Abstract
Coronavirus disease 2019, the infectious disease caused by severe acute respiratory syndrome coronavirus‐2, has resulted in a global pandemic with unprecedented health, societal, and economic impact. The disease often manifests with flu‐like symptoms and is dominated by pulmonary complications, but widely diverse clinical manifestations involving multiple organ systems can result. We posit that viral tropism and the aberrant host immune response mediate the protean findings and severity in this disease. In general, extrapulmonary manifestations are a harbinger of or contemporaneously associate with disease progression, but in the case of some extrapulmonary findings (gastrointestinal and dermatologic), may track with milder disease. The precise underlying pathophysiological mechanisms remain incompletely elucidated, and additional immune phenotyping studies are warranted to reveal early correlates of disease outcomes and novel therapeutic targets.
Keywords: generalized infection, immune response, immune system, immunopathology, innate immunity, pathogenesis
1. INTRODUCTION: MECHANISTIC PERSPECTIVES
Although the ongoing coronavirus disease 2019 (COVID‐19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is most commonly dominated by lung involvement, it is a great imitator, affecting multiple organ systems in a myriad of ways (Figure 1). These diverse manifestations may be related to viral tropism and host immune responses although the precise mechanisms have not been elucidated. The first step in infection requires a virus binding to a host cell through its target receptor. 1 SARS‐CoV‐2 uses the angiotensin‐converting enzyme 2 (ACE2) for entry in concert with the transmembrane serine protease TMPRSS2 for spike protein priming (and potentially other coreceptors) that are widely distributed among many cells and tissues including alveolar epithelial type 2 cells, nasal goblet secretory cells, esophageal epithelial cells, gastric glandular cells, enterocytes from ileum and colon, cholangiocytes, macrophages, myocytes, kidney proximal tubules, glomerular parietal cells, vascular endothelial cells, and keratinocytes. 2 , 3 Infection with SARS‐CoV‐2 triggers a local immune response, including recruitment of innate immune populations and the generation of viral‐specific adaptive B and T cell responses, that in most cases, resolve infection with minimal inflammation and lung damage (Figure 1A). 1 Within 3 weeks after symptom onset, all patients test positive for antiviral immunoglobulin‐G (IgG); seroconversion for IgG and IgM can occur sequentially or simultaneously. 4 Alternatively (Figure 1B), in dysregulated immune responses, the direct cytopathic effect of SARS‐CoV‐2 can induce pyroptosis, a highly inflammatory form of programmed cell death, releasing endogenous danger signals including pathogen‐associated molecular patterns (PAMPs; eg, viral RNA) and damage‐associated molecular patterns (DAMPS; eg, ATP, nucleic acids and ASC oligomers) 1 that are recognized by epithelial cells, macrophages and endothelial cells both locally and in distant parts of the body. A cascade of local inflammation ensues characterized by secretion of pro‐inflammatory cytokines and chemokines that attract monocytes, macrophages, and T cells that mediate extensive pathology, culminating in acute respiratory distress syndrome (ARDS) 1 , 5 The “cytokine storm” spills into the circulation, as reflected by higher serum levels of interleukin (IL)‐6, IL‐8, IL‐17, G‐CSF, GM‐CSF, IP10, MCP1, MIP1α, tumor necrosis factor‐α and inflammatory chemokines (CCL2, CCL3, CXCL10) in patients with severe COVID‐19, which can lead to septic shock and multiorgan failure, 6 , 7 Macrophages show the highest number of interactions and potential crosstalk with ACE2‐expressing cells in multiple organs, 3 and inflammation may upregulate ACE2 expression on macrophages, 5 further amplifying viral sensing and tissue injury. Postmortem analyses of secondary lymph nodes and spleen implicate CD169+ macrophages that contain viral nucleocapsid protein as mediators of activation‐induced cell death (AICD) of lymphocytes. 8 T cell exhaustion with higher expression of PD‐1 and Tim‐3 has been demonstrated in patients with advanced COVID‐19 disease. 6 As reviewed here, the dysregulation of innate immune and hemostatic mechanisms, two critical emergency response pathways that maintain homeostasis under controlled circumstances, 9 have mechanistic underpinnings for the development of COVID‐19‐related complications.
Figure 1.
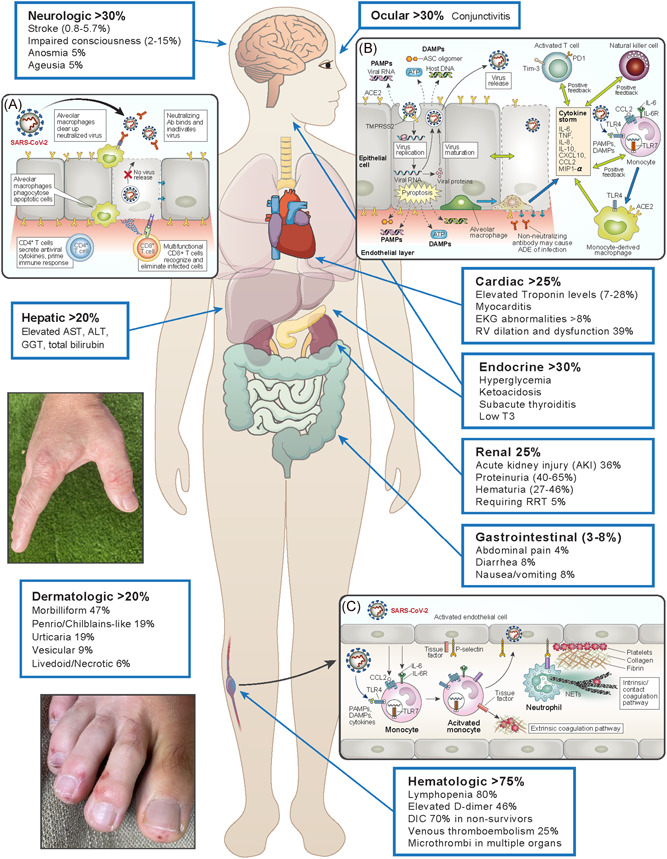
Clinical manifestations and possible mechanisms of severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) infection.The first step in infection requires virus binding and entry to a host cell through angiotensin‐converting enzyme 2 (ACE2)/transmembrane serine protease (TMPRSS2), expressed widely throughout the body. 1 , 2 , 3 The median incubation period is approximately 4–5 days before symptom onset, with fever and dry cough as the typical presenting symptoms; other symptoms include difficulty breathing, muscle and/or joint pain, headache/dizziness, and diarrhea. 1 Within 6 days of symptom onset, SARS‐CoV‐2 viral load reaches its peak. In the majority of patients, a healthy immune response (inset A) results in neutralizing antibodies that bind and inactivate SARS‐CoV‐2 and alveolar macrophages phagocytose neutralized viral particles and apoptotic cells, followed by generation of viral‐specific T cell responses, eliminating infected cells and preventing cell‐to‐cell viral spread with minimal inflammation and lung damage. However, when the cytopathic effect of SARS‐CoV‐2 overwhelms the first line of innate immune response (inset B), a form of programmed cell death known as pyroptosis leads to release of damage‐associated molecular patterns (DAMPs) and pathogen‐associated molecular patterns (PAMPs) that are recognized both locally and distally in the body. Inflammation characterized by chemo‐attraction to lung tissue and activation of immune cells leads to a “cytokine storm” that is reflected by high circulating levels (interleukin [IL‐6], IFN‐γ, MCP1, MIP1α, and IP‐10) and can result in septic physiology. Further, non‐neutralizing antibodies produced by B cells may worsen organ damage through antibody‐dependent enhancement (ADE) and increase hyperinflammatory responses 1 , 6 (adapted from 1 ). In addition to the well‐characterized pulmonary manifestations, SARS‐CoV‐2 induces protean clinical findings that include neurologic, ocular, cardiac, gastrointestinal/hepatic, renal, dermatologic (Pernio/Chilblains‐like acral eruption on hand and foot are shown), and hematologic abnormalities. The increased risk of thrombotic phenomenon, including microthrombi within many organs, is possibly related to direct viral infection of endothelium and indirect inflammatory‐mediated mechanisms, including neutrophil extracellular traps (NETs) which have the potential to initiate and propagate inflammation and thrombosis (inset C). 5 , 10 Circulating cytokines, DAMPs, and PAMPs trigger activation of blood monocytes to induce tissue factor (TF) membrane expression. Endothelial cells take up viral particles and produce chemo‐attractants that recruit monocytes and upregulate adhesion molecules. Tissue factor activates the extrinsic coagulation pathway that leads to fibrin deposition and blood clotting. Neutrophils are recruited by activated endothelial cells and can release neutrophil extracellular traps (NETs), scaffolds comprised of nuclear DNA studded with histones and granule proteins representing in many cases a process of cell death, 11 that induce the coagulation contact pathway and amplify platelet‐mediated clotting (adapted from Merad and Martin 5 )
2. CO‐INFECTIOUS COMPLICATIONS
The decreased effector function and frequency of cells involved in pathogen clearance coupled with prolonged hospitalization of many patients with COVID‐19 potentially impact the risk of coinfection with other viruses, bacteria, and fungus. Procalcitonin, advocated as a marker for severe bacterial infections, 12 is not elevated in patients with noncomplicated COVID‐19 at admission but its progressive increase is associated with a nearly 5‐fold higher risk of evolution towards a more severe course of the disease. 13 Although it is known that rates of secondary infection are significantly increased in patients with severe COVID‐19, for example, half of the nonsurvivors experienced a secondary infection in a series from Wuhan, China, 14 the overall rate of bacterial or fungal co‐infection in total COVID‐19 cases is estimated to be 8%. 15 Despite this, more than 70% of patients with COVID‐19 received empiric antimicrobial therapy, frequently broad‐spectrum and in noncritical care settings. 15 Remarkably, in one report from Wuhan, among patients in the intensive care unit (ICU) who died, >55% of COVID‐19 patients were coinfected with carbapenem‐resistant Acinetobacter baumanni, although the 17% rate of infection in the recovering COVID‐19 ICU to‐ward transfer patient group is also very high. 16 In a series from New York City, 17 bacteremia was noted in almost 12% of patients on invasive mechanical ventilation versus less than 2% in those not ventilated. These studies likely underestimate the actual rate of coinfection in this population since the diagnosis of the hospital‐ or ventilator‐associated pneumonia may depend on invasive procedures such as endotracheal aspirate or bronchoalveolar lavage, which may not be performed due to the risk of exposure of healthcare providers. Approximately 21% of patients with SARS‐CoV‐2 will test positive for another respiratory pathogen, including rhinovirus/enterovirus and respiratory syncytial virus, which is comparable to the rate observed in symptomatic patients (cough, fever, dyspnea) who are negative for SARS‐CoV‐2. 18
3. HEMATOLOGIC FINDINGS
Numerous hematologic findings and complications of SARS‐CoV‐2 infection have been identified. Lymphopenia, which may result from tissue recruitment of lymphocyte apoptosis (more pronounced for CD8+ T cells) 5 following viral‐receptor binding or indirectly through cytokine activation, is a cardinal laboratory finding that occurs in >80% of patients, and lower lymphocyte count is associated with the risk of ARDS. 17 , 19 , 20 Coagulation disorders are also frequently encountered, particularly among those with severe disease. 12 , 14 , 21 In a multicenter retrospective study of 560 patients during the first 2 months of the pandemic in China, 260 (46.4%) had elevated d‐dimers (>0.5 mg/L). 22 Elevated d‐dimers (fibrin degradation products) correlate with mortality, and non‐survivors had a significant rise in serial values over the course of their hospitalization while survivors' d‐dimers remained relatively low. 14 In one series, 23 more than 70% of patients who died of COVID‐19 complications fulfilled the clinical criteria (elevated d‐dimer, fibrinogen, prolonged PT, and thrombocytopenia) 24 for disseminated intravascular coagulation (DIC), with a median time from admission to DIC manifestation of 4 days. As COVID‐19 spread to Europe and the United States, mounting data indicated another concerning clinical problem: increased venous thromboembolism (VTE) rates in patients with COVID requiring ICU care despite appropriate pharmacologic prophylaxis. In the first cohort of ICU patients with COVID‐19 reported from the Netherlands (n = 184), where low molecular heparin prophylaxis was given to all patients, the cumulative incidence of VTE was 27%, and an additional 4% of patients developed an arterial event. 25 In a follow‐up to that report, the cumulative incidence at 14 days of the combined endpoint increased to 49%, with 65 (35%) patients diagnosed with pulmonary embolism (PE), 26 the majority involving segmental or more proximal arteries. Importantly, diagnostic testing for VTE was only performed in patients with suggestive symptomatology, so the actual rate of deep venous thrombosis (DVT) may have been underestimated. Another Dutch study evaluated the cumulative incidence of symptomatic and asymptomatic patients in the ICU and the general wards 27 with the majority of patients receiving standard pharmacologic prophylaxis. Among patients in the ICU, 47% developed VTE (28% symptomatic) with a cumulative incidence of 59% (34% symptomatic) at 21 days. Of note, none of the 19 patients who presented and remained on full‐dose anticoagulation developed VTE; only 3% of patients on the regular wards developed VTE with a cumulative incidence of 9.2% at 21 days. Moreover, microthrombi have been identified in the brain, heart, liver, and kidneys of patients with COVID‐19. 5 The pathophysiologic basis likely includes cytokine‐, PAMP‐, and DAMP‐mediated activation of endothelium, platelets, monocytes, and possibly complement. 5, , 9 Activated endothelial cells recruit neutrophils that can form neutrophil extracellular traps (NETs), scaffolds comprised of nuclear DNA studded with histones and granule proteins representing in many cases a process of cell death, 11 that induce the coagulation contact pathway and amplify platelet‐mediated clotting. 5 Tissue factor (CD142 or coagulation factor III pathway) is expressed on monocytes in response to pro‐inflammatory cytokines as well as on endothelial cells, promoting the transformation of prothrombin into thrombin, converting circulating fibrinogen into fibrin and resulting in tissue damage and microangiopathic pathology 5 , 9 (Figure 1C).
4. CARDIAC MANIFESTATIONS
Cardiac complications are common in SARS‐CoV‐2 infection (~20‐25%) 14 , 28 and the prevalence and risk of death of severe COVID‐19 is higher in older patients with chronic comorbidities (eg, arterial hypertension, diabetes, cardiac, and cerebrovascular disorders). 29 Patients presenting with evidence of cardiac injury are more prone to have coagulation disorders (7.3%) as compared to those without cardiac signs (1.8%). 30 Between 7% and 28% of SARS‐CoV‐2 infected patients have elevated troponin levels 21 , 28 , 30 , 31 that may be the result of direct myocarditis through viral infection, cytokine storm, and coronary microvascular ischemia (endothelium with viral inclusion bodies, inflammation and resultant dysfunction, including vasoconstriction 31 , 32 ). Troponin levels have prognostic implications: those with elevated levels at baseline have a greater risk of severe disease, increased intensive care unit admissions, and significantly higher mortality. 27 , 28 , 30 In a cohort study, the presence of elevated troponin levels was associated with mortality (hazard ratio 4.26; 95% CI 1.92–9.49). In a single‐center retrospective study of 187 COVID‐19 patients, the risk of death increased linearly stratified by the presence of elevated troponin‐T and/or presence of prior cardiovascular disease (7.62% [no cardiovascular disease history]; 13% [cardiovascular disease history]; 37.5% [elevated troponin only]; 69% [elevated troponin and cardiovascular disease history]). 28 Therefore, troponin elevation may even carry prognostic significance in the absence of cardiovascular disease; nonsurvivors showed a trend of progressive increase in troponin levels, suggesting that elevation may reflect the progression of COVID‐19 disease to a severe stage. 33 Cardiac arrhythmias are also frequent, occurring in ~17% of patients. 21 Common electrocardiogram findings included atrial premature contractions (~8%), abnormal intraventricular conduction (~12%), left ventricular hypertrophy (15.5%), and repolarization abnormalities (40%). Multivariable logistic regression analysis that included age, electrocardiographic and clinical characteristics identified characteristics associated with increased odds of death: atrial premature contractions (odds ratio [OR], 2.57), right bundle branch or intraventricular block (OR, 2.61), ischemic T‐wave inversion (OR, 3.49), and nonspecific repolarization (OR, 2.31). 34 Echocardiographic assessment in 100 consecutively admitted COVID‐19 also revealed a high prevalence of abnormalities (68%) with right ventricular dilatation and dysfunction (39%) being the most common, followed in frequency by left ventricular diastolic (16%) and systolic (10%) dysfunction. 35
5. GASTROINTESTINAL AND HEPATIC FINDINGS
Patients with COVID‐19 can present with gastrointestinal (GI) symptoms which may precede respiratory manifestations of disease by 1–2 days. 21 A recent meta‐analysis reported the overall pooled prevalence for abdominal pain (3.6%), diarrhea (7.7%), and nausea or vomiting (7.8%) 36 . GI symptoms, without any respiratory involvement is rare, and seen in only about 3% of patients. 37 , 38 Patients with GI symptoms are more likely to have a longer disease duration (≥1 week) compared to those without (33% vs. 22%), although they may be less likely to require ICU admission. 39 In particular, diarrhea has been associated with a slightly longer interval between the symptom onset and hospital admission, possibly reflecting a delayed recognition of GI symptoms as being COVID‐19 related. 37 Diarrhea lasts an average duration of 5.4 days (with a frequency of 4.3 ± 2.2 bowel movements per day) and is usually self‐limited. 40 Patients with GI symptoms are more likely to have positive viral RNA stool tests compared to those without GI symptoms (73% vs. 14%, p = .033). 40 Nevertheless, there are conflicting data on whether detection of viral RNA confers infectivity, as this may be indicative of live virus or inactivated viral particles. 40 , 41 , 42 However, considering that more than 20% of patients with SARS‐COV‐2 demonstrate positive viral RNA in feces after respiratory tract tests have converted to negative, the fecal‐oral transmission could be an additional route for viral spread, 43 warranting enhanced control measures to prevent spread. 44 Liver biochemistry test abnormalities are also frequently noted in patients with COVID‐19: elevated aspartate aminotransferase or alanine aminotransferase in 15% and hyperbilirubinemia in ~17%. 36 Patients with abnormal liver enzymes were more likely to have higher inflammatory indices such as C‐reactive protein and procalcitonin and fevers, and a higher proportion were receiving treatment with lopinavir/ritonavir compared to those with normal LFTs. 45 Abnormal liver tests at presentation are prognostically significant, associated with a greater than 2‐fold risk of developing severe disease or requiring ICU admission. 45 , 46 , 47
6. ENDOCRINOLOGIC MANIFESTATIONS
Endocrinologic manifestations are frequent in patients presenting with SARS‐CoV‐2 infection. The prevalence of diabetes mellitus (DM) amongst COVID‐19 studies varies substantially (~5%–44%) and may reflect estimated prevalence in the respective population 48 , 49 Hyperglycemia and DM are positively associated with COVID‐19 disease severity and risk of death. In 1099 patients from China, 26.9% of patients with DM had ICU admission, mechanical ventilation, or death compared to 6.1% of non‐DM patients. 22 Early data from the United States (US) showed that 24%–35% of hospitalized patients had DM. 49 , 50 , 51 Meta‐analyses have found DM to be consistently associated with risk of severe disease or mortality (OR, 1.90–3.21), 6 , 7 , 22 and a large whole‐population study confirmed DM as a risk factor for in‐hospital COVID‐19‐related death after adjustment for demographics and comorbidities. 52 Acute hyperglycemia itself is a manifestation of COVID‐19 with negative prognostic significance. 52 , 53 , 54 Hyperglycemia without pre‐existing DM occurred in 14.3% of hospitalized patients in a US series, and in‐hospital mortality was 41.7% in this group. 53 Ketoacidosis also may complicate COVID‐19 in patients with DM, noted in more than 10% of hospitalized cases. 55 The underlying mechanisms include the low‐grade inflammation that characterizes obesity that is further exacerbated by the cytokine storm induced by SARS‐CoV‐2 infection. Moreover, there is an expression of ACE2 in pancreatic islets 56 , 57 which is enhanced in patients with DM/hyperglycemia compared to controls without DM, 57 raising the possibility that direct damage to pancreatic islets through SARS‐CoV‐2 ‐ACE2 binding may contribute to hyperglycemia.
Thyroid dysfunction may accompany SARS‐CoV2 infection, either as biochemical thyrotoxicosis (20%–56%) and hypothyroidism (5%). 57 , 58 , 59 Lower TSH and triiodothyronine (T3) levels are associated with severity of illness and mortality from COVID‐19. 7 , 57 , 58 , 59 However, pre‐existing hypothyroidism was not associated with an increased risk of hospitalization, mechanical ventilation, or death, 60 suggesting risk may not be due to underlying thyroid disease per se. Subacute thyroiditis (SAT) has been reported in patients (particularly women) with classic neck pain and thyrotoxicosis, fever, cardiovascular manifestations, and elevated inflammatory markers several weeks after resolution SARS‐CoV‐2 infection. 60 , 61 , 62 Because the initial symptoms of COVID‐19 may have been mild in patients presenting with SAT, assessment for preceding SARS‐CoV‐2 exposure may be warranted in such patients.
7. RENAL COMPLICATIONS
Acute kidney injury (AKI) in patients with COVID‐19 is a frequent complication that may be caused by direct infection of the kidney by SARS‐COV‐2 with tubular injury, pigment casts due to rhabdomyolysis, endothelial damage, glomerular thrombi, 63 , 64 cytokine storm, and/or hemodynamic changes due to severe sepsis and multiorgan failure. 7 In the largest series published in the US (n = 5449), the incidence of AKI was 36.6% of which 14.3% required renal replacement therapy (RRT) with a mortality rate of 35% in those who developed AKI. 65 Older age, black race, diabetes mellitus, cardiovascular disease, hypertension, and need for ventilation and vasopressor medications were associated with a higher risk for developing AKI and associated with higher mortality. AKI has been reported to occur from 24 h to 15 days after admission. 14 , 65 , 66 Additionally, a high frequency of other renal abnormalities have been demonstrated in a few studies, with 40‐65% presenting with proteinuria, 27‐46% with hematuria, and 44% with both. 65 , 66 , 67 Presence of AKI, proteinuria, or hematuria, have been independently associated with a higher risk of mortality. 7 , 14 , 66 , 67 Currently the only treatment for COVID‐19 with AKI is supportive management and RRT, with the delivery of the latter potentially being compromised by the higher risk of thrombosis of central lines and dialysis access related to SARS‐CoV‐2 infection‐related hypercoagulability. Before seeking medical care, patients may be experiencing fever and GI symptoms including nausea, vomiting, and diarrhea making them prone to intravascular volume depletion and pre‐renal AKI. In these patients, early aggressive volume resuscitation with balanced solutions may prevent the development of acute tubular injury due to prolonged severe renal hypoperfusion. However, caution should be taken when administering fluids as too little may promote hyper‐coagulation and worsen AKI, while a positive fluid balance may worsen gas exchange and is an independent predictor of worse outcomes in critically ill patients. Similar to all AKI, the best timing of RRT remains controversial. 68 , 69 , 70 , 71 In the absence of an urgent indication, a delayed approach to RRT initiation may be considered during the COVID pandemic when RRT resources are stretched thin, 72 should be individualized and considered in patients in whom metabolic and fluid demand surpasses the capacity of the kidney. 73
8. DERMATOLOGIC FINDINGS
The cutaneous manifestations of SARS‐CoV‐2 infection occur frequently (>20%), are quite varied, and can be grouped into five main distinctive morphologic categories: morbilliform (maculopapular), urticarial, vesicular, pernio/chilblains‐like, and necrotic/livedoid. 74 Other less common cutaneous findings include a papulosquamous, pityriasis rosea‐like eruption, a perifollicular papular eruption, erythema multiforme‐like lesions, axillary erythema or purpura, a Dengue fever‐like petechial eruption, an enanthem, and edematous papules of the dorsal hands or other extensor surfaces. The majority of dermatologic findings occur either concurrently (56%) or after (37%) other manifestations of COVID‐19. 74 Morbilliform eruptions are the most common reported cutaneous finding in COVID‐19 patients, representing 47% of all cutaneous eruptions in the Spanish cohort 74 and 16% of all patients in the Italian study. 75 The morphology was not uniform with some patients showing the additional findings of purpura, petechiae, pityriasis rosea‐like scale, or edema leading to a pseudovesicular pattern (mainly on the dorsal hands) (Figure 1). These patterns can be common to other viral exanthems and are not considered specific for COVID‐19. Pernio/Chilblains‐like eruptions are characterized by acral erythema and sometimes vesicles, pustules, or purpura on the hands and feet; the toes are the most frequently involved sites (Figure 1). This pattern occurred in 19% of the Spanish COVID‐19 cases with cutaneous findings. 74 Of note, this pattern is described mostly in the young, portends a good prognosis, and may show a delayed onset. Pain or pruritus may be present, but patients may lack other characteristic signs and symptoms of COVID‐19. If more typical COVID‐19 symptoms are present, they are typically mild. Furthermore, patients may test negative for COVID‐19 at the time of this eruption; it has been suggested that a robust Type I interferon response (which is deficient in patients who develop severe COVID‐19 disease 76 ) may limit viral replication but induce this late auto‐inflammatory manifestation. 77 In contrast, livedoid/necrotic lesions, seen in 6% of the Spanish cohort, 74 particularly in elderly individuals, were associated with coagulopathies and a 10% mortality.
9. NEUROLOGIC COMPLICATIONS
Neurological manifestations of SARS‐CoV‐2 infection are common, being reported in about 36% of hospitalized patients 78 including central and peripheral nervous system findings. Although similar neurological manifestations have been reported in general viral and specific SARS infection, SARS‐CoV‐2 seems to have a greater propensity to affect parts of the nervous system involved with sensory processes. Impairments of taste, smell (anosmia, ageusia), 79 and vision occur at much higher rates than in other viral infection, an observation that may be tied to unique tropism to neuronal cells. 80 In general, neurological manifestations are more common with severe infection compared to non‐severe as it relates to stroke (5.7% vs. 0.8%), impaired consciousness (14.8% vs. 2.4%), and skeletal muscle injury (19.3% vs. 4.8%). 78 However, patients without typical symptoms (fever, cough, anorexia, and diarrhea) of COVID‐19 may have neurologic findings as their presenting symptoms, and most neurologic manifestations occur early in the illness (with the median time to hospital admission of 1–2 days). The combination of anorexia and diarrhea coupled with a loss of smell, taste, and fever is 99% specific for SARS‐CoV‐2 infection. 81 Most patients presenting with stroke in the setting of SARS‐CoV‐2 infection will be older with the usual stroke risk factors including hypertension, diabetes, and dyslipidemia, although case series have reported increasing rates of young patients with ischemic stroke, particularly large vessel occlusion. 82 In addition, about one‐third of patients have ocular findings (eg, conjunctival hyperemia, chemosis, epiphora, or increased secretions) particularly in patients with more severe disease. 83
In summary, it is important for the clinician to consider and identify the broad range of manifestations related to SARS‐CoV‐2 infection which can precede or be neglected due to respiratory impairment. Early consideration is paramount to avoid delayed diagnosis/misdiagnosis or missing the opportunity to treat and prevent further transmission. In general, extrapulmonary manifestations are a harbinger of or contemporaneously associate with disease progression, but in the case of some GI and dermatologic findings, may track with milder disease. The precise underlying pathophysiological mechanisms remain incompletely elucidated, and additional immune phenotyping studies are warranted to reveal early correlates of disease outcomes and novel therapeutic targets.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
All authors discussed the layout of the manuscript; each author wrote their respective sections according to specialty.
ACKNOWLEDGMENT
Hugo R. Rosen is supported by R01AI120622, R01 DK117004, R01 AI127463, and 2P30 DK48522.
Rosen HR, O'Connell C, Nadim MK, et al. Extrapulmonary manifestations of severe acute respiratory syndrome coronavirus‐2 infection. J Med Virol. 2021;93:2645–2653. 10.1002/jmv.26595
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Tay MZ, Poh CM, Renia L, MacAry PA, Ng LFP. The trinity of COVID‐19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020;526(1):135‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Long QX, Liu BZ, Deng HJ, et al. Antibody responses to SARS‐CoV‐2 in patients with COVID‐19. Nat Med.2020;26:845‐848. [DOI] [PubMed] [Google Scholar]
- 5. Merad M, Martin JC. Pathological inflammation in patients with COVID‐19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20:355‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cao X. COVID‐19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20(5):269‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jin Y, Yang H, Ji W, et al. Virology, epidemiology, pathogenesis, and control of COVID‐19. Viruses. 2020;12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lillicrap D. Disseminated intravascular coagulation in patients with 2019‐nCoV pneumonia. J Thromb Haemost. 2020;18(4):786‐787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zuo Y, Zuo M, Yalavarthi S, et al. Neutrophil extracellular traps and thrombosis in COVID‐19. medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaplan MJ, Radic M. Neutrophil extracellular traps: double‐edged swords of innate immunity. J Immunol. 2012;189(6):2689‐2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Jong E, van Oers JA, Beishuizen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open‐label trial. Lancet Infect Dis. 2016;16(7):819‐827. [DOI] [PubMed] [Google Scholar]
- 13. Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID‐19): a meta‐analysis. Clin Chim Acta. 2020;505:190‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rawson TM, Moore LSP, Zhu N, et al. Bacterial and fungal co‐infection in individuals with coronavirus: a rapid review to support COVID‐19 antimicrobial prescribing. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang G, Hu C, Luo L, et al. Clinical features and short‐term outcomes of 221 patients with COVID‐19 in Wuhan, China. J Clin Virol. 2020;127:104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid‐19 in New York City. N Engl J Med. 2020;382:2372‐2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim D, Quinn J, Pinsky B, Shah NH, Brown I. Rates of co‐infection between SARS‐CoV‐2 and other respiratory pathogens. JAMA. 2020;323:2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Terpos E, Ntanasis‐Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID‐19. Am J Hematol. 2020;95:834‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li T, Lu H, Zhang W. Clinical observation and management of COVID‐19 patients. Emerg Microbes Infect. 2020;9(1):687‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Taylor F, Jr. , Toh CH, Hoots K, Wada H, Levi M. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86(5):1327‐1330. [PubMed] [Google Scholar]
- 25. Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:145‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Klok FA, Kruip MJHA, van der Meer NJM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID‐19: an updated analysis. Thromb Res. 2020;191:148‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Middeldorp S, Coppens M, Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost. 2020;18:1995‐2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5:811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moccia F, Gerbino A, Lionetti V, et al. COVID‐19‐associated cardiovascular morbidity in older adults: a position paper from the Italian Society of Cardiovascular Researches. GeroScience. 2020;42:1021‐1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol. 2020;5:802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet. 2020;395(10234):1417‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McCullough SA, Goyal P, Krishnan U, Choi JJ, Safford MM, Okin PM. Electrocardiographic findings in COVID‐19: insights on mortality and underlying myocardial processes. J Card Fail. 2020;26:626‐632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Szekely Y, Lichter Y, Taieb P, et al. The spectrum of cardiac manifestations in coronavirus disease 2019 (COVID‐19) ‐ a systematic echocardiographic study. Circulation. 2020;142:342‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sultan S, Altayar O, Siddique SM, et al. AGA institute rapid review of the GI and liver manifestations of COVID‐19, meta‐analysis of international data, and recommendations for the consultative management of patients with COVID‐19. Gastroenterology. 2020;159:320‐334.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pan L, Mu M, Yang P, et al. Clinical characteristics of COVID‐19 patients with digestive symptoms in Hubei, China: a descriptive, cross‐sectional, multicenter study. Am J Gastroenterol. 2020;115(5):766‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jin X, Lian JS, Hu JH, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus‐infected disease 2019 (COVID‐19) with gastrointestinal symptoms. Gut. 2020;69(6):1002‐1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nobel YR, Phipps M, Zucker J, et al. Gastrointestinal Symptoms and COVID‐19: Case‐Control Study from the United States. Gastroenterology. 2020;159:373‐375.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Han C, Duan C, Zhang S, et al. Digestive symptoms in COVID‐19 patients with mild disease severity: clinical presentation, stool viral RNA testing, and outcomes. Am J Gastroenterol. 2020;115:916‐923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang W, Xu Y, Gao R, et al. Detection of SARS‐CoV‐2 in different types of clinical specimens. JAMA. 2020;323:1843‐1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID‐2019. Nature. 2020;581:465‐469. [DOI] [PubMed] [Google Scholar]
- 43. Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS‐CoV‐2. Gastroenterology. 2020;158(6):1831‐1833.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bonato G, Dioscoridi L, Mutignani M. Faecal‐oral transmission of SARS‐COV‐2: practical implications. Gastroenterology. 2020;159:1621‐1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fan Z, Chen L, Li J, et al. Clinical features of COVID‐19‐related liver functional abnormality. Clin Gastroenterol Hepatol. 2020;18(7):1561‐1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hajifathalian K, Krisko T, Mehta A, et al. Gastrointestinal and hepatic manifestations of 2019 novel coronavirus disease in a large cohort of infected patients from New York: clinical implications. Gastroenterology. 2020;159:1137‐1140.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cai Q, Huang D, Yu H, et al. COVID‐19: abnormal liver function tests. J Hepatol. 2020;73:566‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Apicella M, Campopiano MC, Mantuano M, Mazoni L, Coppelli A, Del Prato S. COVID‐19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8(9):782‐792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID‐19 in China. Clin Res Cardiol. 2020;109(5):531‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Team CC‐R. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 ‐ United States, February 12‐March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):382‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Holman N, Knighton P, Kar P, et al. Risk factors for COVID‐19‐related mortality in people with type 1 and type 2 diabetes in England: a population‐based cohort study. Lancet Diabetes Endocrinol. 2020;8(10):823‐833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bode B, Garrett V, Messler J, et al. Glycemic characteristics and clinical outcomes of COVID‐19 patients hospitalized in the United States. J Diabetes Sci Technol. 2020;14(4):813‐821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sardu C, D'Onofrio N, Balestrieri ML, et al. Outcomes in patients with hyperglycemia affected by COVID‐19: can we do more on glycemic control? Diabetes Care. 2020;43(7):1408‐1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li J, Wang X, Chen J, Zuo X, Zhang H, Deng A. COVID‐19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metab . 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liu F, Long X, Zhang B, Zhang W, Chen X, Zhang Z. ACE2 expression in pancreas may cause pancreatic damage after SARS‐CoV‐2 infection. Clin Gastroenterol Hepatol. 2020;18(9):2128‐2130.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Taneera J, El‐Huneidi W, Hamad M, Mohammed AK, Elaraby E, Hachim MY. Expression profile of SARS‐CoV‐2 host receptors in human pancreatic islets revealed upregulation of ACE2 in diabetic donors. Biology. 2020;9(8):215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lania A, Sandri MT, Cellini M, Mirani M, Lavezzi E, Mazziotti G. Thyrotoxicosis in patients with COVID‐19: the THYRCOV study. Eur J Endocrinol. 2020;183(4):381‐387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen M, Zhou W, Xu W. Thyroid function analysis in 50 patients with COVID‐19: a retrospective study. Thyroid. 2020. 10.1089/thy.2020.0363 [DOI] [PubMed] [Google Scholar]
- 60. Gerwen M, Alsen M, Little C, et al. Risk factors and outcomes of COVID‐19 in New York City; a retrospective cohort study. J Med Virol. 2020. 10.1002/jmv.26337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Brancatella A, Ricci D, Cappellani D, et al. Is subacute thyroiditis an underestimated manifestation of SARS‐CoV‐2 infection? Insights from a case series. J Clin Endocrinol Metab. 2020;105(10):dgaa537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Brancatella A, Ricci D, Viola N, Sgro D, Santini F, Latrofa F. Subacute thyroiditis after Sars‐COV‐2 infection. J Clin Endocrinol Metab. 2020;105(7):2367‐2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Su H, Yang M, Wan C, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID‐19 in China. Kidney Int. 2020;98:219‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Farkash EA, Wilson AM, Jentzen JM. Ultrastructural evidence for direct renal infection with SARS‐CoV‐2. J Am Soc Nephrol. 2020;31:1683‐1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hirsch JS, Ng JH, Ross DW, et al. Acute kidney injury in patients hospitalized with COVID‐19. Kidney Int. 2020;98:209‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in‐hospital death of patients with COVID‐19. Kidney Int. 2020;97(5):829‐838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pei G, Zhang Z, Peng J, et al. Renal involvement and early prognosis in patients with COVID‐19 pneumonia. J Am Soc Nephrol. 2020;31:1157‐1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gaudry S, Hajage D, Schortgen F, et al. Initiation strategies for renal‐replacement therapy in the intensive care unit. N Engl J Med. 2016;375(2):122‐133. [DOI] [PubMed] [Google Scholar]
- 69. Barbar SD, Clere‐Jehl R, Bourredjem A, et al. Timing of renal‐replacement therapy in patients with acute kidney injury and sepsis. N Engl J Med. 2018;379(15):1431‐1442. [DOI] [PubMed] [Google Scholar]
- 70. Zarbock A, Kellum JA, Schmidt C, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically Ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA. 2016;315(20):2190‐2199. [DOI] [PubMed] [Google Scholar]
- 71. Gaudry S, Hajage D, Benichou N, et al. Delayed versus early initiation of renal replacement therapy for severe acute kidney injury: a systematic review and individual patient data meta‐analysis of randomised clinical trials. Lancet. 2020;395(10235):1506‐1515. [DOI] [PubMed] [Google Scholar]
- 72. Goldfarb DS, Benstein JA, Zhdanova O, et al. Impending shortages of kidney replacement therapy for COVID‐19 patients. Clin J Am Soc Nephrol. 2020;15:880‐882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ostermann M, Joannidis M, Pani A, et al. Patient selection and timing of continuous renal replacement therapy. Blood Purif. 2016;42(3):224‐237. [DOI] [PubMed] [Google Scholar]
- 74. Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34(5):e212‐e213. [DOI] [PubMed] [Google Scholar]
- 76. Blanco‐Melo D, Nilsson‐Payant BE, Liu WC, et al. Imbalanced host response to SARS‐CoV‐2 drives development of COVID‐19. Cell. 2020;181(5):1036‐1045.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID‐19) infection‐induced chilblains: a case report with histopathologic findings. JAAD Case Rep. 2020;6:489‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Vaira LA, Salzano G, Deiana G, De Riu G. Anosmia and ageusia: common findings in COVID‐19 patients. Laryngoscope. 2020;130:1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kosaisavee V, Suwanarusk R, Chua ACY, et al. Strict tropism for CD71(+)/CD234(+) human reticulocytes limits the zoonotic potential of Plasmodium cynomolgi. Blood. 2017;130(11):1357‐1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chen A, Agarwal A, Ravindran N, To C, Zhang T, Thuluvath PJ. Are gastrointestinal symptoms specific for COVID‐19 infection? A prospective case‐control study from the United States. Gastroenterology. 2020;159:1161‐1163.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Oxley TJ, Mocco J, Majidi S, et al. Large‐vessel stroke as a presenting feature of Covid‐19 in the young. N Engl J Med. 2020;382(20):e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wu P, Duan F, Luo C, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID‐19) in Hubei Province, China. JAMA Ophthalmol. 2020;138(5):575‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


