhan, China. In early February 2020, the World Health Organization declared that the disease caused by SARS‐CoV‐2 was named as coronavirus disease‐19 (COVID‐19). The clinical manifestations of COVID‐19 include fever, dry cough, fatigue, muscle soreness and shortness of breath, and in a small number of patients it might progress to acute respiratory distress syndrome (ARDS) and multiple organ failure 1 . As of 10 May 2020, 221 countries and regions reported as many as 4 million confirmed cases. Cao et al. 2 summarized 31 studies about the clinical manifestations of COVID‐19, and found that the incidence of required intensive care was 29.3%, the incidence of ARDS was 28.8%, the incidence of multiple organ dysfunction syndrome was 8.5% and the mortality rate was 6.8%.
The mortality rate of COVID‐19 is affected by multiple factors, so we can find that the mortality rate varied widely in different countries or at different periods of the outbreak. The first study of the initial 41 confirmed cases with COVID‐19 in China showed that six of 41 (15%) patients died 3 , but the estimated final mortality rate is approximately 5.5% according to the public data. However, the real‐time mortality rate in European and American countries ranged from 3.3% to 13.3% when this article was written. Although the mortality rate varied widely in different countries, the death cases seemed to have some striking common characteristics, such as advanced age and comorbidities 4 , 5 . Chen et al. 4 summarized the clinical characteristics of 113 deceased patients, and found that the median age of deceased patients (68 years) was significantly older than recovered patients (51 years), hypertension and other cardiovascular comorbidities were more frequent among deceased patients (54 [48%] and 16 [14%]) than recovered patients (39 [24%] and 7 [4%]). Chen et al. 5 found that advanced age is the strongest risk factor for a fatal outcome, and dyspnea, coronary heart disease and cerebrovascular disease are also independent risk factors. Therefore, further revelation of the pathophysiological mechanisms of disease progression in COVID‐19 patients with comorbidities is urgently required for lowering the mortality rate.
Insufficient glycemic control (hyperglycemia) is mainly caused by low awareness and control rates of diabetes mellitus, which is the leading chronic non‐infectious disease, with approximately half a billion people affected worldwide 6 . Many studies proved that hyperglycemia could affect the cellular immune response 7 , improve the infection susceptibility and infection‐related mortality 8 , 9 . Hyperglycemia has already been proved to promote the progression and worsen the outcomes of other similar severe corona viral infections, including the severe acute respiratory syndrome and the Middle East respiratory syndrome 10 , 11 .
Two retrospective studies carried out in Wuhan showed that diabetes mellitus is an independent risk factors for mortality of adult inpatients with COVID‐19; in one of the studies, the relative risk was 2.85, and in the other study, the relative risk was 1.58 12 , 13 . One possible explanation for the huge difference between the relative risk of the two studies is that some diabetes mellitus patients were misgrouped because of low awareness rate of diabetes, and another possible explanation is that the glycemic control before admission was different in the diabetes mellitus patients of the two studies.
Hemoglobin A1c (HbA1c) is an objective indicator that can be used as a measure of diabetic control, reflecting long‐term glucose concentrations over the preceding months 14 . In the present study, to avoid the misgrouping of patients, we used HbA1c as the main grouping criteria, and tried to find the differences between patients with sufficient glycemic control and insufficient glycemic control, to explore whether HbA1c levels can predict the relative risk of poor outcomes, and how the relative risk changes with each 1% increase of HbA1c in COVID‐19 patients.
METHODS
Study design and participants
The present retrospective study included 77 laboratory‐confirmed adult inpatients admitted to two intensive care units of Sino‐French Branch of Tongji Hospital (Wuhan, China), a designed hospital for severe or critically ill patients with COVID‐19, and had definite outcomes (discharged or died) during February 2020. All 77 patients were initially presented as severe patients (n = 40) or critically ill patients (n = 37) at the time of admission. All the patients were categorized based on HbA1c level as HbA1c <6.5% (sufficient group, n = 49) or HbA1c ≥6.5% (insufficient group, n = 28). We chose 6.5% as the cut‐off point for sufficient degree of glycemic control for the following reasons: (i) HbA1c ≥6.5% could be used as a diagnostic marker for diabetes according to the Standards of Medical Care in Diabetes released by the American Diabetes Association 15 , based on this, we could diagnose many newly diagnosed diabetes patients; and (ii) the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) study showed that intensive control (HbA1c <6.5%) reduced the incidence of combined major macrovascular and microvascular events 16 .
The therapeutic schedule was formulated according to the patient’s condition and not influenced by the study. Finally, we retrospectively evaluated and analyzed the clinical data of the treatment course and outcome.
The study protocol was approved by the Ethics Commission of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. All participants provided oral informed consent; written informed consent was waived due to the urgent outbreak of this communicable disease.
Definitions
Case definition
According to the Guidelines for Diagnosis and Management of COVID‐19 (6th edition, in Chinese) released by the National Health Commission of China 17 , all 77 patients met the following diagnostic criteria: (i) epidemiology history; (ii) fever or other respiratory symptoms and typical computed tomography (CT) appearance of viral pneumonia; and (iii) real‐time reverse transcription polymerase chain reaction (RT–PCR) detection for SARS‐CoV‐2 ribonucleic acid (RNA) is positive.
The clinical classifications of COVID‐19 are as follows. For severe cases, meeting any of the following: (i) respiratory distress or respiratory rates ≥30 breaths/min; (ii) SpO2 ≤93% at a rest state; (iii) arterial partial pressure of oxygen : fraction of inspired oxygen ratio ≤300; and (iv) patients with >50% lesions progression within 24–48 h in pulmonary imaging. For critically ill cases, meeting any of the following: (i) respiratory failure occurs and mechanical ventilation is required; (ii) shock occurs; and (iii) complicated with other organ failure that requires monitoring and treatment in intensive care unit.
ARDS
The diagnosis of ARDS needed to meet the following criteria: (i) acute (<7 days) onset or exacerbation of dyspnea; (ii) X‐ray or CT examination showed bilateral lung infiltration, and could not be explained by pleural effusion, atelectasis and nodules; (iii) rule out respiratory failure as a result of heart failure and fluid overload; and (iv) arterial partial pressure of oxygen : fraction of inspired oxygen ≤300 mmHg.
Secondary respiratory infection
The diagnostic criteria of secondary respiratory infection are as follows: (i) purulent sputum; (ii) significant elevation of neutrophils, C‐reactive protein (CRP) and procalcitonin (PCT); (iii) typical CT imaging features determined by experienced radiologists, such as consolidation, air bronchography sign and cavity; and (iv) antibiotic therapy was effective.
Data collection
We collected demographic data, comorbidities, clinical symptoms and signs, vital signs, laboratory measurements findings, and clinical outcome from the electronic medical records using data collection forms, and the forms were checked independently by two researchers.
Laboratory measurements
Real time RT–PCR assay for SARS‐CoV‐2
All the patients were confirmed by a real‐time RT–PCR‐positive result from the upper respiratory tract swab specimens by local Chinese Center for Disease Control and Prevention before admission. After admission to Tongji Hospital, re‐examination for SARS‐CoV‐2 PCR was carried out. In brief, throat swab samples were collected and stored in virus preservation solution. Total RNA was extracted within 2 h using the respiratory sample RNA isolation kit according to the manufacturer’s instruction (Zhongzhi, Wuhan, China). Then, two target genes including open reading frame 1ab (ORF1ab) and nucleocapsid protein (N) of SARS‐CoV‐2 were detected using real‐time RT–PCR according to the manufacturer’s instructions (Bio‐germ, Shanghai, China).
Clinical laboratory measurements
The HbA1c test was routinely carried out for all the patients using high‐performance liquid chromatography. Other laboratory values were obtained by routine tests, including complete blood count, urine routine test, serum biochemical indexes (including renal and liver function, electrolytes, lactate dehydrogenase [LDH] and lipid), coagulation function test, N‐terminal brain natriuretic propeptide (NT‐proBNP), cardiac troponin I (cTnI) and inflammation markers (including high‐sensitivity CRP [hsCRP], PCT, serum ferritin and erythrocyte sedimentation rate). Examinations frequency was determined by the state of illness.
Statistical analysis
Data are presented as the mean ± standard error or median (interquartile range [IQR]) for continuous variables, and number (percentage) for categorical variables. Comparisons between patients with sufficient (HbA1c <6.5) and insufficient (HbA1c ≥6.5) glycemic control were analyzed using Student’s t‐test, Mann–Whitney U‐test, χ2‐test or Fisher’s exact test. Spearman’s correlation analysis was used to explore the relationship of HbA1c with selected covariates. Bivariate Cox proportional hazards regression analysis was used to evaluate the association of baseline risk factors with in‐hospital death in patients with COVID‐19. The selection of covariates in multivariate Cox proportional hazards regression analysis was mainly based on the results of univariate analysis and risk factors reported in previous literature. SPSS version 20.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Statistical significance was defined by a two sided P‐value <0.05.
RESULTS
Demographics and baseline characteristics of adult patients with COVID‐19
The present study cohort included 77 hospitalized patients with confirmed COVID‐19. They were all identified as severe cases or critically ill cases at admission. Demographics and baseline characteristics at admission are presented in Table 1. The mean age was 63.6 ± 3.6 years, and 48 (62%) were men. Comorbidities were present in 56 (73%) patients. Hypertension and diabetes were the most common comorbidities, followed by chronic cardiovascular disease and chronic respiratory disease. The most common manifestations at onset were fever (61 [79%]), dry cough (61 [79%]), chest tightness (30 [36%]), fatigue (28 [36%]), diarrhea (21 [27%]) and myalgia (21 [27%]). As Tongji Hospital is the designated hospital for the treatment of severe cases with confirmed COVID‐19, patients admitted to the hospital often manifested with dyspnea (44 [57%]). Less common symptoms were headache, hemoptysis, nausea or vomiting, anorexia and palpitations.
Table 1.
Demographics and baseline characteristics of COVID‐19 patients with sufficient and insufficient glycemic control
| Characteristics | Total (n = 77) | HbA1c <6.5 (n = 49) | HbA1c ≥6.5 (n = 28) | P‐value |
|---|---|---|---|---|
| Age (years) | 63.6 ± 3.6 | 62.2 ± 3.1 | 66.2 ± 2.5 | 0.230 |
| Sex | 0.213 | |||
| Male | 48 (62%) | 28 (57%) | 20 (71%) | |
| Female | 29 (38%) | 21 (43%) | 8 (29%) | |
| Comorbidity | ||||
| Hypertension | 32 (42%) | 19 (39%) | 30 (61%) | 0.512 |
| Diabetes | 33 (43%) | 5 (10%) | 28 (100%) | 0.000* |
| Cardiovascular disease | 15 (20%) | 11 (22%) | 4 (14%) | 0.552 |
| Chronic respiratory disease | 8 (10%) | 6 (12%) | 2 (7%) | 0.703 |
| Chronic kidney disease | 7 (9%) | 6 (19%) | 1 (4%) | 0.412 |
| Chronic liver disease | 1 (1%) | 0 (0%) | 1 (2%) | 1.000 |
| Malignancy | 1 (1%) | 0 (0%) | 1 (4%) | 0.364 |
| Signs and symptoms | ||||
| Fever | 61 (79%) | 40 (82%) | 21 (71%) | 0.490 |
| Cough | 61 (79%) | 38 (78%) | 23 (82%) | 0.633 |
| Fatigue | 28 (36%) | 21 (43%) | 7 (25%) | 0.117 |
| Myalgia | 21 (27%) | 15 (31%) | 6 (21%) | 0.384 |
| Headache | 10 (13%) | 7 (14%) | 3 (11%) | 0.739 |
| Chest tightness | 30 (39%) | 18 (37%) | 12 (43%) | 0.633 |
| Dyspnea | 44 (57%) | 28 (57%) | 16 (57%) | 1.000 |
| Hemoptysis | 6 (8%) | 4 (8%) | 2 (7%) | 1.000 |
| Diarrhea | 21 (27%) | 12 (25%) | 9 (32%) | 0.468 |
| Nausea or vomiting | 11 (14%) | 8 (16%) | 3 (11%) | 0.737 |
| Anorexia | 11 (14%) | 7 (14%) | 4 (14%) | 1.000 |
| Palpitation | 8 (10%) | 7 (14%) | 1 (4%) | 0.246 |
| Vital signs on admission | ||||
| Systolic pressure (mm Hg) | 132 ± 2.8 | 129 ± 3.4 | 135 ± 4.8 | 0.340 |
| Heart rate (b.p.m.) | 94 ± 6.3 | 89 ± 9.1 | 101 ± 5.0 | 0.012* |
| Respiratory rate, per min | 22 (20–26) | 22 (20–25) | 23 (20–30) | 0.398 |
| Percutaneous oxygen saturation (%) | 96 (91–99) | 97 (95–99) | 93 (87–97) | 0.001* |
Data are presented as the mean ± standard error or median (interquartile range) for continuous variables, and n (%) for categorical variables. P‐values comparing patients with glycated hemoglobin (HbA1c) <6.5 and HbA1c ≥6.5 are from Student’s t‐test, Mann–Whitney U‐test, χ2‐test or Fisher’s exact test.
COVID‐19, coronavirus disease 2019; HbA1c, glycated hemoglobin.
P < 0.05.
Patients were categorized based on HbA1c levels, as HbA1c <6.5 (sufficient control, n = 49) or HbA1C ≥6.5 (insufficient control, n = 28). Five (10%) patients in the group of HbA1c <6.5 were diabetes patients with excellent glycemic control, and all the patients with HbA1c ≥6.5 had diabetes as expected. Compared with patients with HbA1c <6.5, patients with HbA1c ≥6.5 manifested with a higher heart rate (101 ± 5.0 vs 89 ± 9.1 b.p.m., P = 0.012) and lower percutaneous oxygen saturation (93% [IQR 87–97] vs 97% [IQR 95–99], P = 0.001) at hospital admission, presenting more severe hypoxemia. In the correlation analysis, percutaneous oxygen saturation was also inversely correlated with HbA1c levels (r = −0.387, P = 0.001). However, age, sex, other comorbidities, signs and symptoms at onset, systolic pressure, and respiratory rate were similar across groups.
Baseline laboratory parameters of adult patients with COVID‐19
Laboratory findings at hospital admission are summarized in Table 2. Of all the patients, median levels of lymphocyte count (0.8 × 109/L [IQR 0.6–1.2], decreased), hemoglobin (123 g/L [IQR 112–138], decreased), albumin (32 g/L [IQR, 28–37], decreased), LDH 351 U/L [IQR 248–516], increased), fibrinogen (4.6 g/L [IQR 3.2–5.6], increased), D‐dimer (1.8 μg/mL [IQR 0.8–9.2], increased), hsCRP (41 mg/L [IQR 6–114], increased), serum ferritin (797 μg/L [IQR 446–1,708], increased), PCT (0.16 ng/mL [IQR 0.09–0.80], increased) and erythrocyte sedimentation rate (34 mm/h [IQR 15–62], increased) were deviated from normal reference.
Table 2.
Baseline laboratory findings of COVID‐19 patients with sufficient and insufficient glycemic control
| Normal range | Total (n = 77) | HBA1c <6.5 (n = 49) | HBA1c ≥6.5 (n = 28) | P‐value | |
|---|---|---|---|---|---|
| Hematological | |||||
| WBC count (×109/L) | 3.5–9.5 | 6.7 (4.8–10.5) | 6.0 (4.7–8.7) | 7.9 (5.9–13.4) | 0.019* |
| Neutrophil count (×109/L) | 1.8–6.3 | 4.9 (3.1–9.2) | 4.1 (2.8–7.5) | 6.5 (4.4–11.9) | 0.010* |
| Lymphocyte count (×109/L) | 1.1–3.2 | 0.8 (0.6–1.2) | 0.8 (0.7–1.3) | 0.7 (0.5–1.0) | 0.049* |
| Hemoglobin (g/L) | 130–175 | 123 (112–138) | 121 (106–131) | 128 (115–141) | 0.089 |
| Platelet count (×109/L) | 125–350 | 188 (146–280) | 182 (133–267) | 221 (147–338) | 0.242 |
| Biochemical | |||||
| ALT (U/L) | ≤41 | 27 (17–46) | 26 (15–47) | 29 (18–45) | 0.578 |
| AST (U/L) | ≤40 | 33 (20–51) | 28 (20–56) | 35 (19–50) | 0.703 |
| Albumin (g/L) | 35.0–52.0 | 32 (28–37) | 33 (28–38) | 31 (28–33) | 0.164 |
| TB (mmol/L) | ≤26 | 10 (7–18) | 10 (6–17) | 11 (8–19) | 0.211 |
| BUN (mmol/L) | 3.1–8.0 | 5.5 (4.0–10.9) | 4.7 (3.6–6.5) | 6.2 (5.3–10.0) | 0.164 |
| Cr (μmol/L) | 59–104 | 75 (60–97) | 69 (58–94) | 77 (64–98) | 0.870 |
| Potassium (mmol/L) | 3.5–5.1 | 4.4 (4.1–4.8) | 4.4 (4.1–4.9) | 4.5 (4.1–4.8) | 0.634 |
| Sodium (mmol/L) | 136–145 | 141 (137–143) | 140 (138–143) | 141 (138–144) | 0.870 |
| TG (mmol/L) | <1.7 | 1.4 (1.0–2.0) | 1.2 (1.0–2.0) | 1.6 (1.3–1.9) | 0.069 |
| TC (mmol/L) | <5.2 | 3.6 (3.0–4.1) | 3.5 (3.0–4.2) | 3.7 (3.1–4.1) | 0.676 |
| LDH (U/L) | 135–225 | 351 (248–516) | 301 (236–489) | 426 (273–679) | 0.090 |
| HsCTnI (pg/mL) | ≤34.2 | 10.7 (4.3–54.9) | 8.7 (3.9–54.9) | 18.35 (7.5–94.9) | 0.464 |
| NT‐proBNP (pg/mL) | <285 | 223 (116–1,911) | 212 (97–2,070) | 687 (181–1,706) | 0.502 |
| Coagulation function | |||||
| PT (s) | 11.5–14.5 | 14.4 (13.6–15.5) | 14.3 (13.4–15.1) | 14.5 (13.9–15.8) | 0.089 |
| PTA (%) | 75–125 | 86 (73–95) | 87 (78–98) | 84 (71–92) | 0.117 |
| Fibrinogen (g/L) | 2–4 | 4.6 (3.2–5.6) | 4.4 (3.2–5.2) | 5.1 (2.9–6.2) | 0.225 |
| D‐dimer (μg/mL) | <0.5 | 1.8 (0.8–9.2) | 1.5 (0.5–9.6) | 2.4 (1.1–9.0) | 0.114 |
| Inflammation indicators | |||||
| HsCRP (mg/L) | <1 | 41 (6–114) | 30 (4–100) | 52 (22–175) | 0.025* |
| Serum ferritin (μg/L) | 30–400 | 797 (446–1,708) | 716 (289–1,533) | 1287 (731–2,735) | 0.023* |
| PCT (ng/mL) | 0.02–0.05 | 0.16 (0.09–0.80) | 0.18 (0.12–0.84) | 0.14 (0.08–0.79) | 0.546 |
| ESR (mm/h) | 0–15 | 34 (15–62) | 24 (12–72) | 34 (23–62) | 0.449 |
Data are presented as the median (interquartile range). P‐values comparing patients with glycated hemoglobin (HbA1c) <6.5 and HbA1c ≥ 6.5 are from the Mann–Whitney U‐test. *P < 0.05.
WBC count, white blood cell count; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; COVID‐19, coronavirus disease 2019; Cr, creatinine; ESR, erythrocyte sedimentation rate; HsCRP, high‐sensitivity C‐reactive protein; HsCTnI, hypersensitive cardiac troponin; LDH, lactate dehydrogenase; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; TB, total bilirubin; TC, total cholesterol; TG, triglycerides; PCT, procalcitonin; PT, prothrombin time; PTA, prothrombin activity.
Compared with patients with HbA1c <6.5, patients with HbA1c ≥6.5 showed significantly increased levels of multiple indicators of inflammation, such as white blood cell count, neutrophil count, hsCRP and serum ferritin (P < 0.05 for each), as well as a decreased level of lymphocyte count (P = 0.049). However, there was no difference in the injury indicators of other organs (hepatic, cardiac and nephritic) and coagulation function between the two groups.
Complications and outcomes of adult patients with COVID‐19
As shown in Table 3, the common complications in all the patients were secondary respiratory infections (58 [75%]), ARDS (31 [40%]) and acute liver injury (30 [39%]), followed by acute kidney injury (21 [27%]), acute cardiac injury (18 [23%]), hyperkalemia (13 [17%]), disseminated intravascular coagulation (12 [16%]) and heart failure (9 [12%]). Patients with HbA1c ≥6.5% were more likely to develop secondary respiratory infections (25 [89%] vs 33 [67%], P = 0.032) and ARDS (17 [61%] vs 14 [29%], P = 0.006) than patients with HbA1c <6.5%. Thus, patients with HbA1c ≥6.5% were more likely to use corticosteroids, non‐invasive mechanical ventilation and invasive mechanical ventilation (P < 0.05 for each).
Table 3.
Complications and outcome of COVID‐19 patients with sufficient and insufficient glycemic control
| Total (n = 77) | HbA1c <6.5 (n = 49) | HbA1c ≥6.5 (n = 28) | P‐value | |
|---|---|---|---|---|
| Complications | ||||
| Acute respiratory distress syndrome | 31 (40%) | 14 (29%) | 17 (61%) | 0.006* |
| Acute cardiac injury | 18 (23%) | 10 (20%) | 8 (29%) | 0.416 |
| Heart failure | 9 (12%) | 7 (14%) | 2 (7%) | 0.348 |
| Acute kidney injury | 21 (27%) | 13 (27%) | 8 (29%) | 0.847 |
| Disseminated intravascular coagulation | 12 (16%) | 6 (12%) | 6 (21%) | 0.285 |
| Acute liver injury | 30 (39%) | 18 (37%) | 12 (43%) | 0.596 |
| Hyperkalemia | 13 (17%) | 9 (18%) | 4 (14%) | 0.646 |
| Secondary infection | 58 (75%) | 33 (67%) | 25 (89%) | 0.032* |
| Treatment | ||||
| Antibiotics | 50 (65%) | 29 (59%) | 21 (75%) | 0.162 |
| Antiviral treatment | 59 (77%) | 37 (76%) | 22 (79%) | 0.760 |
| Corticosteroids | 27 (35%) | 13 (27%) | 14 (50%) | 0.038* |
| Intravenous immunoglobin | 19 (25%) | 12 (25%) | 7 (25%) | 0.960 |
| Non‐invasive mechanical ventilation | 23 (30%) | 9 (18%) | 14 (50%) | 0.004* |
| Invasive mechanical ventilation | 21 (27%) | 9 (18%) | 12 (43%) | 0.020* |
| Renal replacement therapy | 4 (5%) | 2 (4%) | 2 (7%) | 0.619 |
| Severity and mortality | ||||
| Severe cases at admission | 40 (52%) | 31 (63%) | 9 (32%) | 0.009* |
| Critically ill cases at admission | 37 (48%) | 18 (37%) | 19 (68%) | |
| Discharge | 53 (69%) | 38 (78%) | 15 (54%) | 0.029* |
| Death | 24 (31%) | 11 (22%) | 13 (46%) | |
Data are presented as n (%) for categorical variables. P‐values comparing patients with glycated hemoglobin (HbA1c) <6.5 and HbA1c ≥6.5 are from the χ2‐test or Fisher’s exact test. *P < 0.05.
COVID‐19, coronavirus disease 2019.
Of all the 77 patients, 40 (52%) were severe cases and 37 (48%) were critically ill cases at admission; and finally, 53 (69%) were discharged and 24 (31%) died during the follow‐up period. The proportions of critically ill cases (19 [68%] vs 18 [37%], P = 0.009) and non‐survivors (13 [46%] vs 11 [22%], P = 0.029) were significantly higher in the group of patients with HbA1c ≥6.5% than in the group of patients with HbA1c <6.5%.
Association of HbA1c with in‐hospital death in adult patients with COVID‐19
Bivariate Cox proportional hazards regression analysis was used to evaluate the association of HbA1c with in‐hospital death in adult patients with COVID‐19. In the univariate analysis (Table 4), the hazard ratio (HR) of HbA1c associated with in‐hospital death was 1.300 (95% confidence interval [CI] 1.044–1.617, P = 0.019) for per 1% increase. Meanwhile, age, the presence of cardiovascular disease or chronic respiratory disease, percutaneous oxygen saturation, white blood cell count, lymphocyte count, alanine aminotransferase, sodium, LDH, NT‐proBNP, hypersensitive cTnI (hscTnI), prothrombin time, D‐dimer, hsCRP, serum ferritin and PCT were also associated with the risk of in‐hospital death of patients with COVID‐19.
Table 4.
Univariate Cox proportional hazards regression analysis of baseline factors associated with in‐hospital death
| Variables | HR (95% CI) | P‐value |
|---|---|---|
| Age (years) | 1.051 (1.014–1.089) | 0.007* |
| Sex | ||
| Male | – | |
| Female | 0.516 (0.204–1.300) | 0.160 |
| Hypertension | ||
| No | – | 0.428 |
| Yes | 0.709 (0.303–1.658) | |
| Cardiovascular disease | ||
| No | – | 0.014* |
| Yes | 2.955 (1.247–7.002) | |
| Chronic respiratory disease | ||
| No | – | 0.044* |
| Yes | 2.793 (1.028–7.587) | |
| Diabetes | ||
| No | – | 0.128 |
| Yes | 1.906 (0.831–4.368) | |
| Chronic kidney disease | ||
| No | – | 0.335 |
| Yes | 0.043 (0.000–25.655) | |
| Chronic liver disease | ||
| No | – | 0.614 |
| Yes | 0.047 (0.000–6,799.725) | |
| Percutaneous oxygen saturation (%) | 0.967 (0.948–0.986) | 0.001* |
| White blood cell count (×109/L) | 1.268 (1.177–1.367) | 0.000* |
| Lymphocyte count (×109/L) | 0.047 (0.009–0.248) | 0.000* |
| Alanine aminotransferase (U/L) | 1.004 (1.001–1.007) | 0.004* |
| Creatinine (μmol/L) | 1.000 (0.098–1.002) | 0.929 |
| Sodium (mmol/L) | 1.164 (1.088–1.247) | 0.000* |
| Lactate dehydrogenase (per 100 U/L) | 1.226 (1.134–1.324) | 0.000* |
| NT‐proBNP (per 100 pg/mL) | 1.008 (1.005–1.012) | 0.000* |
| HsCnTI (per 10 mg/L) | 1.001 (1.000–1.001) | 0.001* |
| Prothrombin time (s) | 1.431 (1.264–1.620) | 0.000* |
| D‐dimer (μg/mL) | 1.083 (1.038–1.130) | 0.000* |
| HsCRP (per 10 mg/L) | 1.086 (1.042–1.132) | 0.000* |
| Serum ferritin (per 100 μg/L) | 1.008 (1.004–1.013) | 0.000* |
| Procalcitonin (ng/mL) | 1.039 (1.014–1.065) | 0.002* |
| Erythrocyte sedimentation rate (mm/h) | 0.994 (0.979–1.009) | 0.431 |
| Glycated hemoglobin (%) | 1.300 (1.044–1.617) | 0.019* |
CI, confidence interval; HR, hazard ratio; HsCnTI, hypersensitive cardiac troponin I; HsCRP, high‐sensitivity C‐reactive protein; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide.
P < 0.05.
In the multivariate Cox proportional hazards regression analysis (Table 5), in model 1, after adjustment for age and sex the HR of HbA1c associated with in‐hospital death was 1.301 (95% CI 1.034–1.639, P = 0.025) per 1% increase; in model 2, after adjustment for age, sex, and the presence of cardiovascular disease and chronic respiratory disease, the HR of HbA1c associated with in‐hospital death was 1.363 (95% CI 1.082–1.717, P = 0.009) per 1% increase; in model 3, after adjustment for age, sex, alanine aminotransferase, creatinine and hscTnI, the HR of HbA1c associated with in‐hospital death was 1.400 (95% CI 1.080–1.814, P = 0.011) per 1% increase; in model 4, after adjustment for age, alanine aminotransferase, hscTnI, LDH and lymphocyte count, the HR of HbA1c associated with in‐hospital death was 1.626 (95% CI 1.234–2.142, P = 0.001) per 1% increase; in model 5, after adjustment for age, LDH, lymphocyte count, hsCRP and D‐dimer, the HR of HbA1c associated with in‐hospital death was 1.562 (95% CI 1.141–2.137, P = 0.005) per 1% increase; in model 6, after adjustment for age, LDH, lymphocyte count, NT‐proBNP and sodium, the HR of HbA1c associated with in‐hospital death was 1.556 (95% CI 1.185–2.044, P = 0.001) per 1% increase; and in model 7, after adjustment for age, LDH, lymphocyte count, NT‐proBNP and serum ferritin, the HR of HbA1c associated with in‐hospital death was 1.577 (95% CI 1.157–2.150, P = 0.004) per 1% increase. The summarized HR of HbA1c independently associated with in‐hospital death in all the models were delineated in the forest plots (Figure 1).
Table 5.
Multivariate Cox proportional hazards regression analysis of baseline factors associated with in‐hospital death
| Mode | HR (95% CI) | P‐value |
|---|---|---|
| Not adjusted HbA1c (per 1%_ | 1.300 (1.044–1.617) | 0.019* |
| Model 1 | ||
| HbA1c (per 1%) | 1.302 (1.034–1.639) | 0.025* |
| Age (per 1 year) | 1.055 (1.017–1.095) | 0.004* |
| Female vs male (ref) | 0.397 (0.146–1.085) | 0.072 |
| Model 2 | ||
| HbA1c (per 1%) | 1.363 (1.082–1.717) | 0.009* |
| Age (per 1 year) | 1.045 (1.004–1.089) | 0.031* |
| Female vs male (ref) | 0.461 (0.167–1.275) | 0.136 |
| CVD, yes vs no (ref) | 2.533 (1.108–6.306) | 0.046* |
| CRD, yes vs no (ref) | 2.799 (0.967–8.104) | 0.058 |
| Model 3 | ||
| HbA1c (per 1%) | 1.400 (1.080–1.814) | 0.011* |
| Age (per 1 year) | 1.065 (1.015–1.110) | 0.009* |
| Female vs male (ref) | 0.474 (0.154–1.462) | 0.194 |
| ALT (per 10 U/L) | 1.039 (1.002–1.077) | 0.039* |
| Cr (per 10 μmol/L) | 0.995 (0.962–1.029) | 0.753 |
| HsCnTI (per 100 pg/mL) | 1.007 (1.003–1.011) | 0.002* |
| Model 4 | ||
| HbA1c (per 1%) | 1.626 (1.234–2.142) | 0.001* |
| Age (per 1 year) | 1.069 (1.015–1.126) | 0.011* |
| ALT (per 10 U/L) | 0.986 (0.945–1.029) | 0.526 |
| HsCnTI (per 100 pg/mL) | 1.000 (0.994–1.005) | 1.000 |
| LDH (per 100 U/L) | 1.251 (1.088–1.440) | 0.002* |
| Lymphocyte count (per 1 × 109/L) | 0.042 (0.004–0.437) | 0.008* |
| Model 5 | ||
| HbA1c (per 1%) | 1.562 (1.141–2.137) | 0.005* |
| Age (per 1 year) | 1.057 (0.999–1.118) | 0.053 |
| LDH (per 100 U/L) | 1.132 (1.003–1.277) | 0.044* |
| Lymphocyte count (per 1 × 109/L) | 0.011 (0.001–0.206) | 0.003* |
| HsCRP (per 10 mg/L) | 1.038 (0.981–1.099) | 0.197 |
| D‐dimer (per 1 μg/mL) | 1.040 (0.968–1.118) | 0.285 |
| Model 6 | ||
| HbA1c (per 1%) | 1.556 (1.185–2.044) | 0.001* |
| Age (per 1 year) | 1.035 (0.983–1.088) | 0.189 |
| LDH (per 100 U/L) | 1.153 (1.046–1.272) | 0.004* |
| Lymphocyte count (per 1 × 109/L) | 0.036 (0.004–0.333) | 0.003* |
| NT‐proBNP (per 100 pg/mL) | 1.005 (0.001–0.339) | 0.018* |
| Sodium (per 1 mmol/L) | 1.043 (0.974–1.117) | 0.232 |
| Model 7 | ||
| HbA1c (per 1%) | 1.577 (1.157–2.150) | 0.004* |
| Age (per 1 year) | 1.039 (0.985–1.096) | 0.164 |
| LDH (per 100 U/L) | 1.196 (1.063–1.345) | 0.003* |
| Lymphocyte count (per 1 × 109/L) | 0.011 (0.001–0.183) | 0.002* |
| NT‐proBNP (per 100 pg/mL) | 1.005 (1.000–1.009) | 0.034* |
| Serum ferritin (per 100 μg/L) | 0.999 (0.993–1.006) | 0.861 |
*P < 0.05.
ALT, alanine aminotransferase; CI, confidence interval; Cr, creatinine; CRD, chronic respiratory disease; CVD, cardiovascular disease; HbA1c, glycated hemoglobin; HR, hazard ratio; HsCnTI, hypersensitive cardiac troponin I; HsCRP, high‐sensitivity C‐reactive protein; LDH, lactate dehydrogenase; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide.
Figure 1.
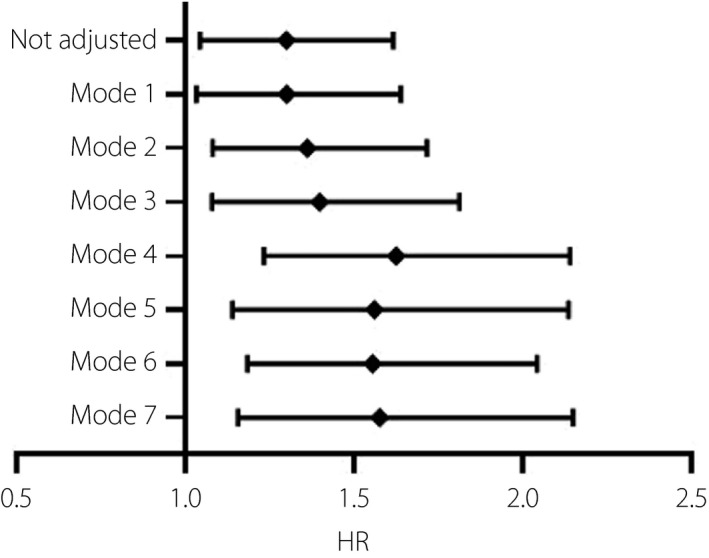
Forest plots of multivariate Cox proportional hazards regression analyzing the risk assessment of glycated hemoglobin on in‐hospital death. Model 1: adjusted for age and sex. Model 2: adjusted for age, sex, and the presence of cardiovascular and chronic respiratory disease. Model 3: adjusted for age, sex, alanine aminotransferase, creatinine and hypersensitive cardiac troponin I. Model 4: adjusted for age, alanine aminotransferase, hypersensitive cardiac troponin I, lactate dehydrogenase (LDH) and lymphocyte count. Model 5: adjusted for age, LDH, lymphocyte count, high‐sensitivity C‐reactive protein and D‐dimer. Model 6: adjusted for age, LDH, lymphocyte count, N‐terminal pro‐brain natriuretic peptide and sodium. Model 7: adjusted for age, LDH, lymphocyte count, N‐terminal pro‐brain natriuretic peptide and serum ferritin. HR, hazard ratio.
DISCUSSION
A previous study carried out by Wang et al. 18 showed that HbA1c was associated with inflammation, hypercoagulability and low SaO2 in COVID‐19 patients; meanwhile, both the HbA1c level and mortality rate were higher in patients with a history of diabetes, no regression analysis was used to evaluate whether HbA1c was an independent risk factor for poor prognosis of COVID‐19 in their study. In the present study, for the first time, we explored the effects of blood glucose on complications, severity and mortality rate of COVID‐19 patients from the perspective of HbA1c, which reflects the long‐term glycemic control degree before admission. Compared with patients with HbA1c <6.5%, patients with HbA1c ≥6.5% showed a higher heart rate, lower percutaneous oxygen saturation and higher levels of multiple indicators of inflammation, as well as a lower level of lymphocyte count at hospital admission. Thus, patients with HbA1c ≥6.5% were more likely to develop secondary respiratory infections and ARDS than patients with HbA1c <6.5%, resulting in a higher proportion of critically ill cases and non‐survivors. After adjustment for potential risk factors, HbA1c was independently associated with in‐hospital death.
In a large number of previous studies about the relationship between glycemic control and the outcome of infection diseases, patients were grouped according to diabetes medical history. However, Menke et al. 19 reported that the overall awareness rate of diabetes in the USA was 74.8%. In China and India, as the two most populous developing countries in the world, the awareness rates of diabetes were 30 and 25%, respectively 20 , therefore, low awareness rate is a great adverse factor for diabetes management. In the present study, we grouped the patients according to HbA1c level instead of diabetes medical history, and hoped that using this grouping method might significantly reduce the likelihood of misgrouping, and obtain a more accurate understanding of the relationship between long‐term glycemic control prior to admission and the prognosis of COVID‐19. In addition, because of the low awareness of diabetes and based on the results of the present study, we advise that the HbA1c test is necessary for the management of COVID‐19, especially for patients with advanced age, who were considered to have a greater relative risk of mortality 5 , 21 . For the patients with elevated HbA1c, decreasing the conversion rates of severe and critical illnesses through close monitoring and intensive treatment might be beneficial to reduce the mortality rate. It is not a coincidence that a previous study carried out by Li et al. 22 agreed with the present finding that it is necessary to screen hospitalized COVID‐19 patients for diabetes, as they found that newly diagnosed diabetes (unawareness of diabetes before admission, fasting glucose ≥7 mmol/L and/or HbA1c ≥6.5% after admission) is associated with a higher risk of mortality than known diabetes in hospitalized patients with COVID‐19.
As shown in the results, we investigated the differences of the risk factors that might influence the outcome between the sufficient glycemic control group (HbA1c <6.5%) and the insufficient glycemic control group (HbA1c ≥6.5%). Previous studies have proved that a significant reduction in lymphocytes is the most prominent abnormality, which could be observed in 39–92.6% COVID‐19 patients through routine blood analysis 23 . Meanwhile, absolute count of lymphocytes was inversely correlated with the virus RNA load, ARDS development, need for intensive care unit care, severity and death among COVID‐19 patients 13 , 24 , 25 , 26 . In the present study, a significant numeric difference in lymphocyte count between the sufficient control group and insufficient control group was also noted, which might be an explanation for the correlation between glycemic control and the severity and outcome of COVID‐19 patients.
Neutrophils play an irreplaceable role in resisting microbial infections. In general, the degree of neutrophils elevation is positively correlated with the severity of the infections, especially bacterial infections, including primary bacterial infections and bacterial infections secondary to viral infections. In addition, neutrophils are the main source of chemokines and cytokines 13 ; in the pathophysiological course of severe infection, markedly increased cytokines released from elevated inflammatory cells, including neutrophils, might lead to a cytokine storm, which can result in multiple organ dysfunction, and is considered to be one of the leading causes of disease progression and death in COVID‐19 patients 1 , 3 , 13 . The positive correlation between elevation of neutrophils and increased risk of fatal outcome has been proved 13 , 26 . CRP and ferritin are emerging biomarkers when infection occurs, markedly increased CRP and ferritin suggest a serious infection and severe secondary inflammatory response. Elevated CRP is more common in severe COVID‐19 patients, Guan et al. 27 found that more severe cases showed a more significant increase of CRP compared with the non‐severe cases (81.5% vs 56.4%). Meanwhile, a study carried out by Evangelos et al. summarized several clinical studies about the correlation between CRP and the outcome of COVID‐19, and showed that higher CRP was positively correlated with ARDS development, myocardial injury and death 23 . Regarding ferritin, similar to CRP, it has also been found to be elevated more commonly in severe cases, and be correlated with poor progression and prognosis of COVID‐19 23 .
Similar to the aforementioned previous studies, neutrophils, CRP and ferritin were also found to be more significantly elevated in the insufficient control group, and be positively correlated with death in the present study. In addition to that, the present study proved that ARDS development, secondary bacterial infection and low percutaneous oxygen saturation were significantly more common in patients with insufficient glycemic control. All the aforementioned findings showed that the patients with insufficient glycemic control might suffer from greater severity of pulmonary infection and ventilation dysfunction. Not coincidentally, another study carried out by Guo et al. 28 analyzed the chest CT scan data using a CT imaging scores system, and found that pulmonary infection was significantly more severe in COVID‐19 patients with diabetes than in those without diabetes. Based on the findings, we speculated that COVID‐19 patients with insufficient glycemic control are more likely to suffer from severe pulmonary infection and the consequent ARDS, which were probably the leading cause of death.
Acute injury of multiple organs outside the respiratory system, especially the liver, kidney, heart, and hematopoietic and coagulation system, were reported in many studies, meanwhile, acute injury of multiple organs was proved to be more common in severe COVID‐19 patients and predictors of poor prognosis 3 , 4 , 13 , 29 . In the present study, a significant difference of the incidence of liver and heart injuries and coagulation disorders between survival and non‐survival patients was observed. However, there was no difference of the incidence of organ injuries between the sufficient glycemic control group and the insufficient glycemic control group, which suggested that long‐term glycemic control levels before admission might not be associated with acute injury of multiple organs outside the respiratory system. This finding was also supported by the study carried out by Guo et al. 28 , in which they showed that no difference of the incidence of extrapulmonary organ injuries between the diabetes group and non‐diabetes group was found when other comorbidities were not eliminated. These findings showed again that severe pulmonary infection and consequent ARDS, but not extrapulmonary organ injuries, were probably the leading cause of death in COVID‐19 patients with insufficient glycemic control.
There were some limitations of the present study that should be acknowledged. First, we used a retrospective, single‐center dataset for analysis. Second, sample size was limited during follow up, as clinical outcomes had not yet been determined for more patients during the observation period. Third, the present study mainly focused on patients with severe and critical illness. Further validation is required with another larger sample size, multicentered study and, ideally, involving patients of different ethnic origins, and patients with mild and moderate symptoms.
In conclusion, the incidence of critically ill cases and mortality rate were significantly higher in the insufficient glycemic control group (HbA1c ≥6.5%), and HbA1c was a significant independent risk factor associated with in‐hospital death of patients with COVID‐19. Severe pulmonary infection and consequent ARDS, but not extrapulmonary organ injuries, might be the primary cause of death in patients with insufficient glycemic control. Routine examination of HbA1c, and timely identification and treatment of pulmonary secondary respiratory infections and ARDS might be beneficial to improve the prognosis of COVID‐19 patients with insufficient glycemic control.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
We gratefully acknowledge all the healthcare workers on the front line and all the patients involved in the study. This research was funded by the National Natural Science Foundation of China (81700727 to LL; 81700207 to BZ).
J Diabetes Investig 2021; 12: 1064–1073
References
- 1. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 2020; 130: 2620–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cao Y, Liu X, Xiong L, et al. Imaging and clinical features of patients with 2019 novel coronavirus SARS‐CoV‐2: a systematic review and meta‐analysis. J Med Virol 2020; 92: 1449–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020; 368: m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen R, Liang W, Jiang M, et al. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest 2020; 158: 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maddaloni E, Buzzetti R. Covid‐19 and diabetes mellitus: unveiling the interaction of two pandemics. Diabetes Metab Res Rev 2020; 36: e33213321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Delamaire M, Maugendre D, Moreno M, et al. Impaired leucocyte functions in diabetic patients. Diabet Med 1997; 14: 29–34. [DOI] [PubMed] [Google Scholar]
- 8. Shah BR, Hux JE. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care 2003; 26: 510–513. [DOI] [PubMed] [Google Scholar]
- 9. Rao KSS, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause‐specific death. N Engl J Med 2011; 364: 829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Booth CM, Matukas LM, Tomlinson GA, et al. Clinical features and short‐term outcomes of 144 patients with SARS in the greater Toronto area. JAMA 2003; 289: 2801–2809. [DOI] [PubMed] [Google Scholar]
- 11. Alqahtani FY, Aleanizy FS, Ali EHMR, et al. Prevalence of comorbidities in cases of Middle East respiratory syndrome coronavirus: a retrospective study. Epidemiol Infect 2018; 208: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Internal Med 2020; 180: 934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rollins KE, Varadhan KK, Dhatariya K, et al. Systematic review of the impact of HbA1c on outcomes following surgery in patients with diabetes mellitus. Clin Nutr 2016; 35: 308–316. [DOI] [PubMed] [Google Scholar]
- 15. Classification and Diagnosis of Diabetes . Standards of medical care in diabetes‐2019. Diabetes Care 2019; 42(Suppl. 1): S13–S28. [DOI] [PubMed] [Google Scholar]
- 16. Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358: 2560–2572. [DOI] [PubMed] [Google Scholar]
- 17. New Coronavirus Pneumonia Prevention and Control Program (6th ed.) (in Chinese). 2020, 462. Available from: http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2/fil463es/b218cfeb1bc54639af227f922bf6b817.pdf Accessed March 9, 2020.
- 18. Wang Z, Du Z, Zhu F. Glycosylated hemoglobin is associated with systemic inflammation, hypercoagulability, and prognosis of COVID‐19 patients. Diabetes Res Clin Pract 2020; 164: 108214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Menke A, Casagrande S, Geiss L, et al. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA 2015; 314: 1021–1029. [DOI] [PubMed] [Google Scholar]
- 20. Bikbov MM, Fayzrakhmanov RR, Kazakbaeva GM, et al. Prevalence, awareness and control of diabetes in Russia: The Ural Eye and Medical Study on adults aged 40+ years. PLoS One 2019; 14: e215636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang F, Shi S, Zhu J, et al. Analysis of 92 deceased patients with COVID‐19. J Med Virol 2020; 92: 2511–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li H, Tian S, Chen T, et al. Newly diagnosed diabetes is associated with a higher risk of mortality than known diabetes in hospitalized patients with COVID‐19. Diabetes Obes Metab 2020; 22: 1897–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Terpos E, Ntanasis‐Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID‐19. Am J Hematol 2020; 95: 834–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu Y, Liao W, Wan L, et al. Correlation between relative nasopharyngeal virus RNA load and lymphocyte count disease severity in patients with COVID‐19. Viral Immunol 2020. 10.1089/vim.2020.0062. [DOI] [PubMed] [Google Scholar]
- 25. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med 2020; 8: 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guo W, Li M, Dong Y, et al. Diabetes is a risk factor for the progression and prognosis of COVID‐19. Diabetes Metab Res Rev 2020: e3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang D, Yin Y, Hu C, et al. Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS‐CoV‐2, discharged from two hospitals in Wuhan, China. Crit Care 2020; 24: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]


