Abstract
Objective
Recent anecdotal reports and cadaveric simulations have described aerosol generation during endonasal instrumentation, highlighting a possible risk for transmission of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) during endoscopic endonasal instrumentation. This study aims to provide a greater understanding of particle generation and exposure risk during endoscopic endonasal instrumentation.
Study Design
Prospective quantification of aerosol generation during office‐based nasal endoscopy procedures.
Methods
Using an optical particle sizer, airborne particles concentrations 0.3 to 10 microns in diameter, were measured during 30 nasal endoscopies in the clinic setting. Measurements were taken at time points throughout diagnostic and debridement endoscopies and compared to preprocedure and empty room particle concentrations.
Results
No significant change in airborne particle concentrations was measured during diagnostic nasal endoscopies in patients without the need for debridement. However, significant increases in mean particle concentration compared to preprocedure levels were measured during cold instrumentation at 2,462 particles/foot3 (95% CI 837 to 4,088; P = .005) and during suction use at 2,973 particle/foot3 (95% CI 1,419 to 4,529; P = .001). In total, 99.2% of all measured particles were ≤1 μm in diameter.
Conclusion
When measured with an optical particle sizer, diagnostic nasal endoscopy with a rigid endoscope is not associated with increased particle aerosolization in patient for whom sinonasal debridement is not needed. In patients needing sinonasal debridement, endonasal cold and suction instrumentation were associated with increased particle aerosolization, with a trend observed during endoscope use prior to tissue manipulation. Endonasal debridement may potentially pose a higher risk for aerosolization and SARS‐CoV‐2 transmission. Appropriate personal protective equipment use and patient screening are recommended for all office‐based endonasal procedures.
Level of Evidence
3 Laryngoscope, 131:E1415–E1421, 2021
Keywords: COVID‐19, nasal endoscopy, aerosol‐generating procedures, optical particle sizer, droplet quantification
INTRODUCTION
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) pandemic has overwhelmed hospital staff and resources, leading to drastic modifications to healthcare delivery around the world. 1 , 2 , 3 , 4 Mitigation of viral transmission between patients and staff has become a paramount concern. As clinics around the world “reopen” in the era of COVID‐19, a greater understanding of high‐risk activities will be crucial in the development and implementation of protocols to address and minimize viral spread.
At the pandemic's onset, initial anecdotal reports documented concerns for the risk of direct transmission to healthcare workers during endoscopic endonasal surgery. 5 Although these initial reports have since been clarified, 6 concerns remain over a potential increase in viral exposure risk during such procedures. In the outpatient clinic setting, endonasal procedures commonly occur in rooms with significantly less environmental controls compared to standard operating rooms. Generation of aerosolized particles during office‐based endonasal procedures could pose a potential exposure risk to clinic staff due to high traffic level through patient rooms and lower air‐exchange rates compared to operating rooms. 7 , 8
Aerosol generating procedures (AGPs) are typically defined as any procedure, which presents an increased exposure risk to healthcare workers from baseline levels. 9 , 10 , 11 In general, manipulation of the upper or lower airway is understood to increase the risk of particle aerosolization. 12 , 13 The generation of airborne and droplet particles risks potential exposure of clinic staff to pathogens and thus warrants further investigation.
Recent simulations performed in the laboratory setting by Workman et al. describe aerosol generation during powered endonasal instrumentation in cadaveric models. Specifically, their simulation demonstrated an increase in aerosolization of respiratory droplets (>30 microns) and airborne particles (1–10 microns). 14 , 15 Workman et al. also performed a limited number of simulations on healthy volunteers which also demonstrated aerosol generation with the solo use of nasal endoscopes. These simulations suggest a potential exposure risk to operators during endonasal instrumentation; however, cadaveric simulations may not accurately demonstrate true aerosol generation during clinical procedures; due to patient respiration and presence of mucous and crusting after endoscopic nasal surgeries.
The safety of patients and clinic staff requires a better understanding of particle aerosolization during office‐based endoscopic endonasal procedures. Understanding aerosol generation in the clinic setting will help clinics design and implement safety protocols to maximize environmental controls, including appropriate use of personal protective equipment (PPE). This investigation looks to build upon the findings of Workman et al. by measuring airborne particle concentrations during live patient procedures in a traditional outpatient clinic setting. 14 , 15
METHODS
Study Design
Institutional Review Board exemption #20–1377 was obtained for this study—no identifying patient information was collected. The study was conducted in a single outpatient clinic building with non‐negative pressure rooms and standard air exchange rates for local commercial buildings. Airborne particle concentrations were measured during 1) diagnostic nasal endoscopies and 2) nasal endoscopies with debridement. All clinic patients were screened via phone, 24 hours prior to their clinic appointment for COVID‐19 symptoms or contact exposure. No COVID‐19 testing was performed for clinic visits. Postoperative patients had a documented single negative COVID‐19 test result, via nasopharyngeal swab and PCR that was completed 24–48 hours prior to surgery. 4‐mm rigid endoscopes were used during 28 adult patient endoscopies and 2.9 mm rigid endoscopes were used during two pediatric patient endoscopies. Anesthetic aerosol sprays were not used prior to endoscopy. Nasal endoscopy with debridement was performed on postoperative patients and/or postradiation therapy patients. A 10 French disposable suction was used during the suction portion of sinonasal debridement, with Tobey and alligator forceps used for the mechanical portion of sinonasal debridement. Verbal consent was obtained from patients prior to measurements.
Data Collection
Airborne particle concentrations were measured in particles per cubic foot (p/ft3) with an Extech VPC300 Particle Counter (FLIR Commercial Systems Inc.), calibrated to standards established by the National Institute of Standards and Technology. An internal pump regulates airflow to 2.83 L/min; particles pass through an internal laser system and the degree of scattered light is used to calculate particle size and number. The device measures airborne particles of 0.3, 0.5, 1.0, 2.5, 5.0, and 10 microns (um) in size; counting efficiency varies by particle size, with 50% efficiency at 0.3 μm; however, particles >0.45 μm in diameter are counted with an efficiency approaching 100%. Individual sample measurements were collected over a fixed 20‐second interval, allowing for consistent sampling for each instrument timepoint. However, the duration of instrument use during endoscopy procedures often varied and longer instrument use allowed for the collection of additional sampling measurements. The time between sample measurements during instrument transition ranged between 2 and 5 seconds.
Baseline particle concentration measurements were obtained in empty clinic rooms to assess the effect of native patient breathing on airborne particle concentrations. Empty room measurements were collected prior to patient entry, with the door closed, and measurements were taken within an 18‐inch radius from the exam chair headrest. Clinic aerosol measurements were then collected during diagnostic nasal endoscopies and nasal endoscopies with suction and mechanical debridement. Sample measurements were collected within an 18‐inch radius from the patient's head to detect aerosol generation. A sampling radius of 18 inches was deemed appropriate when considering the distance from the patient's head to the surgeon's head often measures within 2–3 feet (i.e., one arm's length).
Separate particle measuring protocols were implemented for the two nasal endoscopy procedures. Any interruption during the patient visit or sampling protocol was followed by a 1‐3‐minute waiting period before the continued collection of sample measurements. Sampling interruptions were defined as any event that may alter airborne particle levels such as door openings or patient coughing, sneezing, or talking during the procedure.
Measurement Protocol for Diagnostic Nasal Endoscopy
The following protocol was used for collecting airborne particle measurements during diagnostic nasal endoscopies:
The patient enters the clinic room with a mask on, takes a seat in the exam chair and the exam room door is closed.
The patient's facemask is then removed.
After a 1‐3‐minute waiting period, preprocedure concentration measurements are collected.
Following the completion of preprocedure measurements, the physician inserts the rigid endoscope into the patient's nasal cavity and particle concentration measurements are collected until termination of endoscope use.
2–5 minutes after termination of endoscope use, postprocedure measurements are collected prior to the replacement of the patient's facemask.
Measurement Protocol for Nasal Endoscopy with Debridement
The following protocol was used for collecting airborne particle measurements during nasal endoscopies with debridement:
The patient enters the clinic room with a facemask on, takes a seat in the exam chair and the exam room door is closed.
The patient's facemask is then removed.
After 1–3 minutes, preprocedure concentration measurements are collected.
Following termination of preprocedure measurements, the physician inserts the rigid endoscope into the patient's nasal cavity and air concentration measurements are collected until termination of endoscope use.
Following termination of endoscope use and prior to activation of the suction generator, particle concentrations are measured during endonasal cold instrumentation, with either Tobey or Blakesley forceps.
Following termination of cold instrumentation, the suction generator is activated, and particle concentrations are measured during endonasal suction debridement.
2–5 minutes after termination of suction used, postprocedure measurements are collected prior to the replacement of the patient's facemask.
Statistical analysis
Descriptive statistics were calculated for total particle concentration (cumulatively between 0.3 and 10.0 um) at different time points during the clinic procedures. Changes in mean particle concentration were summarized for clinic procedures involving 1) diagnostic nasal endoscopies and 2) nasal endoscopies with debridement (cold instrumentation and suction). Two‐sided t‐tests were used to estimate the mean difference (MD) and 95% confidence intervals (CI) for particle concentration at different points during the procedures. A linear regression model was used for direct comparison of particle effect with different instrument types, adjusting for preprocedure concentration. A significance level of P < .05 was used for all testing. Stata 16.0 (StataCorp LP, College Station, TX) was used for all analyses.
RESULTS
Comparison of Empty Room and Preprocedure Aerosol Concentrations
Sample measurements collected in empty patient rooms demonstrated a mean particle concentration of 3,864 p/ft3. Sample measurements collected prior to the initiation of both diagnostic and debridement nasal endoscopies demonstrated a mean particle concentration of 5,780 p/ft3. To assess the effect of native unmasked patient breathing on airborne particle measurements, a comparison of empty room measurements to preprocedure measurements demonstrated a mean difference of 1,915 p/ft3 (95% CI 85 to 3,745; P = .041).
Diagnostic Nasal Endoscopy
A total of 33 sample measurements were collected during 11 diagnostic nasal endoscopies. The mean particle concentration during diagnostic endoscopy was measured at 6,021 p/ft3 with a nonsignificant mean difference of −173 p/ft3 (95% CI −1,139 to 793; P = .698) compared to preprocedure concentrations; no statistically significant changes in particle concentration were measured during diagnostic nasal endoscopies (Table I).
TABLE I.
Changes in Particle Concentration During Diagnostic Endoscopy.
| Prescope (Mean, SD) | During Scope (mean, SD) | Postscope (mean, SD) | Mean Difference* (95% CI) | P Value* |
|---|---|---|---|---|
| 6,194 (2,505) | 6,021 (2,952) | 6,014 (3,229) | −173 (−1,139 to 793) | .698 |
For prescope versus during scope; n = 11 procedures total.
Nasal Endoscopy with Debridement
A total of 86 sample measurements were collected during 19 nasal endoscopies with debridement. The mean particle concentration during cold instrumentation was observed at 8,002 p/ft3, with a significant mean increase of 2,462 p/ft (95% CI 837 to 4,088; P = .005) from preprocedure levels (Table II). The mean particle concentration during suction use was observed at 8,514, with a significant mean increase of 2,973 p/ft (95% CI from 1,419 to 4,529; P = .001) compared preprocedure levels (Fig. 1). Endoscope use prior to tissue manipulation during endoscopies with debridement was associated with a mean particle concentration of 7,169 p/ft3 and a nonsignificant but trended mean increase of 1,629 p/ft3 (95% CI −96 to 3,354; P = .063) from preprocedure levels (Fig. 2). Direct comparison of mean particle concentrations during cold instrumentation versus suction demonstrated a nonsignificant mean difference of 511 p/ft3 (95% CI −2,051 to 3,073; P = .687). This nonsignificant effect persisted when adjusting for preprocedure concentration in a linear regression model, with a mean difference of 974 p/ft3 (95% CI −1,383 to 3,332; P = .406).
TABLE II.
Changes in Particle Concentration During Nasal Endoscopy With Debridement.
| Timing | Particle Concentration (Mean, SD) | Mean Difference (95% CI) * | P Value* |
|---|---|---|---|
| Preprocedure | 5,539 (2,725) | Reference | N/A |
| Scope | 7,169 (2,685) | 1,629 (−96 to 3,354) | .063 |
| Cold instrumentation | 8,002 (3,418) | 2,462 (837 to 4,088) | .005 |
| Suction | 8,514 (3,449) | 2,973 (1,419 to 4,529) | .001 |
| Postprocedure | 5,816 (2,417) | 276 (−1,120 to 1,673) | .683 |
Mean difference is for each instrument versus the preprocedure concentration; n = 19 observations for pre‐ and postprocedure and for suction, 15 for cold instrumentation, 14 for scope.
Fig. 1.
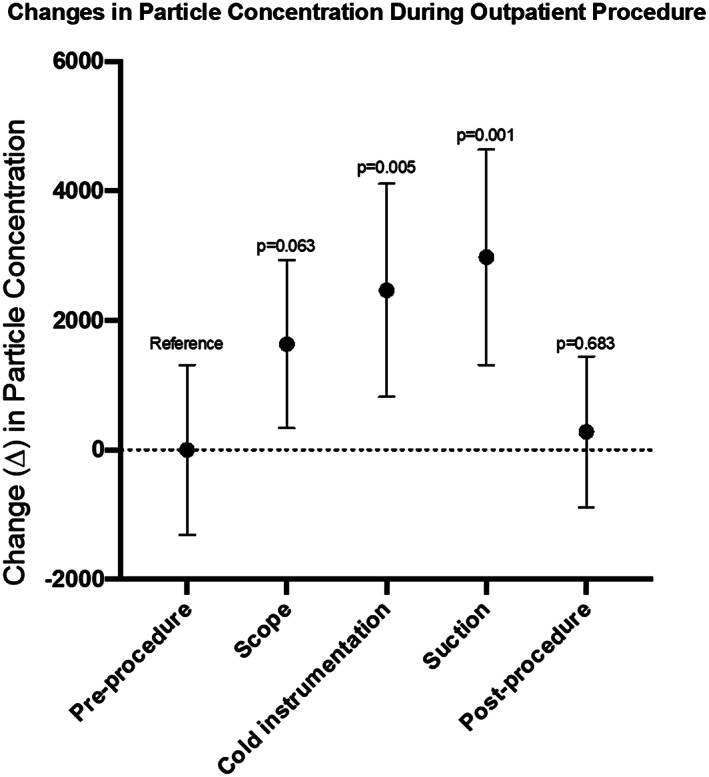
Changes in mean particle concentration during nasal endoscopy with debridement. Preprocedure measurements demonstrated a mean particle concentration of 5,539 particle/foot3 (p/ft3), which were normalized to zero for comparison of mean particle concentrations of subsequent instrumentation. Endoscope use was associated with a mean particle concentration of 7,169 p/ft3 and a mean difference of 1,629 p/ft3 (95% CI −96 to 3,354; P = .063) from preprocedure concentrations. Cold instrumentation was associated with a mean particle concentration of 8,002 p/ft3 and a mean difference of 2,462 p/ft3 (95% CI 837 to 4,088; P = .005) from preprocedure concentrations. Suction use was associated with a mean particle concentration of 8,514 p/ft3 and a mean difference of 2,973 p/ft3 (95% CI 1,419 to 4,529; P = .001) from preprocedure concentrations. Measurements taken at the end of the procedure demonstrated a mean particle concentration of 5,816 p/ft3 with a mean difference of 276 p/ft3 (95% CI −1,120 to 1,673; P = .683).
Fig. 2.
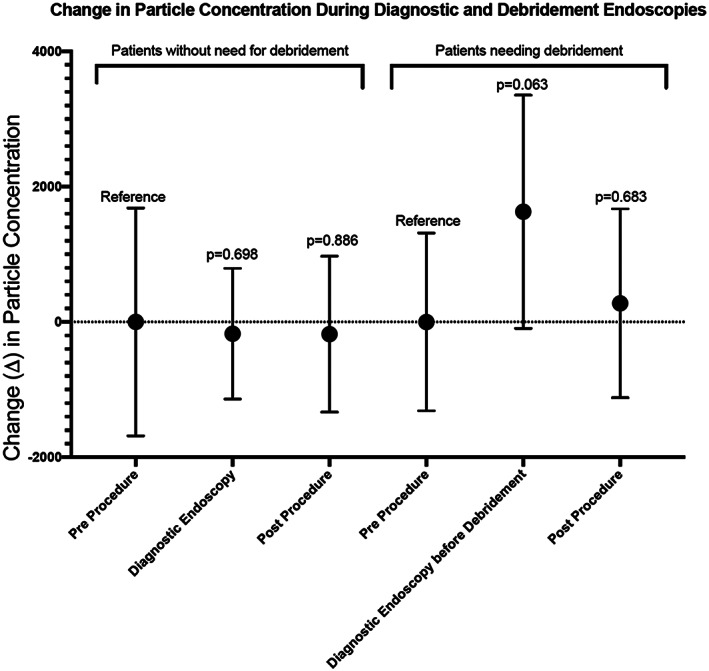
Difference in mean particle concentrations between diagnostic nasal endoscopy and nasal endoscopy with debridement. Zero reference normalized to combined preprocedure data in the cohort. Endoscope use during diagnostic endoscopy was associated with a nonsignificant mean particle difference of 173 p/ft3 (95% CI −1,139 to 793; P = .698) compared to preprocedure levels. Endoscope use prior to tissue manipulation in nasal endoscopy with debridement was associated with a trending mean increase of 1,629 p/ft3 (95% CI −96 to 3,354; P = .063).
Comparison of Endoscope Use in Diagnostic and Debridement Procedures
Patients who require sinonasal debridement undergo diagnostic endoscopy prior to sinonasal debridement. The potential difference in nasal anatomy and mucus/biomass in the nose warranted comparison of endoscope use in patients requiring debridement and patients that do not require debridement. Figure 2 shows a comparison of aerosol concentrations normalized to preprocedure levels, for the two groups of patients during endoscopy without instrumentation. Endoscope use in patients without need for debridement showed virtually no aerosolization difference compared to native breathing prior to scope (P = .698). However, endoscope use in patients that subsequently underwent debridement, demonstrated aerosol concentrations that trended toward significance (P = .063).
Particle Size Distribution
Of the 131 sampling measurements, 99.2% of measured airborne particles were ≤1 μm in diameter, with 72.9% measured at 0.3 μm, 22.4% at 0.5 μm, and 4.0% at 1.0 μm in diameter (Table III). Particle size distribution remained comparable in the three different sampling environments (Fig. 3).
TABLE III.
Mean Particle Concentration (percentage) and Particle Size Measured in Particles/Foot3 at Distinct Timepoints Throughout Diagnostic Nasal Endoscopy and Nasal Endoscopy with Debridement.
| Particle Size (um) | Total | 0.3 | 0.5 | 1 | 2.5 | 5 | 10 |
|---|---|---|---|---|---|---|---|
| Mean particle concentration (Percent) | |||||||
| Empty room | 3,863.7 | 2,938 (76.0) | 810 (21.0) | 102 (2.6) | 12 (0.3) | 1 (0.03) | 0.7 (0.01) |
| Diagnostic nasal endoscopy | |||||||
| Pre | 6,195 | 4,509 (72.8) | 1,388 (22.4) | 250 (4.0) | 36 (0.58) | 7 (0.10) | 5 (0.07) |
| During | 6,022 | 4,444 (73.8) | 1,307 (21.7) | 231 (3.8) | 32 (0.53) | 5 (0.08) | 3 (0.05) |
| Post | 6,014 | 4,393 (73.0) | 1,335 (22.2) | 245 (4.1) | 31 (0.52) | 6 (0.09) | 4 (0.07) |
| Nasal endoscopy with debridement | |||||||
| Pre | 5,539 | 4,014 (72.5) | 1,252 (22.6) | 228 (4.1) | 35 (0.64) | 6 (0.12) | 4 (0.08) |
| Scope | 7,169 | 5,224 (72.9) | 1,603 (22.4) | 288 (4.0) | 43 (0.60) | 7 (0.10) | 4 (0.06) |
| Cold instrumentation | 8,002 | 5,819 (72.7) | 1,799 (22.5) | 335 (4.2) | 41 (0.51) | 5 (0.06) | 3 (0.04) |
| Suction | 8,514 | 6,160 (72.4) | 1,951 (22.9) | 341 (4.0) | 50 (0.59) | 8 (0.09) | 4 (0.05) |
| Post | 5,816 | 4,232 (72.8) | 1,300 (22.3) | 234 (4.0) | 41 (0.70) | 6 (0.10) | 3 (0.05) |
Fig. 3.
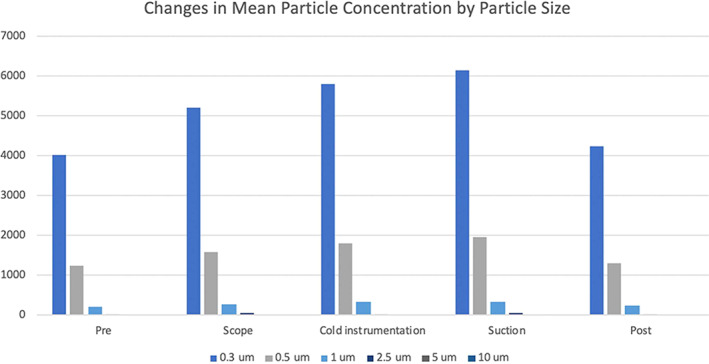
Mean particle concentrations with particle size distribution during nasal endoscopy with debridement. Particle measurements obtained during nasal endoscopies with debridement showed greater than 72% of all measured particles were 0.3 μm in diameter, greater than 22% were measured at 0.5 μm in diameter, and greater than 4% was measured at 1.0 μm in diameter. Particles sizes in the range of 2.5 to 10 μm in diameter composed less than 1% of all particles measured during nasal endoscopies with debridement.
DISCUSSION
Although epidemiological projections of the SARS‐CoV‐2 pandemic remain ever‐changing, we anticipate a sustained risk to clinic operations given the viral transmissibility, latency period, and potential seasonality. 16 , 17 This reality will require diligence by healthcare institutions in identifying and mitigating iatrogenic modes of viral transmission. Cadaveric simulations performed in the laboratory setting by Workman et al. have demonstrated the aerosol generation of the droplet (30–100 μm) and airborne (1–10 μm) size particles during endonasal instrumentation. 14 , 15 The potential for particle aerosolization during office‐based endoscopic endonasal procedures poses an unknown exposure risk for transmission of the SARS‐CoV‐2 virus to clinic staff. 14 , 15 , 18 , 19 Our investigation of office‐based endoscopic endonasal procedures did not show significant increases in aerosol concentrations during diagnostic nasal endoscopies, suggesting a lower risk for airborne particle exposure. However, the use of endonasal cold instruments and suction did show significant increases in airborne particle concentrations during nasal endoscopies with debridement, suggesting a potential exposure risk with both debridement techniques.
Office‐based diagnostic nasal endoscopy does not appear to increase aerosol concentrations when compared to preprocedure concentrations (P = .698). This suggests that the diagnostic use of nasal endoscopes likely does not pose any greater risk than native patient breathing. This lack of particle generation supports the cadaveric simulations performed by Workman et al., but differs however, from the aerosol measurements taken from two healthly volunteers, which showed significant particle aerosolization with endoscope use alone. Our findings likely differ from the Workman et al. simulations due to a larger sample size of patients with a sinonasal disease, history of skull base surgery and/or radiation therapy. 15
Endoscope use during diagnostic procedures did not show increased particle generation, however, endoscope use prior to tissue manipulation in patients needing sinonasal debridement was associated with a nonsignificant, but trending increase in airborne particle concentrations (P = .063) compared to preprocedure levels. From these data, we hypothesize, the diagnostic use of nasal endoscopes in patients requiring sinonasal debridement may pose a higher risk for aerosol generation compared to native patient breathing; the continued investigation is needed to further elucidate this proposed phenomenon. It is reasonable to postulate that the difference in particle generation during endoscope use is due to differences in the nasal cavities of patients requiring sinonasal debridement compared to those that do not. The observed particle effects may be influenced by biomass/nasal crusting, altered nasal architecture, and transient narrowing of nasal passages from postoperative inflammation leading to increased airflow velocity and turbulence.
These findings suggest an important difference in patients undergoing nasal endoscopy, in that, endonasal instrumentation in sinonasal debridement patients may pose a higher risk for an aerosol generation; however, patients who do not require sinonasal debridement, likely do not pose a higher aerosolization risk compared to native patient breathing. However, given the proximity of the sinonasal cavities and the higher viral sheading areas of the nasopharynx, we recommend high‐level PPE use and appropriate precautions for all patient encounters. In addition, the observed increase in particle concentration with suction use is inconsistent with the findings reported during the Workman et al. cadaveric simulations, which showed a comparatively decreased particle effect during suction use in conjunction with high‐speed drilling. In our clinic study, there were no differences between suction and nonsuction instrumentation. 14 , 15 We did not investigate the effects of combined drill and suction use as this is not applicable in the clinic setting; however, our findings suggested comparable aerosolization during endonasal debridement procedures with suction and cold instrumentation.
From these findings, we propose the following stratification for aerosol generation and subsequent exposure risk during nasal endoscopies: 1) low risk during diagnostic endoscopy (similar to native patient breathing), 2) higher risk during diagnostic endoscope use in patients requiring sinonasal debridement, and 3) highest risk during endoscopic nasal debridement with cold instrumentation and suction use.
Particle size distribution remained consistent at all measured time points, and the majority of measured airborne particles, during all nasal endoscopies, ranged from 0.3 to 1.0 μm in diameter. The natural pathway for coronavirus transmission is believed to be through respiratory droplets 20 ; however, recent findings by Liu et al. describe SARS‐CoV‐2 genetic material in aerosols sample in two Wuhan hospitals, adding evidence to the possibility for airborne transmission. 21 In light of these findings, airborne precautions are a reasonable consideration during endonasal procedures in SARS‐CoV‐2‐positive and unknown patients due to the high risk for airborne particle generation.
Overall, our study corroborates the Workman et al. findings, supporting the theory for the potential generation of airborne particles during endonasal instrumentation. 14 , 15 In addition, a recent study performed by Rameau et al. did not show increased particle generation during flexible laryngoscopy in two healthy volunteers compared to baseline levels during native breathing and phonation. 22 Although the nature of tissue manipulation during flexible laryngoscopy likely differs from rigid nasal endoscope use, the Rameau et al. findings in health individuals complement our findings of no significant particle effects during rigid endoscope use in patients without the need for sinonasal debridement. 22
Prior to the global pandemic, office‐based endonasal debridement, with cold instruments and suction use, was commonly performed with minimal PPE. However, these findings highlight the need for enhanced protocols governing PPE use during office‐based nasal endoscopies. Considering the Center for Disease Controls (CDC) current guidelines for AGPs to include the use of N95 or equivalent higher‐level respirator eye protection, gloves, and a gown, 9 our clinic continues to use N95 respirators and face shields during in‐office endonasal procedures. To further mitigate exposure risk, we have installed Hepa‐filters in all clinic rooms and recommend the use of video recorded endoscopy with high definition cameras if possible, to ensure a safer distance between physician and patient.
While this study provides further insight into the potential for aerosolized particle generation during nasal endoscopy procedures, there are several important limitations. Airborne particle quantification was performed with an optical particle sizer (OPS), which detects airborne particles between the sizes of 0.3 and 10 μm. Optical particle sizing is unable to differentiate particles by composition, and therefore, may detect nonpatient generated particulate, such as dust. In addition, OPS is unable to characterize aerodynamic properties such as particle desiccation, diffusion, or settling rates, which may influence airborne transmissibility. Accuracy of the device is limited at the 0.3 μm size, at only 50% counting efficiency. In addition, the dynamics of airborne particle movement are highly sensitive to airflow changes, which can lead to excessive particle background noise and transient, drastic variances in particle measurements. The opening of clinic doors, personnel traffic, and the use of pneumatic‐powered equipment (involved in suction use) can affect clinic room airflow patterns, thus altering particle readings. Finally, all samples were collected in 20‐second intervals at a single position, which provides only a snapshot of particle concentrations.
CONCLUSION
This is the first evaluation of particle aerosolization during endoscopic endonasal procedures performed in the clinic setting. These data suggest that diagnostic nasal endoscopy with rigid scopes does not increase particle aerosolization compared to native breathing in patients that do not require debridement. The use of nasal endoscopes in patients requiring sinonasal debridement trended toward higher risk for aerosol generation. In patients that need sinonasal debridement, cold and suction instrumentation produces a significant increase in measured particle concentration compared to native patient breathing; with no difference between the two techniques. In light of these findings, we believe appropriate use of high‐level PPE and patient screening is paramount in mitigating the potential exposure risk from office‐based diagnostic nasal endoscopies and nasal endoscopies with debridement; however, more sophisticated particle detection methodologies and further clinical studies are warranted to advance our understanding of this phenomenon.
Editor's Note: This Manuscript was accepted for publication on August 31, 2020
The project described was supported by NIH grants KL2TR002490 to A.J.K. and the Howard Holderness Distinguished Medical Scholars program and Pillsbury Medical Student Research Fellowship Grants. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. No authors have any financial conflicts of interest.
BIBLIOGRAPHY
- 1. Livingston E, Desai A, Berkwits M. Sourcing personal protective equipment during the COVID‐19 pandemic. J Am Med Assoc 2020;323:E1–E3. 10.1001/jama.2020.5317. [DOI] [PubMed] [Google Scholar]
- 2. Nacoti M, Ciocca A, Giupponi A. At the epicenter of the Covid‐19 pandemic and humanitarian crises in Italy: changing perspectives on preparation and mitigation. NEJM Catal. 2020;1. 10.1056/CAT.20.0080. [DOI] [Google Scholar]
- 3. Mann DM, Chen J, Chunara R, Testa PA, Nov O, Mann D. COVID‐19 transforms health care through telemedicine: Evidence from the field. 10.1093/jamia/ocaa072 [DOI] [PMC free article] [PubMed]
- 4. Halpern NA, Tan KS, Biostatistician AA. United States Resource Availability for COVID‐19 .
- 5. Patel ZM, Fernandez‐Miranda J, Hwang PH, et al. Letter: precautions for endoscopic transnasal skull base surgery during the COVID‐19 pandemic. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7184431/. Accessed May 28, 2020. [DOI] [PMC free article] [PubMed]
- 6. Huang X, Zhu W, Zhao H, Jiang X. In reply: precautions for endoscopic transnasal skull base surgery during the COVID‐19 pandemic. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7188152/. Accessed May 28, 2020. [DOI] [PMC free article] [PubMed]
- 7. Fineberg H. Rapid expert consultation on the possibility of bioaerosol spread of SARS‐CoV‐2 for the COVID‐19 pandemic (April 1, 2020); 2020. doi: 10.17226/25769 [DOI]
- 8. Brown GS, Mohr AJ. Fate and Transport of microorganisms in air. Yates MV, (Ed.), Manual of environmental microbiology. 4. Washington, DC: ASM Press; 2016:412–420. 10.1128/9781555818821.ch3.2.4. [DOI] [Google Scholar]
- 9. Interim U.S . Guidance for risk assessment and work restrictions for healthcare personnel with potential exposure to COVID‐19|CDC. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html. Accessed May 28, 2020.
- 10. Infection Prevention and Control of Epidemic‐and Pandemic‐Prone Acute Respiratory Diseases in Health Care WHO Interim Guidelines; 2007. Available at: http://www.who.int/csr/sars/infectioncontrol/en/. [PubMed]
- 11. World Health Organization. (2020). Modes of transmission of virus causing COVID‐19: implications for IPC precaution recommendations: scientific brief, 2020. 10.1056/NEJMc2004973.Cheng. [DOI]
- 12. Tran K, Cimon K, Severn M, Pessoa‐Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One Published online 2012;7:e35797. 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mick P, Murphy R. Aerosol‐generating otolaryngology procedures and the need for enhanced PPE during the COVID‐19 pandemic: a literature review. J Otolaryngol Head Neck Surg Published online 2020;49:29. 10.1186/s40463-020-00424-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Workman AD, Welling DB, Carter BS, et al. Endonasal instrumentation and aerosolization risk in the era of COVID‐19: simulation, literature review, and proposed mitigation strategies. Int Forum Allergy Rhinol. Published online 2020;10:798–805. 10.1002/alr.22577. [DOI] [PubMed] [Google Scholar]
- 15. Workman AD, Jafari A, Welling DB, et al. Airborne aerosol generation during endonasal procedures in the era of COVID‐19: risks and recommendations. Otolaryngol Head Neck Surg. 2020;163:465–470. 10.1177/0194599820931805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Projecting the transmission dynamics of SARS‐CoV‐2 through the postpandemic period. Science 2020;368:860–868. 10.1126/science.abb5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oberemok VV, Laikova KV, Yurchenko KA, Fomochkina II, Kubyshkin AV. SARS‐CoV‐2 will continue to circulate in the human population: an opinion from the point of view of the virus‐host relationship. Inflamm Res 2020;69:635–640. 10.1007/s00011-020-01352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. David AP, Jiam NT, Reither JM, Gurrola JG, Aghi M, El‐Sayed IH. Endoscopic skull base and transoral surgery during the COVID‐19 pandemic: minimizing droplet spread with a negative‐pressure otolaryngology viral isolation drape (NOVID). Head Neck Published online 2020;42:1577–1582. 10.1002/hed.26239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS‐CoV‐2 as compared with SARS‐CoV‐1. N Engl J Med. Published online 2020;382:1564–1567. 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fehr AR, Coronaviruses PS. An overview of their replication and pathogenesis. Coronaviruses: Methods and Protocols. New York: Springer; 2015:1–23. 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu Y, Ning Z, Chen Y, et al. Aerodynamic analysis of SARS‐CoV‐2 in two Wuhan hospitals. Nature. Published online 2020;582:557–560. 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- 22. Rameau A, Lee M, Enver N, Sulica L. Is office laryngoscopy an aerosol‐generating procedure? Laryngoscope. Published online July 16 2020;lary.28973. 10.1002/lary.28973. [DOI] [PMC free article] [PubMed] [Google Scholar]


